Abstract
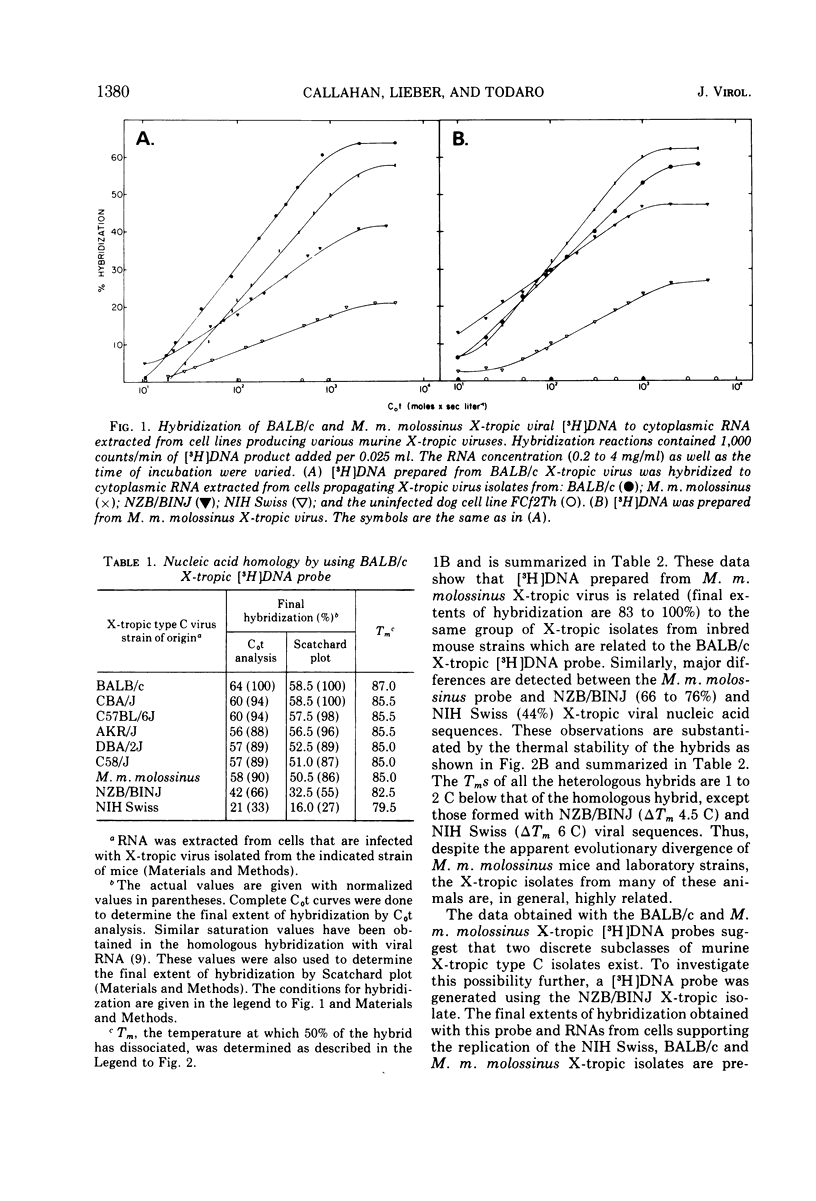
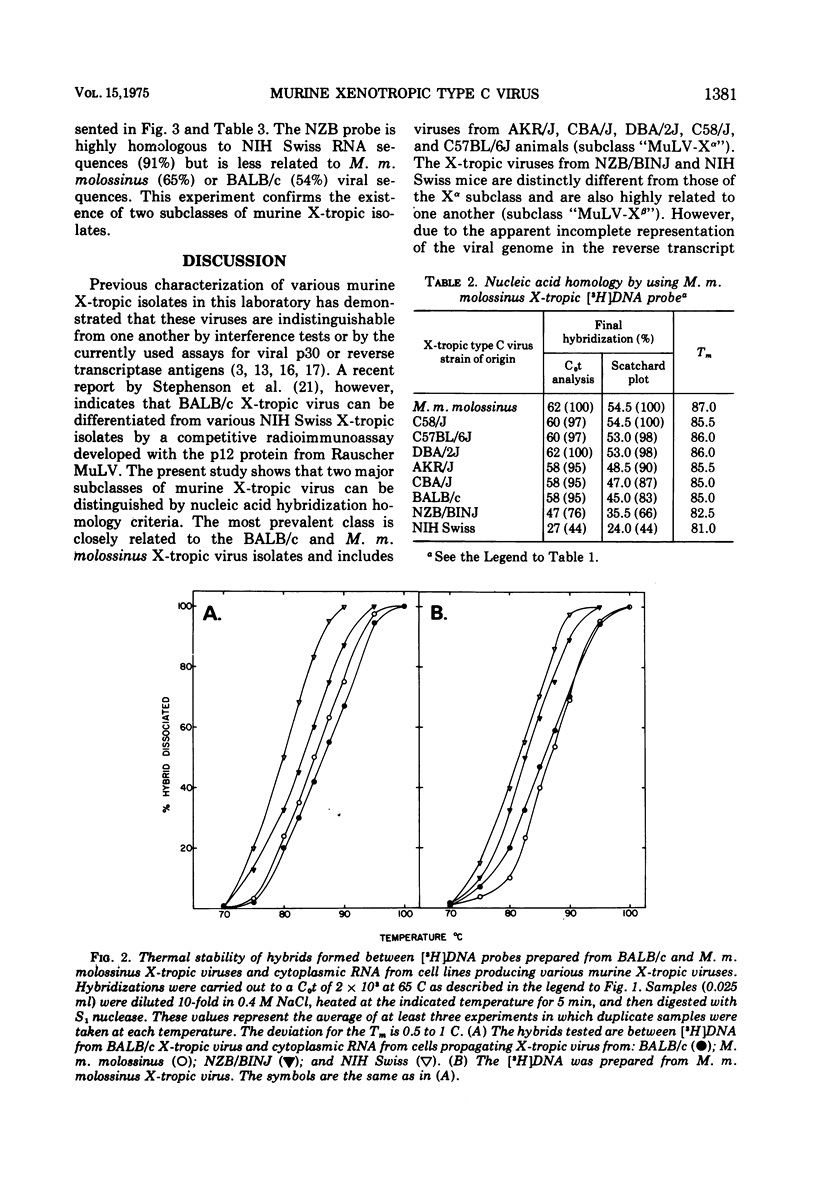
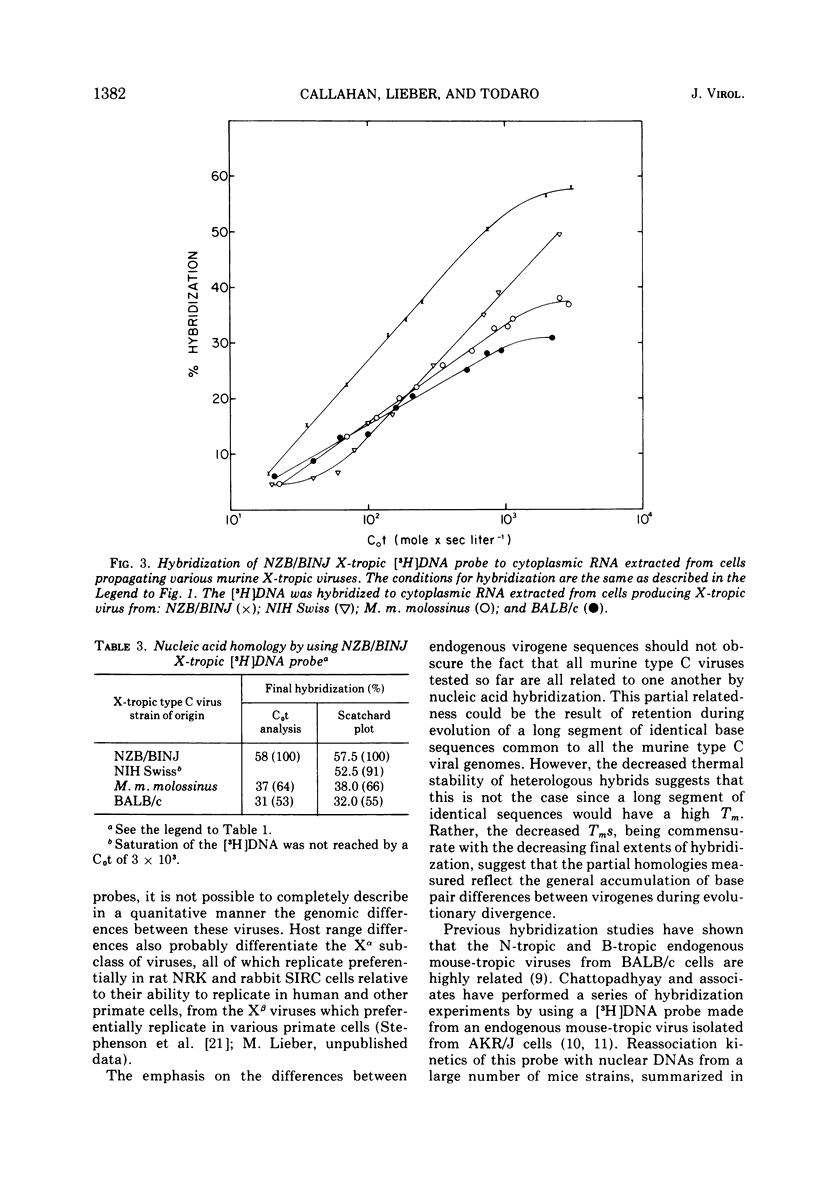
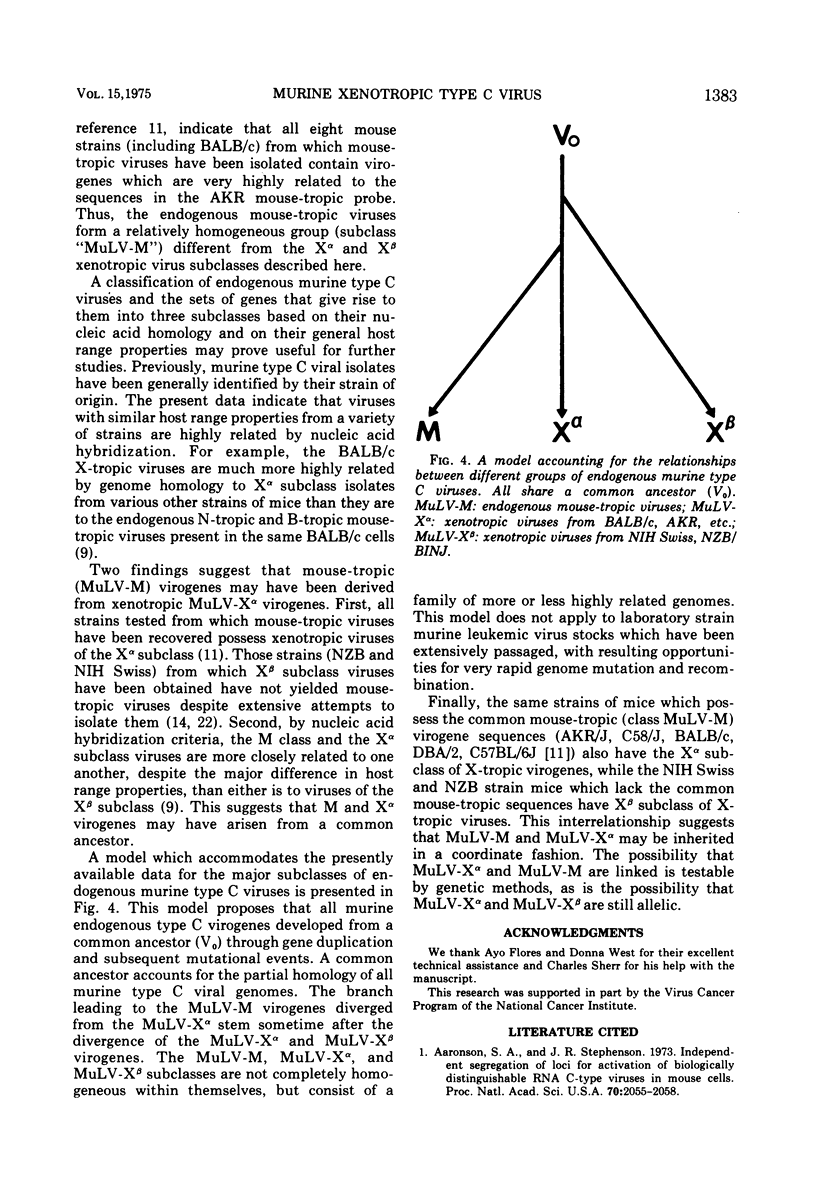
Two major subclasses of xenotropic (X-tropic) murine type C viruses can be distinguished by nucleic acid hybridization. The most frequently encountered subclass (MuLV-X-alpha) includes isolates from BALB/c, C57BL/6J, C58/J, AKR/J, CBA/J, and DBA/2J inbred strains and from the Asian feral mouse subspecies Mus musculus molossinus. The other subclass (MuLV-X-beta) consists of viruses isolated from the NIH Swiss and NZB/BINJ strains. Thus, significant polymorphism exists among the endogenous type C virogenes of a single species, Mus musculus. MuLV-X-alpha genes are found in strains that also have endogenous mouse-tropic viruses (either N-tropic, B-tropic, or both), whereas the MuLV-X-beta subclass is restricted to mouse strains from which mouse-tropic viruses have not yet been isolated. The results are consistent with a model which proposes that mouse-tropic endogenous viruses are derived from the MuLV-X-alpha subclass.