Abstract
Pediatric liver transplantation is the standard of care for treatment of liver failure in children. The aim of this study was to identify the characteristics of pediatric liver transplantation in centers located in Korea and determine factors that influence outcomes. This retrospective study was performed using data from between 1988 and 2010 and included all recipients 18 yr old and younger who underwent pediatric liver transplantation in Korea during that period. Our data sources were hospital medical records and the outcome measure was overall patient survival. Univariate and multivariate statistical analyses were undertaken using the Cox proportional hazards model. Five hundred and thirty-four pediatric liver transplantations were performed in 502 children. Median age and average pediatric end-stage liver disease (PELD) score were 20 months and 18 point, respectively. Biliary atresia (57.7%, 308/534) was the most common cause of liver disease. Eighty-two (15.3%) were deceased donor liver transplantations and 454 (84.7%) were living donor liver transplantations. Retransplantation was performed in 32 cases (6%). Overall, 1-, 5-, and 10-yr patient survival rates were 87.8%, 82.2%, and 78.1%, respectively. In multivariate analysis, independent significant predictors of poor patient survival were chronic rejection and retransplantation. This study presents the epidemiologic data for nearly all pediatric liver transplantation in Korea and shows that the independent prognostic factors in patient survival are chronic rejection and retransplantation.
Keywords: Liver Transplantation, Children, Survival, Rejection, Retransplantation
INTRODUCTION
Liver transplantation is the definite treatment for acute and chronic end-stage liver disease in children (1). The shortage of size-matched liver allografts in deceased donors and high mortality on the waiting list has led to allowing the use of partial hepatic grafts from adult living donors (2). Since the first pediatric liver transplantation performed by Starzl in 1963 (3), the success rate has gradually increased due to the development of new immunosuppressive agents, better preoperative management, and innovative surgical techniques.
Before the 1990s, children in Korea with end-stage liver disease used to die without the therapeutic option of liver transplantation. In Korea, the first pediatric liver transplantation was performed in 1988 and the first living donor liver transplantation was done in 1994 (4). Since 2000, the incidence of pediatric liver transplantation has been about 50 cases annually.
The aim of this retrospective study was to evaluate the epidemiology and to analyze patient outcomes after pediatric liver transplantation in Korea.
MATERIALS AND METHODS
Data collection
A retrospective review of all patients 18 yr of age or younger who underwent pediatric liver transplantation between 1988 and 2011 four transplantation centers in Korea was performed. Multiorgan recipients were excluded. This study ultimately comprised 534 cases.
We sent requests for data to four transplantation centers which has pediatric transplantation program and received data from them. We collected data on variables related to recipient and donor characteristics. Specifically, for the recipients we collected data on age, gender, primary diagnosis, year of liver transplantation, and graft type. For donors we only collected data on age. We then calculated the pediatric end-stage liver disease (PELD) score at the time of liver transplantation (5).
Candidates for deceased donor liver transplantation (DDLT) were assigned, according to their medical condition, to one of the following Korean Organ Network for Organ Sharing (KONOS) categories: Status 1, intensive care unit-bound with expected survival less than 7 days; Status 2, continuously hospitalized; Status 3, at home, but requiring continuous medical care.
Statistical analysis
Descriptive data are reported using parameters such as frequency, mean, mode, and standard deviation. Survival rates were estimated by the Kaplan-Meier method and compared by logrank test. Continuous data are presented as mean with standard deviation and categorical data as a number with percentage. Potential univariate predictors of survival were analyzed using the Cox regression analysis. Variables with a P value of < 0.2 at the univariate level were included in a Cox Multivariable Proportional Hazards Model. The level of significance was set at 0.05. Statistical analysis was performed with SPSS 19.0 statistical software program.
Ethics statement
This study protocol was reviewed and approved by the institutional review board of Samsung Medical Center, Sungkyunkwan University School of Medicine (IRB No. SMC 2011-01-073). Informed consent was waived by the board.
