Abstract
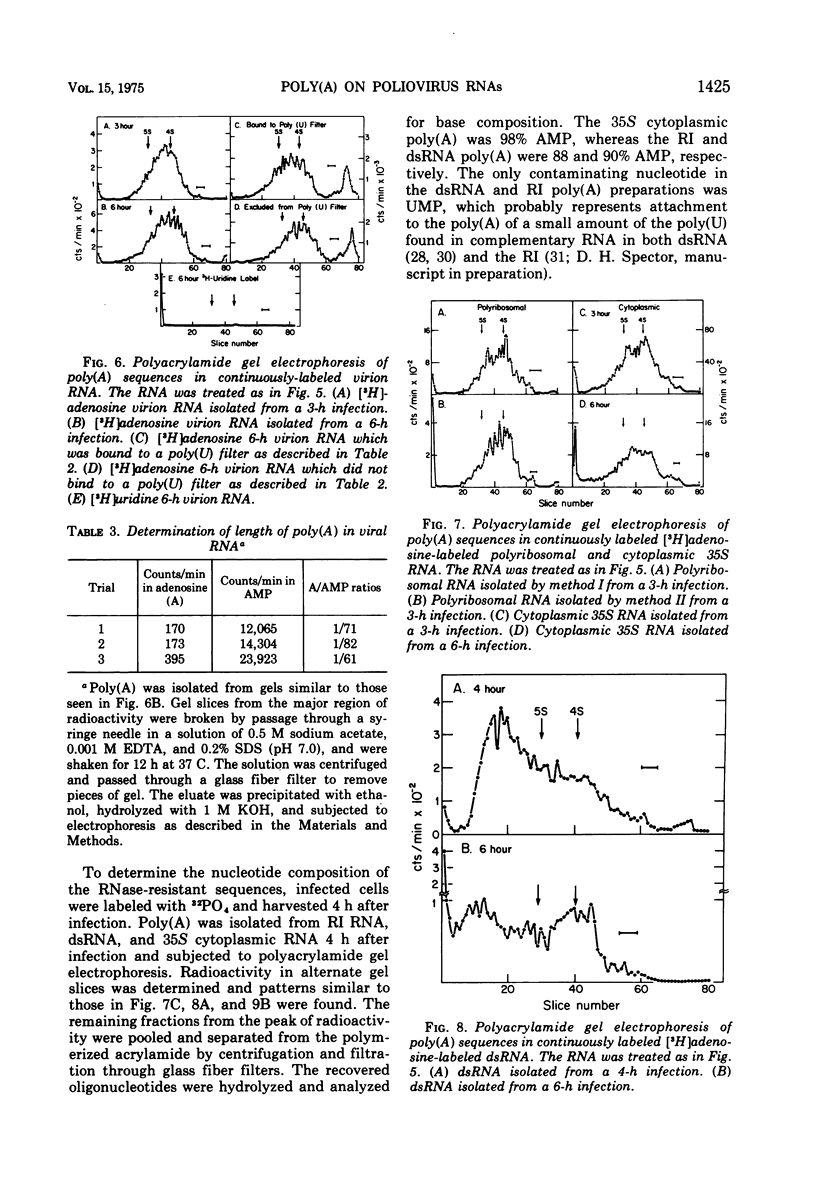
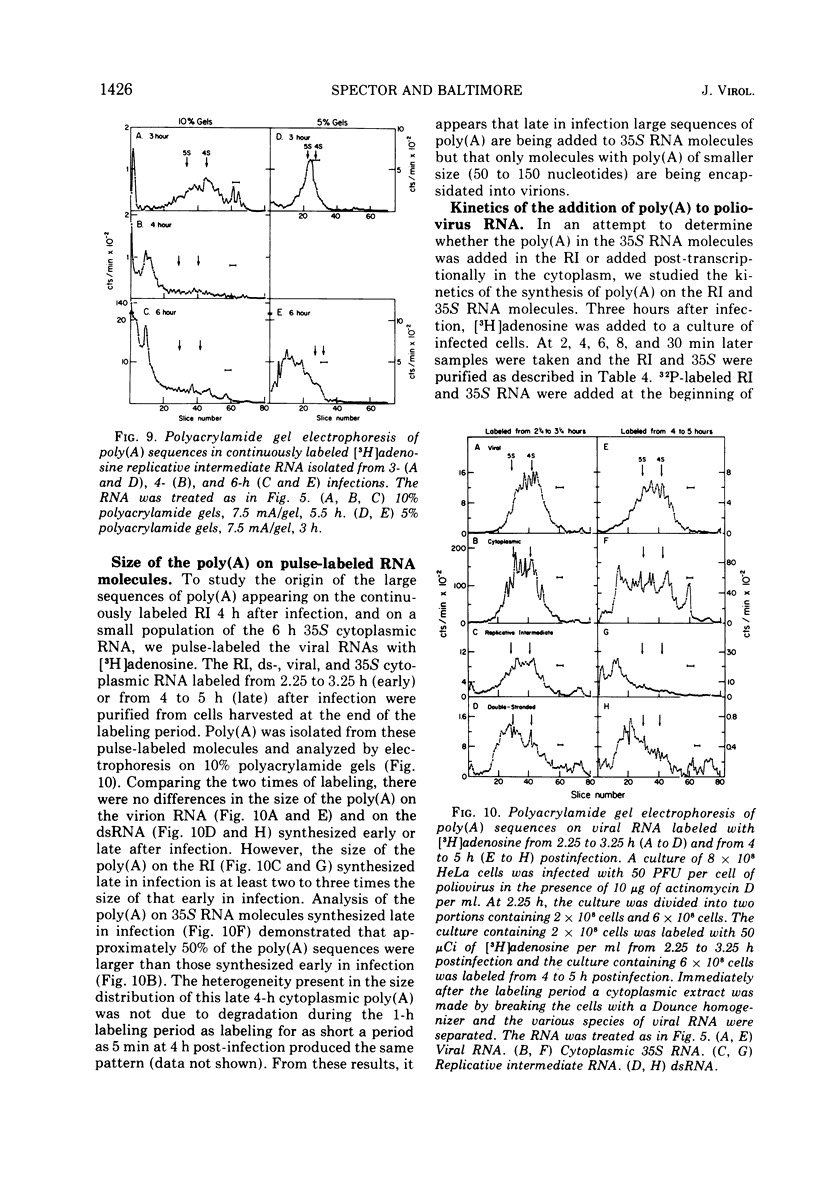
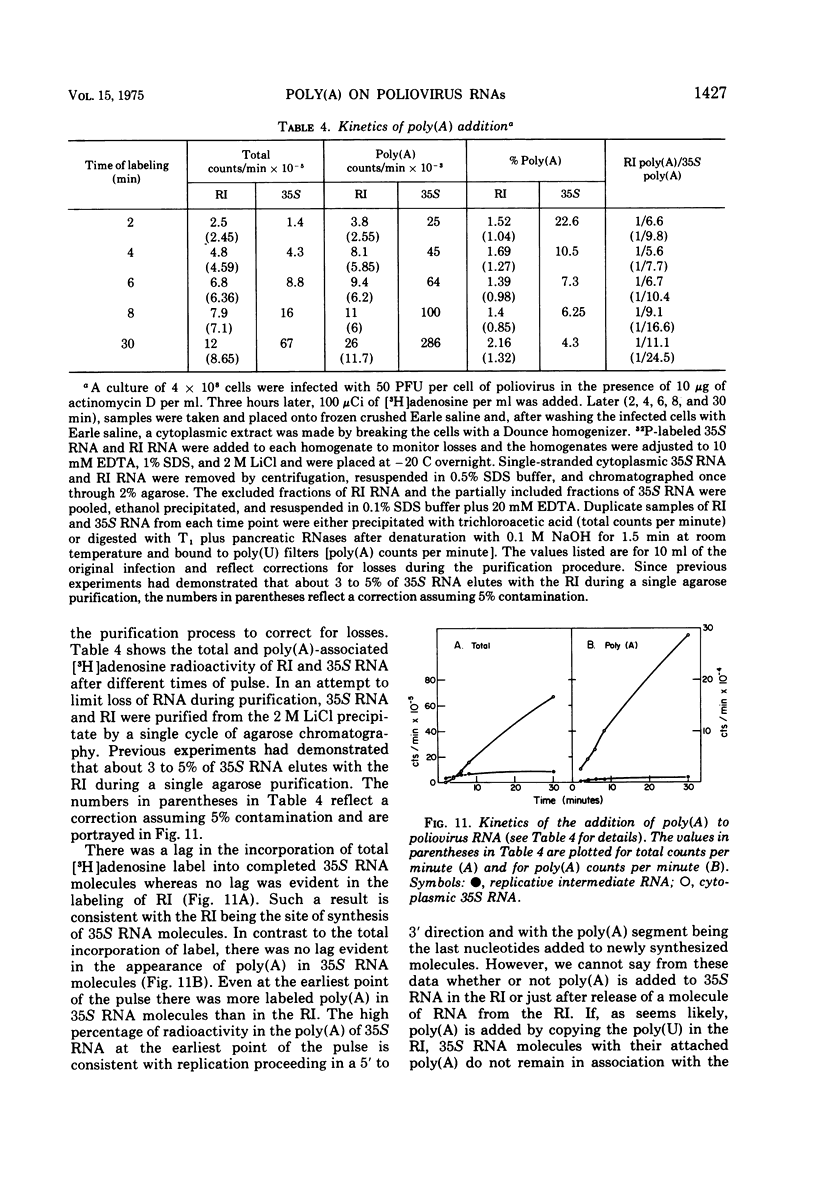
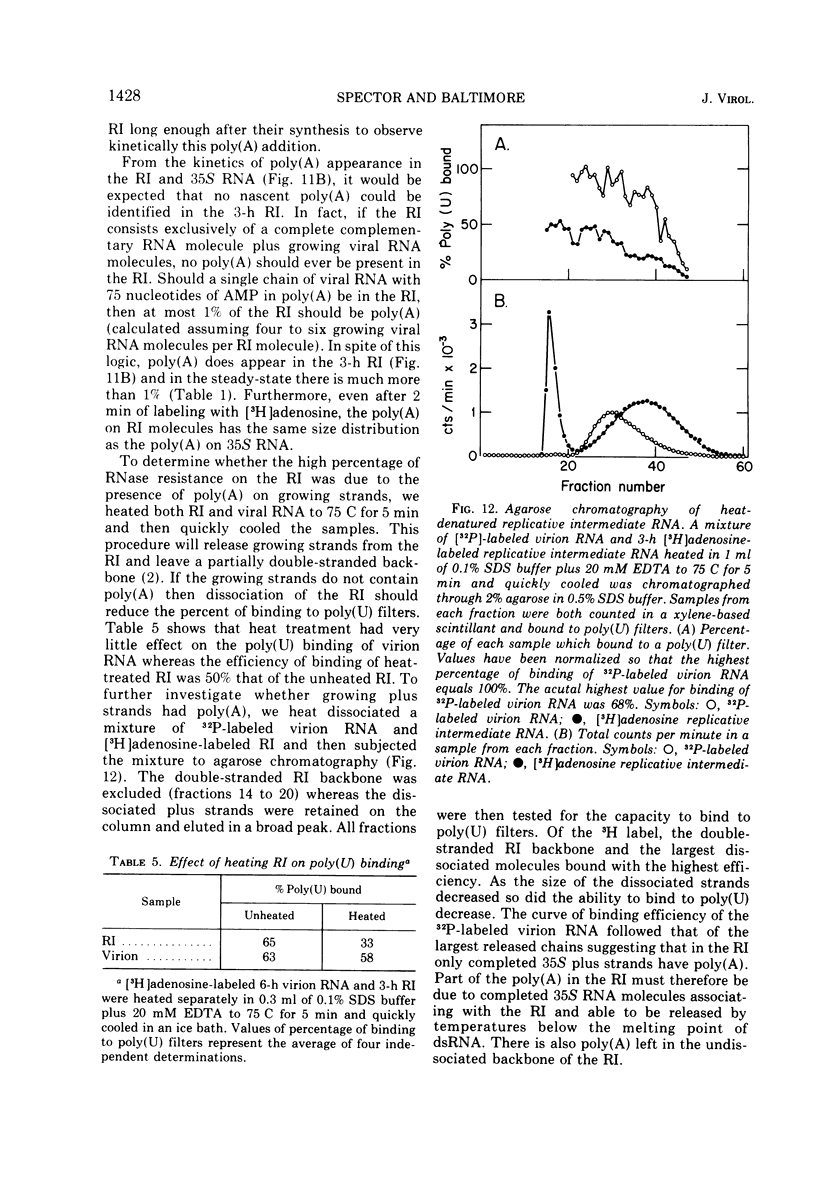
The content, size, and mechanism of synthesis of 3'-terminal poly(A) on the various intracellular species of poliovirus RNA have been examined. All viral RNA species bound to poly(U) filters and contained RNase-resistant stretches of poly(A) which could be analyzed by electrophoresis in polyacrylamide gels. At 3 h after infection, the poly(A) on virion RNA, relicative intermediate RNA, polyribosomal RNA, and total cytoplasmic 35S RNA was heterogeneous in size with an average length of 75 nucleotides. By 6 h after infection many of the intracellular RNA's had poly(A) of over 150 nucleotides in length, but the poly(A) in virion RNA did not increase in size suggesting that the amount of poly(A) which can be encapsidated is limited. At all times, the double-stranded poliovirus RNA molecules had poly(A) of 150 to 200 nucleotides. Investigation of the kinetics of poly(A) appearance in the replicative intermediate and in finished 35S molecules indicated that poly(A) is the last portion of the 35S RNA to be synthesized; no nascent poly(A) could be detected in the replicative intermediate. Although this result