Abstract
Peripheral artery disease (PAD) is an important marker for the risk stratification of patients with coronary artery disease (CAD). We investigated the prevalence of PAD in patients undergoing percutaneous coronary intervention (PCI) with CAD and the relationship between ankle-brachial pressure index (ABPI) and CAD severity. A total of 711 patients undergoing PCI for CAD from August 2009 to August 2011 were enrolled. PAD diagnosis was made using the ABPI. The prevalence of PAD was 12.8%. In PAD patients, mean values of right and left ABPI were 0.71 ± 0.15 and 0.73 ± 0.15. Patients with PAD had a higher prevalence of left main coronary disease (14.3% vs 5.8%, P = 0.003), more frequently had multivessel lesions (74.9% vs 52.1%, P < 0.001) and had higher SYNTAX score (18.2 ± 12.3 vs 13.1 ± 8.26, P = 0.002). Using multivariate analysis, we determined that left main CAD (OR, 2.954; 95% CI, 1.418-6.152, P = 0.004) and multivessel CAD (OR, 2.321; 95% CI, 1.363-3.953, P = 0.002) were both independently associated with PAD. We recommend that ABPI-based PAD screening should be implemented in all patients undergoing PCI with CAD, especially in severe cases.
Keywords: Peripheral Artery Disease, Coronary Artery Disease, Atherosclerosis
INTRODUCTION
Peripheral artery disease (PAD), a manifestation of multifocal atherosclerosis, is an important marker for risk stratification in patients with coronary artery disease (CAD). Early diagnosis of PAD in patients at high risk for cardiovascular disease is important in slowing down the atherosclerosis progression and promoting its regression.
Several studies have demonstrated that the ankle-brachial pressure index (ABPI) strongly correlates with the presence and severity of atherosclerosis in the coronary arteries (1, 2). CAD is prevalent in patients with PAD and patients with both CAD and PAD are at increased risk for cerebrovascular accidents and cardiovascular mortality (3-5). Furthermore, previous studies have reported that PAD is independently associated with increase inhospital and short-term mortality among patients undergoing percutaneous coronary intervention (PCI) for CAD (6, 7).
Although the presence of PAD can be identified through ABPI measurement, PAD detection has often been overlooked, especially in patients without related symptoms such as claudication. Therefore, high-risk patients with asymptomatic PAD often remain underdiagnosed, undertreated, and at increased risk of additional cardiovascular morbidities and mortality. The varied prevalence of PAD in CAD patients has been demonstrated in several epidemiological studies (8-10). However, there is limited data for the frequency of PAD underdiagnosis in patients undergoing PCI, especially in Korean populations.
Furthermore, a few studies started investigating the correlation between ABPI and CAD severity. SYNTAX score (SS), a lesion-based angiographic scoring system, was widely used as a tool quantifying the complexity of CAD (11-13). It was derived from the lesion location, characteristics, tortuosity, and other factors for each lesion, which has been shown to be an effective predictor of clinical outcomes in patients with left main or multivessel disease undergoing PCI. We sought to determine the prevalence of PAD in patients with coronary disease and show the relationship between the ABPI and CAD severity.
MATERIALS AND METHODS
Study design and sample
The study was carried out retrospectively at a single center from August 2009 to August 2011. A total of 711 consecutive patients (75.4% male; mean age 63.4 ± 11.0 yr) with CAD undergoing PCI were enrolled. Patients were excluded from the study if they presented with coronary vasospasm or insignificant coronary artery stenosis. We also excluded 45 patients who presented with ABPI > 1.3 and tibia-brachial pressure index (TBPI) measurement > 0.7, which is associated with diffuse atherosclerotic disease of vessels with calcification and stiffened walls, which in turn hamper PAD detection by ABPI measurement. Among patients with ABPI > 1.3, those who had a TBPI measurement < 0.7 were diagnosed with PAD.
We reviewed the medical reports of enrolled patients to obtain all relevant demographic data, cardiovascular risk factors and coronary angiographic findings. After considering clinical manifestations, electrocardiogram and coronary angiogram results, the CAD cases were classified into the following: stable angina, unstable angina, non-ST segment elevation myocardial infarction and ST segment elevation myocardial infarction. To assess the severity of the CAD cases, we investigated whether multivessel and left main coronary lesions were involved or not. The SYNTAX score (SS) is an anatomic scoring system based on the coronary angiogram, which not only quantifies lesion complexity, but also predicts outcomes after PCI in patients with multivessel CAD or left main disease. The SS allows prospective risk stratification of patients with multivessel CAD undergoing PCI. The SS for each patient was calculated by interventional cardiologists. From the baseline diagnostic angiogram, each coronary lesion producing ≥ 50% diameter stenosis in vessels ≥ 1.5 mm by visual estimation was scored separately using the SS score algorithm from its website (11-13).