RESULTS
Demographics of pediatric liver transplant recipients
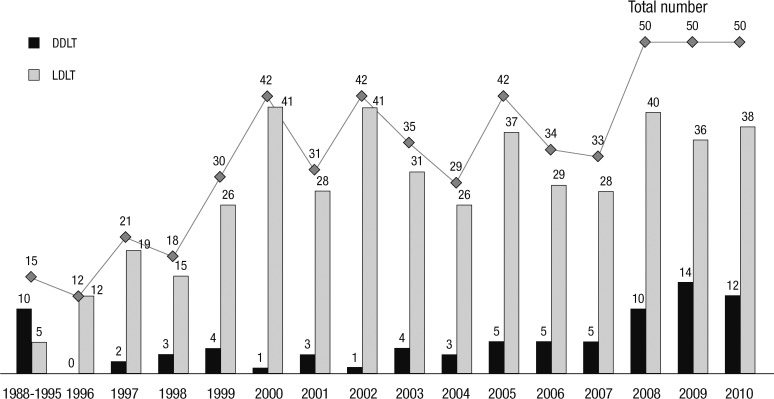
Patient and graft characteristics are summarized in Table 1. Five hundred thirty-four pediatric liver transplantations were performed in 504 children. Retransplantation was performed in 32 cases (6%). Eighty-two (15.4%) of the 536 recipients received liver allografts from deceased donors that were composed of 34 (41.4%) whole size and 21 (25.6%) split grafts. Twenty-seven (32.9%) recipients who underwent deceased donor liver transplantation were not identified. Four hundred and fifty-two recipients (84.6%) received liver allografts from living donors. The yearly cases of pediatric liver transplantation were about 30 to 50 after year 2000 (Fig. 1). The cases of deceased donor liver transplantation (DDLT) abruptly increased after year 2008. The age distribution of the recipients was as follows: 38 were younger than 6 months; 149 were 6 to 12 months; 143 were 1 to 3 yr; 116 were 3 to 12 yr; and 82 were 12 to 18 yr (Fig. 1).
Table 1.
Clinical characteristics of pediatric liver transplantation recipients and donors
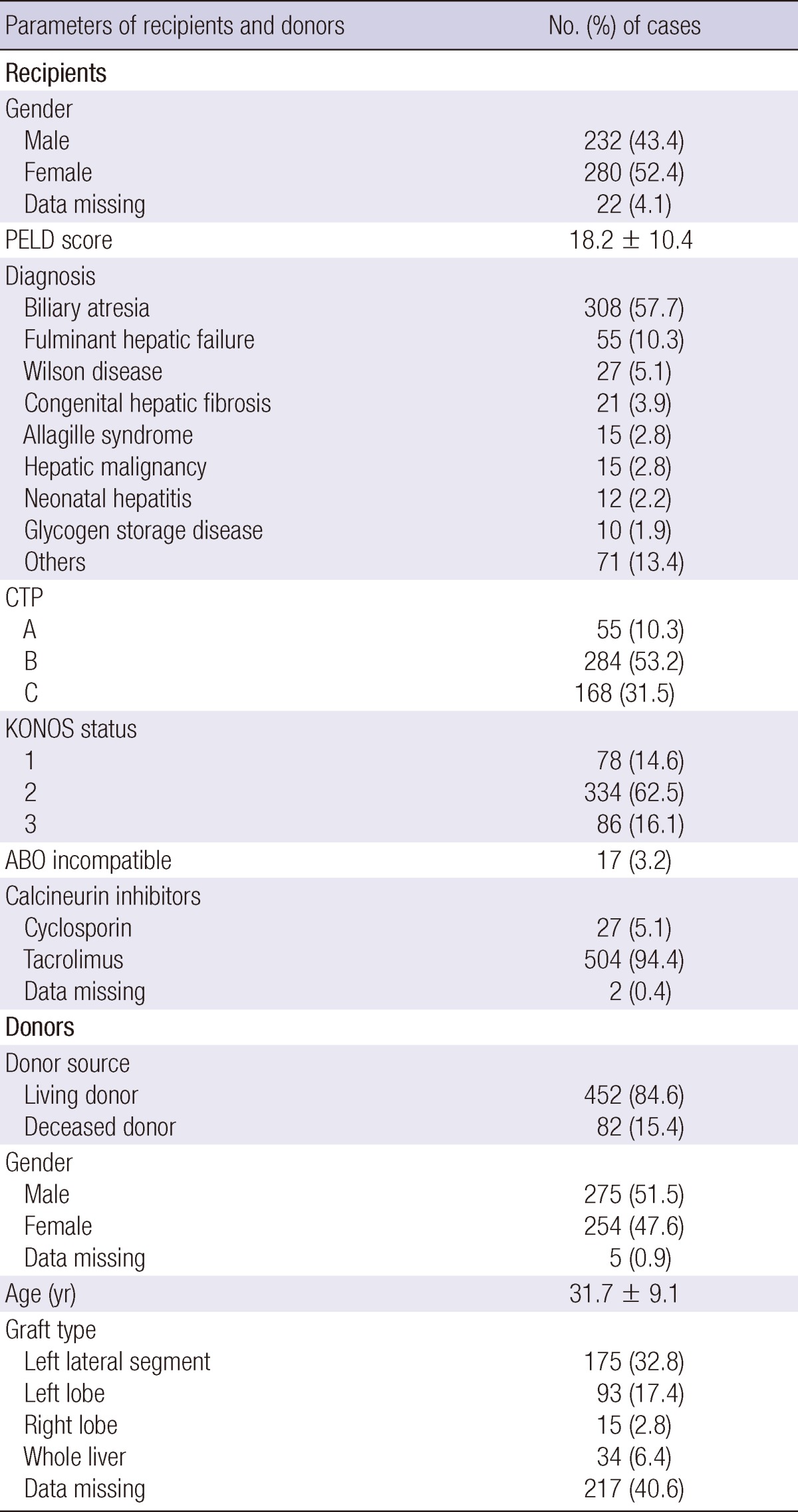