indicates that poliovirus RNA is synthesized 5' leads to 3' like other RNA's, it also suggests that much of the poly(A) found in the replicative intermediate is an artifact possibly arising from the binding of finished 35S RNA molecules to the replicative intermediate during extraction. The addition of poly(A) to 35S RNA molecules was not sensitive to guanidene.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Edmonds M., Nakazato H., Phillips B. A., Vaughn M. H. Polyadenylic acid sequences in the virion RNA of poliovirus and Eastern Equine Encephalitis virus. Science. 1972 May 5;176(4034):526–528. doi: 10.1126/science.176.4034.526. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Girard M., Darnell J. E. Aspects of the synthesis of poliovirus RNA and the formation of virus particles. Virology. 1966 Jun;29(2):179–189. doi: 10.1016/0042-6822(66)90024-9. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968 Mar 14;32(2):359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Action of guanidine on the replication of poliovirus RNA. Virology. 1968 Jul;35(3):408–417. doi: 10.1016/0042-6822(68)90219-5. [DOI] [PubMed] [Google Scholar]
- Dahlberg J. E. Terminal sequences of bacteriophage RNAs. Nature. 1968 Nov 9;220(5167):548–552. doi: 10.1038/220548a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Wall R., Tushinski R. J. An adenylic acid-rich sequence in messenger RNA of HeLa cells and its possible relationship to reiterated sites in DNA. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1321–1325. doi: 10.1073/pnas.68.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard M. In vitro synthesis of poliovirus ribonucleic acid: role of the replicative intermediate. J Virol. 1969 Apr;3(4):376–384. doi: 10.1128/jvi.3.4.376-384.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granboulan N., Girard M. Molecular weight of poliovirus ribonucleic acid. J Virol. 1969 Oct;4(4):475–479. doi: 10.1128/jvi.4.4.475-479.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Balitmore D. Initiation of polyribosome formation in poliovirus-infected HeLa cells. J Mol Biol. 1970 Feb 14;47(3):275–291. doi: 10.1016/0022-2836(70)90302-5. [DOI] [PubMed] [Google Scholar]
- Mendecki J., Lee S. Y., Brawerman G. Characteristics of the polyadenylic acid segment associated with messenger ribonucleic acid in mouse sarcoma 180 ascites cells. Biochemistry. 1972 Feb 29;11(5):792–798. doi: 10.1021/bi00755a018. [DOI] [PubMed] [Google Scholar]
- Noble J., Levintow L. Dynamics of poliovirus-specific RNA synthesis and the effects of inhibitors of virus replication. Virology. 1970 Mar;40(3):634–642. doi: 10.1016/0042-6822(70)90208-4. [DOI] [PubMed] [Google Scholar]