Risk factor stratification
In our study, hypertension was either self reported or defined as having a systolic blood pressure > 140 mmHg. Diabetes mellitus was defined as having a history of type 1 or type 2 diabetes mellitus, treated either pharmacologically or through diet changes. Obesity was defined as having a body mass index (weight [kg]/height2 [m2]) greater than 30. Smoking history was established in patients with at least a 10 pack-year history of tobacco use. Dyslipidemia was determined from medical records and defined as either having used lipid-lowering agents or having laboratory test results with a total cholesterol concentration of 200 mg/dL or more, a low density lipoprotein cholesterol concentration of 130 mg/dL or more, a high density lipoprotein cholesterol concentration of 40 mg/dL or less, or a triglyceride concentration of 150 mg/dL or more. The presence of previous cerebrovascular disease was defined to include ischemic strokes and transient ischemic attacks.
Ankle-brachial pressure index measurement
A 5-mHz Doppler device (Colin, VP 2000, Komaki, Japan) was used to measure the ABPI index. Each patient rested in the supine position for 5 min while having their systolic blood pressure recorded in the brachial arteries and in the lower extremities at the dorsalis pedis and the posterior tibial arteries. The ABPI is a ratio between the higher of two systolic pressures below the ankle and the highest pressure in the brachial portion. The pressures of both legs were measured and ABPI was calculated for both legs. The subjects were considered to have PAD if either leg maximal ABPI was less than 0.9 or more than 1.3 with a 0.7 or lower toe-brachial pressure index. The patients with PAD were grouped into mild (ABPI; 0.7-0.9), moderate (ABPI; 0.4-0.7) and severe (ABPI < 0.4) disease according to the value of ABPI.
Statistical analysis
Continuous variables were expressed as the mean ± SD or the median with an interquartile range. To evaluate differences between patients with or without PAD, we used a Student's unpaired t-test for normally distributed data and Mann-Whitney test for data that were not normally distributed. Categorical variables were compared using chi-square or Fisher's exact tests. Pearson correlation coefficient was used for correlation between SS and ABPI. A comparison of ABPI among three SS groups was performed using the ANOVA with contrast weight and Kruskal-Wallis test. For post-hoc analysis, we used pairwise comparison between the groups. To adjust elevation of the type I error, we used Tukey method using ranks. Multivariate logistic regression analysis was performed to determine the independent predictors of PAD. Mean values, percentages, and odds ratios (OR) are presented with 95% confidence intervals (CI). All statistical analysis was performed using the PASW 17.0 statistical analysis software package (IBM SPSS Inc., Chicago, IL, USA). P values < 0.05 were considered significant.
Ethics statement
This study protocol was reviewed and approved by the institutional review board of Samsung Medical Center (IRB No. 2012-07-012-001), and performed according to the Declaration of Helsinki. All subjects provided their informed written consent before participation.
RESULTS
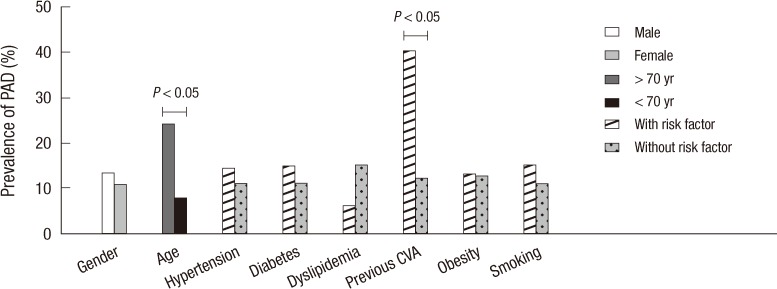
The prevalence of PAD was 12.8% (right ABPI, 0.71 ± 0.15; left ABPI, 0.73 ± 0.15) in 711 patients with CAD undergoing PCI and 23.9% in CAD patients over 70 years. The demographic and clinical characteristics of patients diagnosed with or without PAD are summarized in Table 1. The prevalence of PAD according to the existence of cardiovascular risk factors and co-morbidities is shown in Fig. 1. Patients with PAD were significantly older than those without PAD (64 yr [54-71] vs 71 yr [65-76], P < 0.001). There was no significant difference in prevalence of PAD between genders (13.4% vs 10.9%, P = 0.435). Preexisting conditions of hypertension or diabetes were not different between the groups. However, previous cerebrovascular accidents were more common in patients with PAD (8.8% vs 1.9%, P = 0.002).