PELD, pediatric end-stage liver disease; CTP, Child-Turcotte-Pugh score; KONOS, Korean Organ Network for Organ Sharing.
Fig. 1.
Cases of pediatric liver transplantation in Korea.
Indications for liver transplantation were biliary atresia (n = 308, 57.7%), fulminant hepatic failure (n = 55, 10.3%), Wilson's disease (n = 27, 5.1%), congenital hepatic fibrosis (n = 21, 3.9%), Allagille syndrome (n = 15, 2.8%), hepatic malignancy (n = 15, 2.8%), neonatal hepatitis (n = 12, 2.2%), glycogen storage disease (n = 10, 1.9%), and others (n = 71, 13.4%). The median age of the patients was 20 months (range, 2 month to 18 yr). The mean Child-Pugh score was 8.5 ± 2.2, whereas PELD score was 18.2 ± 10.4. Seventy-eight children (14.6%) were KONOS status 1, 334 (62.5%) were KONOS status 2, and 86 (16.1%) were KONOS status 3. The mean follow-up of the study population was 5.2 yr.
All recipients received steroids as an induction agent and 19 (3.6%) patients received simultaneously received basiliximab as an induction agent. Most recipients (n = 504, 94.4%) received tacrolimus as a calcineurin inhibitor, however, 27 patients (5.1%) received cyclosporine.