- Oberg B., Philipson L. Replicative structures of poliovirus RNA in vivo. J Mol Biol. 1971 Jun 28;58(3):725–737. doi: 10.1016/0022-2836(71)90036-2. [DOI] [PubMed] [Google Scholar]
- PENMAN S., BECKER Y., DARNELL J. E. A CYTOPLASMIC STRUCTURE INVOLVED IN THE SYNTHESIS AND ASSEMBLY OF POLIOVIRUS COMPONENTS. J Mol Biol. 1964 Apr;8:541–555. doi: 10.1016/s0022-2836(64)80010-3. [DOI] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Abelson H. T., Penman S. Mitochondrial protein synthesis: RNA with the properties of Eukaryotic messenger RNA. Proc Natl Acad Sci U S A. 1973 Feb;70(2):350–353. doi: 10.1073/pnas.70.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Requirement of 3'-terminal poly(adenylic acid) for the infectivity of poliovirus RNA. Proc Natl Acad Sci U S A. 1974 Aug;71(8):2983–2987. doi: 10.1073/pnas.71.8.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa-Komaroff L., McDowell M., Baltimore D., Lodish H. F. Translation of reovirus mRNA, poliovirus RNA and bacteriophage Qbeta RNA in cell-free extracts of mammalian cells. Methods Enzymol. 1974;30:709–723. doi: 10.1016/0076-6879(74)30068-7. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Nuclear RNA metabolism. Annu Rev Biochem. 1973;42:329–354. doi: 10.1146/annurev.bi.42.070173.001553. [DOI] [PubMed] [Google Scholar]
- Weith H. L., Gilham P. T. Structural analysis of polynucleotides by sequential base elimination. The sequence of the terminal decanucleotide fragment of the ribonucleic acid from bacteriophage f2. J Am Chem Soc. 1967 Oct 11;89(21):5473–5474. doi: 10.1021/ja00997a042. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Teng M. H., Wimmer E. Poly(U) in poliovirus minus RNA is 5'-terminal. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1101–1109. doi: 10.1016/s0006-291x(74)80397-9. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Poly (A) and poly (U) in poliovirus double stranded RNA. Nat New Biol. 1973 Apr 11;242(119):171–174. doi: 10.1038/newbio242171a0. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Sequence studies of poliovirus RNA. III. Polyuridylic acid and polyadenylic acid as components of the purified poliovirus replicative intermediate. J Mol Biol. 1975 Mar 5;92(3):467–477. doi: 10.1016/0022-2836(75)90292-2. [DOI] [PubMed] [Google Scholar]
- el-Manna M. M., Bruening G. Polyadenylate sequences in the ribonucleic acids of cowpea mosaic virus. Virology. 1973 Nov;56(1):198–206. doi: 10.1016/0042-6822(73)90299-7. [DOI] [PubMed] [Google Scholar]