Table 1.
Clinical characteristics of patients with or without peripheral artery disease (PAD)
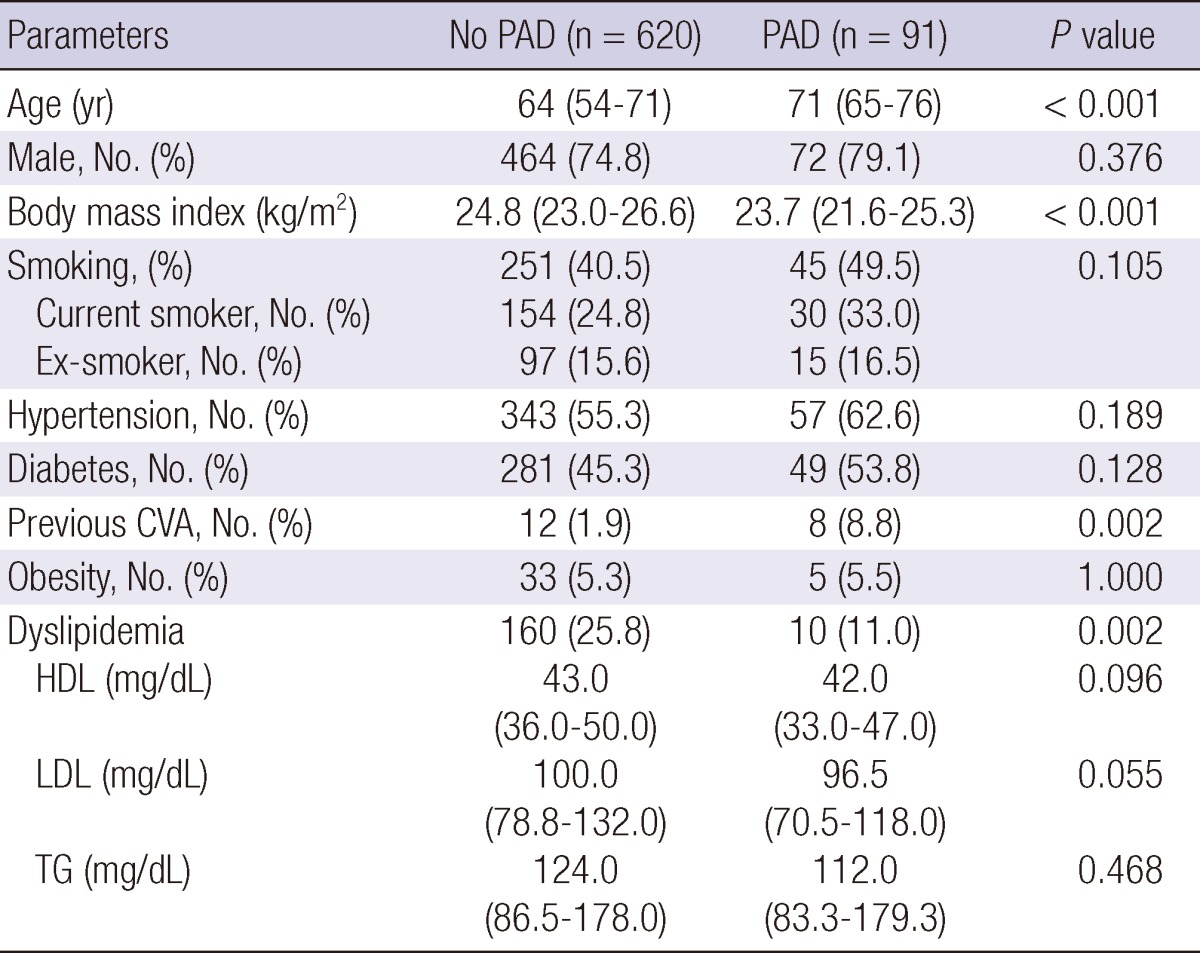
Data was expressed as medians (interquartile range) or percentages. PAD, peripheral artery disease; CVA, cerebrovascular accident; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglyceride.