Survival of patients and grafts
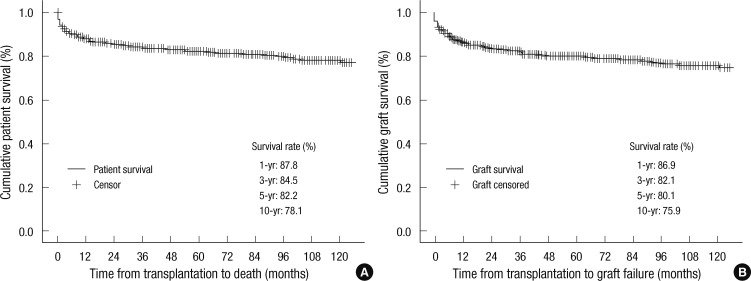
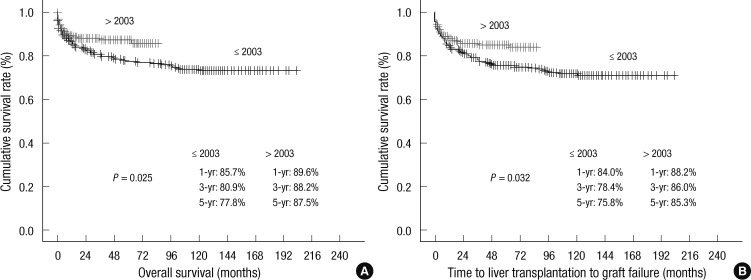
Overall, the 1-yr, 3-yr, 5-yr, and 10-yr patient survival rates in this study were 87.8%, 84.5%, 82.2%, and 78.1%, respectively (Fig. 2). The overall patient survival rates at 1-yr, 3-yr, and 5-yr were 79.5%, 77.9%, and 77.9%, respectively, in the deceased donor liver transplantation group, and 89.2%, 85.7%, and 83.0%, respectively, in the living donor liver transplantation group (P = 0.143). In addition, the 1-yr, 3-yr, and 5-yr patient survival rates for patients who underwent liver transplantation prior to 2003 were 85.7%, 80.9%, and 77.8%, respectively; however, 1-yr, 3-yr, and 5-yr patient survival rates were 89.6%, 88.2%, and 87.5%, respectively, after 2003. The patient survival rates after 2003 were thus superior to those before 2003 (P = 0.025). The mean duration of follow up was 62.6 ± 50.3 months after transplantation. The post-transplant mortality rate was 13.1% (n = 70) in this period and the main causes of death were sepsis (n = 11, 15.7%), chronic rejection (n = 8, 11.4%), primary non-function (n = 7, 10%), and post-transplant lymphoproliferative disease (PTLD) (n = 7, 10%). Forty-three (61.4%) patients died within 6 months postoperatively.
Fig. 2.
Survival of patients (A) and grafts (B) in pediatric liver transplantation.
The graft survival rates at 1-yr, 3-yr, 5-yr, and 10-yr were 86.9%, 82.1%, 80.1%, and 75.9%, respectively. The overall graft survival rates at 1-yr, 3-yr, and 5-yr in patients who underwent liver transplantation prior to 2003 were 84.0%, 78.4%, and 75.8%, respectively; however, the 1-yr, 3-yr, 5-yr graft survival rates were 88.2%, 86%, and 85.3%, respectively, after 2003. Patient graft survival rates after 2003 were thus superior to those of patients before 2003 (P=0.032) (Fig. 3). Thirteen patients (2.4%) underwent retransplantation. The overall patient survival rate at 1-yr was 61.5% in retransplant recipients and 88.5% in primary transplant recipients. The overall survival rates of retransplantation were lower than those of primary transplantation, and there were statistically significant differences between the two groups (P=0.012).
Fig. 3.
Compared survival of patients (A) and grafts (B) in pediatric liver transplantation before and after 2003.
Complications of recipients
Surgical complications included hepatic artery complications (n=20, 3.7%), portal vein complications (n=70, 13.1%), hepatic vein complications (n=37, 6.9%), and biliary complications (n=122, 12.8%). Infectious complications included Epstein-Barr virus (EBV) infection (n=173, 32.4%) and cytomegalovirus (CMV) infection (n=128, 24.0%). Acute and chronic rejection occurred in 208 (39.0%) patients and 15 (2.8%) patients, respectively. Overall, PTLD occurred in 51 patients (9.6%) (Table 2).