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2055–2058. doi: 10.1073/pnas.70.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Todaro G. J. A distinct class of inducible murine type-C viruses that replicates in the rabbit SIRC cell line. Proc Natl Acad Sci U S A. 1974 Mar;71(3):602–606. doi: 10.1073/pnas.71.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Callahan R., Benveniste R. E., Lieber M. M., Todaro G. J. Nucleic acid homology of murine type-C viral genes. J Virol. 1974 Dec;14(6):1394–1403. doi: 10.1128/jvi.14.6.1394-1403.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Evidence that the AKR murine-leukemia-virus genome is complete in DNA of the high-virus AKR mouse and incomplete in the DNA of the "virus-negative" NIH mouse. Proc Natl Acad Sci U S A. 1974 Jan;71(1):167–171. doi: 10.1073/pnas.71.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Qualitative and quantitative studies of AKR-type murine leukemia virus sequences in mouse DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):1085–1101. doi: 10.1101/sqb.1974.039.01.124. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P., Huebner R. J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970 Feb;5(2):221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson I. C., Lieber M. M., Todaro G. J. Mink cell line Mv 1 Lu (CCL 64). Focus formation and the generation of "nonproducer" transformed cell lines with murine and feline sarcoma viruses. Virology. 1974 Jul;60(1):282–287. doi: 10.1016/0042-6822(74)90386-9. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Pincus T. Demonstration of biological activity of a murine leukemia virus of New Zealand black mice. Science. 1970 Oct 16;170(3955):326–327. doi: 10.1126/science.170.3955.326. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB, and other mouse strains. Science. 1973 Dec 14;182(4117):1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Lieber M., Sherr C., Potter M., Todaro G. Isolation of type-C viruses from the Asian feral mouse Mus musculus molossinus. Int J Cancer. 1975 Feb 15;15(2):211–220. doi: 10.1002/ijc.2910150206. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., McCarthy B. J. Analysis of DNA-RNA hybridization data using the Scatchard plot. Biochem Biophys Res Commun. 1973 Dec 10;55(3):805–811. doi: 10.1016/0006-291x(73)91215-1. [DOI] [PubMed] [Google Scholar]
- McConaughy B. L., McCarthy B. J. Related base sequences in the DNA of simple and complex organisms. VI. The extent of base sequence divergence among the DNAs of various rodents. Biochem Genet. 1970 Jun;4(3):425–446. doi: 10.1007/BF00485758. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Aaronson S. A., Arnstein P., Huebner R. J., Tronick S. R. Demonstration of two immunologically distinct xenotropic type C RNA viruses of mouse cells. Virology. 1974 Sep;61(1):56–63. doi: 10.1016/0042-6822(74)90241-4. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Arnstein P., Parks W. P., Lennette E. H., Huebner R. J. A type-C virus in human rhabdomyosarcoma cells after inoculation into NIH Swiss mice treated with antithymocyte serum. Proc Natl Acad Sci U S A. 1973 Mar;70(3):859–862. doi: 10.1073/pnas.70.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E., Lieber M. M., Melnick J. L. Type C viruses of baboons: isolation from normal cell cultures. Cell. 1974 May;2(1):55–61. doi: 10.1016/0092-8674(74)90008-7. [DOI] [PubMed] [Google Scholar]