Fig. 1.
The prevalence of peripheral artery disease (PAD) in patients with or without cardiovascular risk factors and co-morbidities. CVA, cerebrovascular accident.
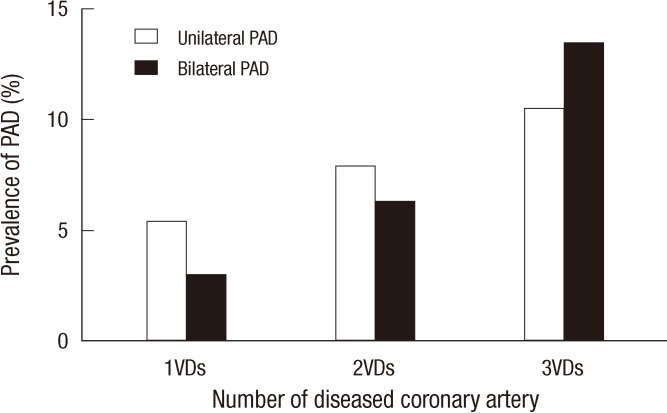
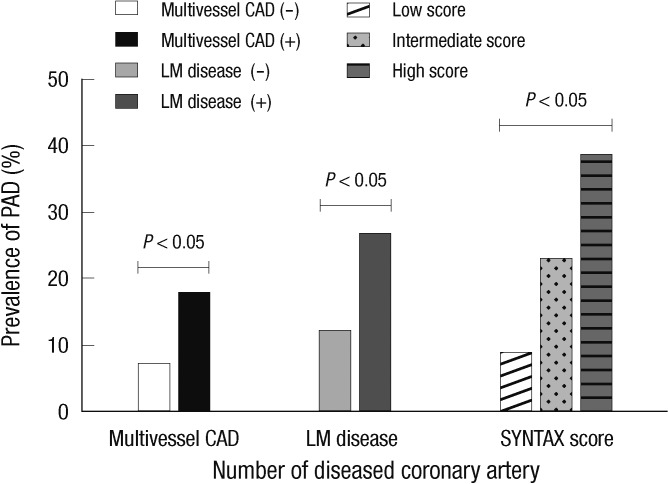
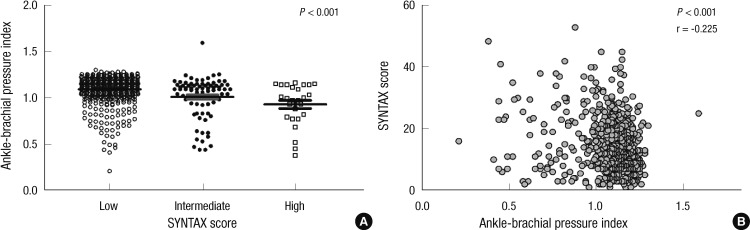
Clinical manifestations and severity of CAD in patients with or without PAD are presented in Table 2. Among patients whose CAD was confirmed by coronary angiography, those with PAD tended to present more often with left main (14.3% vs 5.8%, P = 0.003) and multivessel CAD (74.9% vs 52.1%, P < 0.001) when compared with those who did not have PAD. The overall prevalence of PAD in patients with left main coronary lesion was 26.5%. Moreover, patients with multivessel CAD were more likely to present with moderate to severe PAD (ABPI < 0.7) when compared with patients with single vessel CAD (6.3% vs 2.2%, P = 0.008). PAD seemed more prevalent in patients who presented with atherosclerotic coronary disease than in those with acute coronary syndrome, although the differences were not statistically significant (14.6% vs 12.1%, P = 0.386). The prevalence of bilateral PAD was higher in multivessel CAD patients than those with single vessel disease (5.3% vs 1.9%, P < 0.001) (Fig. 2). The SS tertiles were defined as low (SS < 22), intermediate (SS ≥ 23 and < 32) and high (SS ≥ 33). PAD was more prevalent in patients who presented with multivessel coronary artery disease (17.5%), left main coronary artery disease (26.5%) and higher SS group (38.5%), at statistically significant percentages (Fig. 3). In CAD patients with cerebrovascular accidents, the prevalence of PAD was more than threefold above those with only CAD (40% vs 12.8%, P < 0.001). The value of ABPI was significantly different between the three groups (1.09 ± 0.14, 1.01 ± 0.21, 0.93 ± 0.22, P < 0.001) (Fig. 4A). In the post hoc analysis, the difference of ABPI between patients with the low and intermediate risk group, as well as low and high risk group was statistically significant except comparing of intermediate and high risk group (low and intermediate risk group, P = 0.024; low and high risk group, P < 0.001; intermediate and high risk group, P = 0.072). ABPI showed a significant negative correlation with SS (correlation coefficient=-0.225, P < 0.001) (Fig. 4B).