Table 2.
Recipient outcomes after transplantation
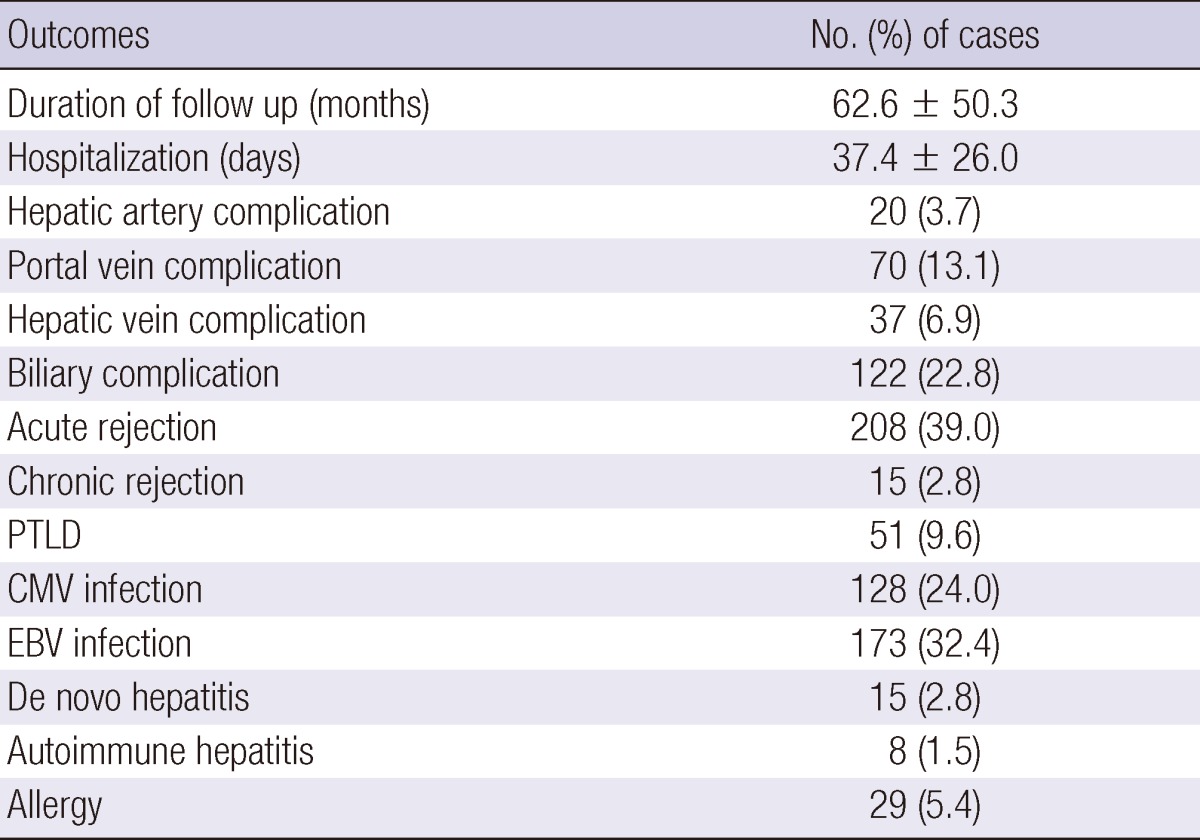
PTLD, post-transplant lymphoproliferative disease; CMV, cytomegalovirus; EBV, Epstein-Barr virus.
Risk factors for patient death
The risk factors for patient death are outlined in Table 3. Among these variables, KONOS status 1, Child-Pugh class A and B, PELD score, KONOS status 3, biliary atresia patients, retransplantation, and the time of pediatric liver transplantation (before 2003) appear to be significantly associated with patient survival. Multivariate analysis reveals that chronic rejection (Hazard ratio [HR], 2.849; 95% Confidence Interval [CI], 1.136-7.145; P=0.026) and retransplantation (HR, 4.837; 95% CI, 1.745-13.414; P= 0.002) are significantly associated with mortality.
Table 3.
Univariate analysis of risk factors for mortality
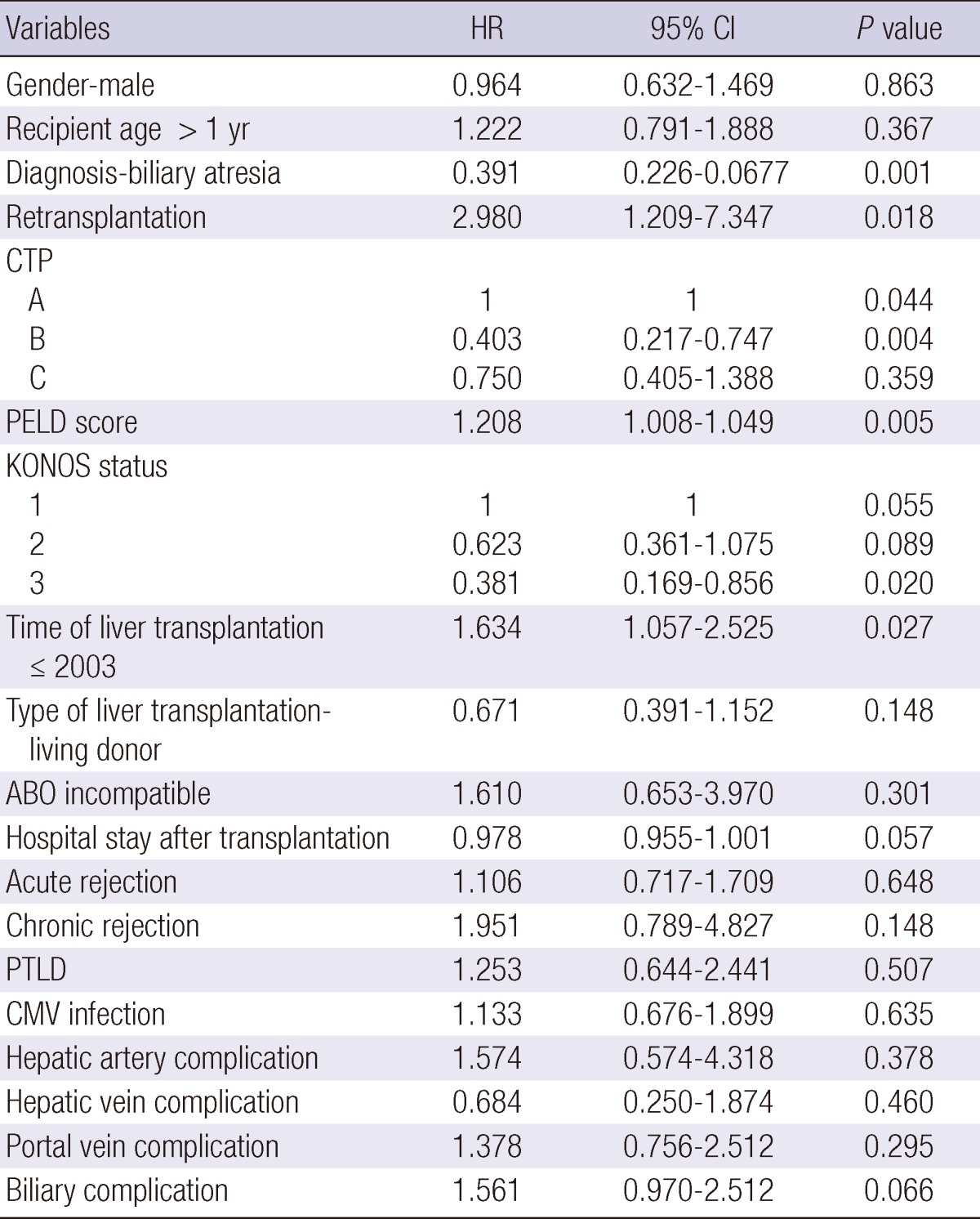
CTP, Child-Turcotte-Pugh; PELD, pediatric end-stage liver disease; KONOS, Korean Organ Network for Organ Sharing; PTLD, post-transplant lymphoproliferative disease; CMV, cytomegalovirus.