Table 2.
Severity of coronary artery disease (CAD) in both patients with or without peripheral artery disease (PAD)
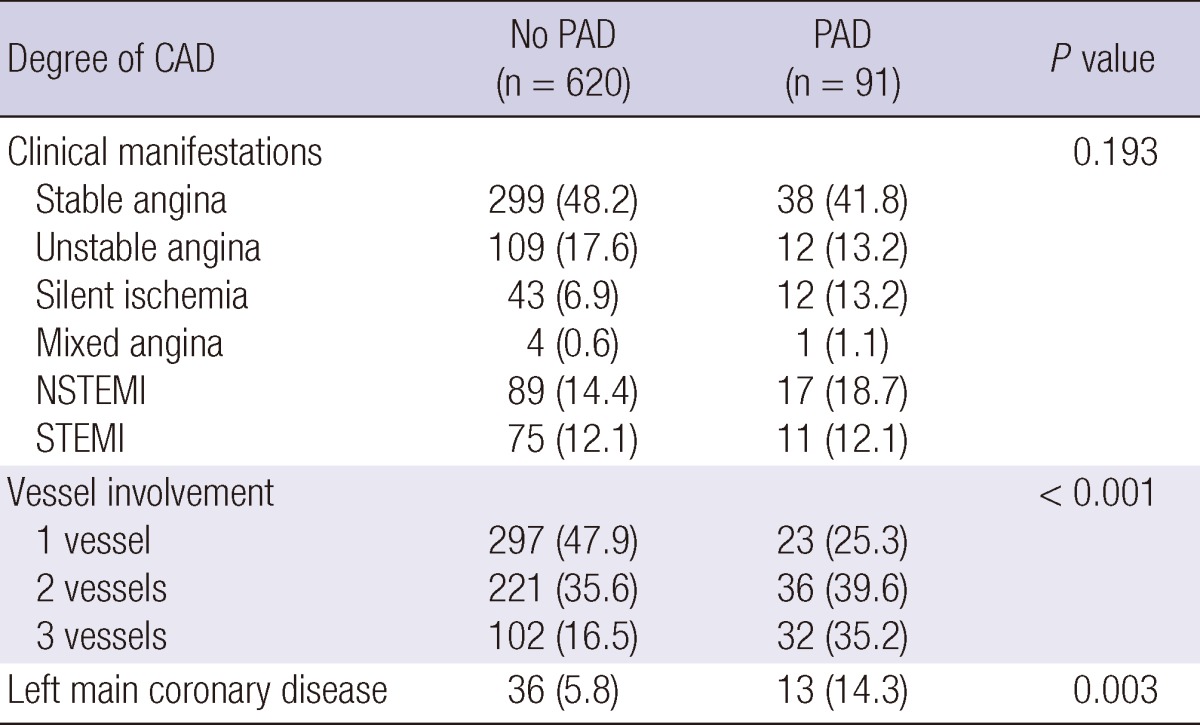
PAD, peripheral artery disease; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
Fig. 2.
The prevalence of unilateral and bilateral PAD according to the number of diseased coronary artery. VDs, vessel disease.
Fig. 3.
The prevalence of peripheral artery disease (PAD) according to the severity of coronary artery disease (CAD). LM, left main coronary disease.
Fig. 4.
(A) The relation between the ankle-brachial pressure index and (B) SYNTAX score.
Using multivariate analysis, we confirmed that the age (OR, 1.090; 95% CI, 1.061-1.120; P < 0.001), smoking history (OR, 2.027; 95% CI, 1.185-3.468; P = 0.010), previous CVA history (OR, 3.320; 95% CI, 1.495-9.226; P = 0.021), left main CAD (OR, 3.117; 95% CI, 1.495-6.497; P = 0.002) and multivessel CAD (OR, 2.257; 95% CI, 1.323-3.852; P = 0.003) were all independently associated with PAD (Table 3).