DISCUSSION
This study describes a population of 534 pediatric liver transplantation recipients between 1988 and 2010 from 4 centers in Korea, representing nearly all of the pediatric liver transplant recipients in Korea. The pediatric liver transplant recipients differ from adult recipients. The most common indications for liver transplantation in the pediatric population are specific to this age group and there are greater technical challenges associated with pediatric liver transplantation (6). Biliary atresia constituted the most common indication for liver transplantation in children: 29.6% (158/534) younger than 1 yr and 46.3% (247/534) younger than 3 yr old.
The Study of Pediatric Liver Transplant (SPLIT) group reported allograft survival rates of 93% at 1 yr, 90% at 3 yr, and 88% at 5 yr (7). In addition, SPLIT group reported overall patient survival rates at 1, 3, and 5 yr of 89.8%, 86.7%, and 84.8%, respectively. Our study reported that graft survival rates at 1, 3, 5, and 10 yr were 86.9%, 82.1%, 80.1%, and 75.9%, respectively, and overall 1-yr, 3-yr, 5-yr, and 10-yr patient survival rates were 87.8%, 84.5%, 82.2%, and 78.1%, respectively. The graft survival and patient survival rates in our study were inferior to the results of SPLIT. However, overall patient survival rates and graft survival rates in patients who underwent pediatric liver transplantation was improved since 2003. These results were nearly as good as the results of SPLIT.
In the SPLIT report, 85.5% of all transplants were from deceased donors and 15.5% were from living donors (8). In Asian countries, liver grafts from living donors were the main transplants because of a shortage of deceased donors due to cultural and ethical reasons. Our study also showed that the incidence of living donors was 84.6%. Living donor liver transplantations (LDLT) have led to a reduction in the pretransplant death rate in children from 20% to almost 0% (9). LDLT provides an excellent graft by minimizing the cold ischemic time. In addition, this procedure is elective and thus allows flexibility in choosing the optimal time for transplantation with regard to the recipient's clinical status. Because of these advantages, worldwide long-term results of LDLT are equal or even superior to those obtained with whole organ or split graft in deceased donors (1).
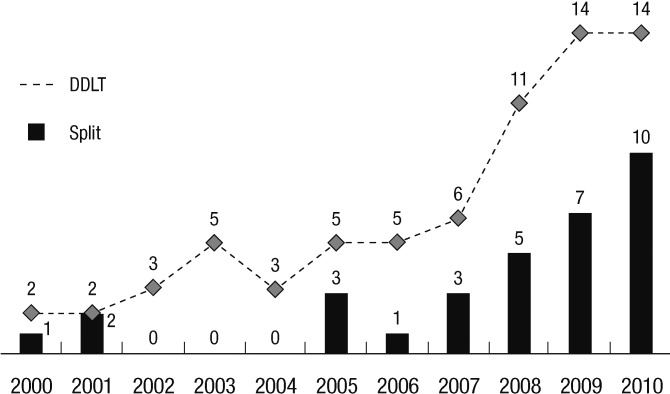
In Korea, the indications for a split graft of left lateral segment for pediatric recipient are stable heart-beating brain death donors, aged between 10 yr to 35 yr, with history of the minimal dose of inotropics (dopamine ≤15 µg/kg/min), a minimal stay in intensive care unit, terminal aspartate transaminase (AST) and alanine transaminase (ALT) levels less than 120 IU/L, and terminal sodium levels less than 160 mg/dL. In addition, the indicators for pediatric liver transplants who would receive a split graft from deceased donor were age ≤15 yr and body weight ≤30 kg, parents were unsuitable donors because of ABO-mismatch, hepatitis B carrier, and anti-HCV positive status. The number of pediatric DDLT reported by the KONOS increased from 2006 to 2010 with increased total deceased donation and pediatric split graft policy (10) (Fig. 4).
Fig. 4.
Pediatric liver transplantation using split graft from deceased donor in Korea (10).
Recently, SPLIT reported that the most significant factors predicting patient and graft loss at 6 months in children listed for transplant were post-transplant surgical complications such as reoperation, hepatic artery thrombosis, portal vein thrombosis, bile leakage, and bowel perforation (8). Our study revealed that chronic rejection and retransplantation were predisposing factors in patient survival, but postoperative complications were not a risk factor for patient survival.
There are several limitations in our study. The primary one was limited clinical data, as the clinical data from other centers were poor. Some data from other centers were inaccurate. We made every effort to ensure data quality, but we were ultimately dependent on others for reliable information. Although, our data spanned nearly 20 yr and provided for a relatively large number of patients, there were drawbacks to using data drawn from such a long period. Improvements in care and technology in postoperative management have taken place, and waiting time, donor availability, immunosuppression, surgical transplantation techniques, and medical, diagnostic, and management methods have all changed during this time. In spite of the limitations, we think that the present study captured nearly all pediatric liver transplant recipients in Korea though data were collected from only 4 centers in Seoul.
In conclusion, pediatric liver transplantation in Korea has been successful and the results have been improved, as shown by our report, which is the first multi-center, nationwide study on pediatric liver transplantation. The independent prognostic factors of patient survival are chronic rejection and retransplantation. This analysis confirms that long-term outcomes for pediatric liver transplant recipients in Korea are excellent.
ACKNOWLEDGMENTS
The authors have no conflicts of interest to disclose.
Footnotes
This study was supported by clinical research fund of The Korean Society of Transplantation.
References
- 1.McDiarmid SV. Current status of liver transplantation in children. Pediatr Clin North Am. 2003;50:1335–1374. doi: 10.1016/s0031-3955(03)00150-0. [DOI] [PubMed] [Google Scholar]
- 2.Otte JB, de Ville de Goyet J, Reding R, Van Obbergh L, Veyckemans F, Carlier MA, De Kock M, Clement de Clety S, Clapuyt P, Sokal E, et al. Pediatric liver transplantation: from the full-size liver graft to reduced, split, and living related liver transplantation. Pediatr Surg Int. 1998;13:308–318. doi: 10.1007/s003830050328. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE, Koep LJ, Schroter GP, Halgrimson CG, Porter KA, Weil R., 3rd Liver replacement for pediatric patients. Pediatrics. 1979;63:825–829. [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KM. Liver transplantation in children. J Korean Pediatr Soc. 2003;46:736–741. [Google Scholar]
- 5.Freeman RB, Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 6.Kim MJ, Choe YH. Indication of pediatric liver transplantation. J Korean Soc Transplant. 2011;25:151–154. [Google Scholar]
- 7.Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, McDiarmid S, Cohen G, Anand R. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a North American Multicenter Registry. Pediatrics. 2008;122:e1128–e1135. doi: 10.1542/peds.2008-1363. [DOI] [PubMed] [Google Scholar]
- 8.McDiarmid SV, Anand R, Martz K, Millis MJ, Mazariegos G. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011;254:145–154. doi: 10.1097/SLA.0b013e31821ad86a. [DOI] [PubMed] [Google Scholar]
- 9.Emre S. Living-donor liver transplantation in children. Pediatr Transplant. 2002;6:43–46. doi: 10.1034/j.1399-3046.2002.1r072.x. [DOI] [PubMed] [Google Scholar]
- 10.KONOS. [access 20 February 2012]. Available from: www.konos.go.kr.