Table 3.
Predictors of peripheral artery disease in patients undergoing PCI
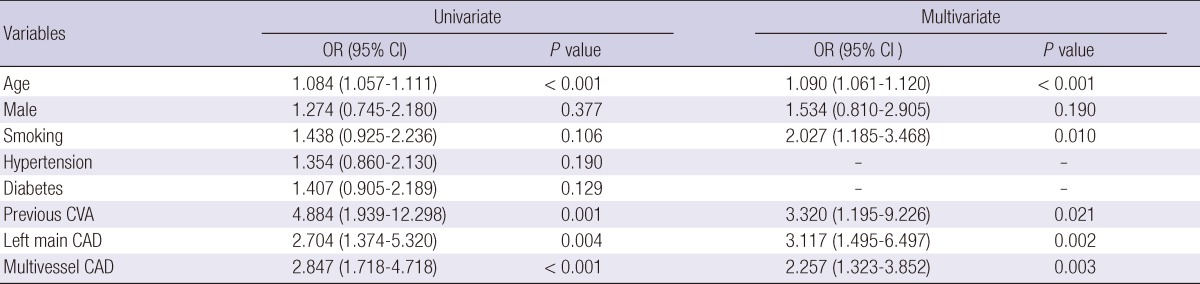
Adjusted covariates include age, gender, history of smoking, previous CVA, left main CAD and multivessel CAD. OR, odds ratio; 95% CI, 95 percentile confidence interval; CVA, cerebrovascular accident; CAD, coronary artery disease.
DISCUSSION
The reported prevalence of PAD in patients undergoing PCI with CAD varies from 5% to 40% in previous studies (14-16). The broad range of the PAD prevalence is most likely due to the patients' diverse population backgrounds, demographics, and manifestations of CAD. Our study demonstrates the discrete prevalence of PAD in Korean patients undergoing PCI for CAD and the association between PAD and CAD severity. Compared to Western population (6), the prevalence of PAD in Korean patients undergoing PCI was higher. Although the overall prevalence of PAD was about 13% in our results, patients with high cardiovascular risk factors such as old age, smoking, and atherosclerotic burden showed significantly higher incidences of PAD (17). Considering that the patients with CAD and PAD who undergo PCI experience reduced procedural success and increased short- and long-term mortality (6, 7), this high prevalence of PAD in Korean patients undergoing PCI arouse the physicians to the importance of early detection and proper management of polyvascular disease.
In our analysis, patients with PAD had more severe CAD manifestations, showing a higher frequency of left main and multivessel coronary disease. With multivariate analysis, the severity of CAD was independently associated with a lower ABPI. These findings would support that the increased morbidities and mortality in PAD patients receiving PCI might be a consequence of the more extensive atherosclerotic burden.
As is well known, SS is an independent prognostic factor of the 1-yr risk of death, myocardial infarction in patients with acute coronary syndrome following PCI. However, our study used SS as the parameter of CAD severity and compared the correlation of ABPI and those SS because the scoring system reflects the complexity of the coronary lesions.
ABPI measurement is widely accepted as a useful tool for the identification of asymptomatic PAD and as an important marker for systemic atherosclerosis (19, 20). However, ABPI may have limited value in patients with diabetes or chronic renal failure on hemodialysis as they tend to have calcified vessel walls and with other factor increasing vessel stiffness. To overcome these limitations, we measured TBPI addition to ABPI. TBPI is not widely used by itself because of its low sensitivity. However, the reference of TBPI is helpful to diagnose PAD in patients with low TBPI value and high ABPI value. Significantly low TBPI values (< 0.7) in patients with high ABPI values (> 1.3) may identify PAD (21, 22). Measurements both ABPI and TBPI as screening for asymptomatic PAD in subjects with high cardiovascular risk may increase early diagnosis of PAD, allowing aggressive secondary preventive measures to be taken.
Our study has several limitations. The present study was conducted retrospectively in a single center, which may have led to selection bias. CVA according to ischemic mechanism did not investigate. Additionally, we were unable to investigate additional medical history that could have affected the PAD. However, the fact that this was a relatively large population-based analysis increases our confidence in the results.
In conclusion, PAD of the lower leg is highly prevalent in patients receiving PCI with CAD and strongly associated with disease severity. The high prevalence of PAD in patients who undergo PCI confirms the importance of active screening for PAD using ABPI. We emphasize that clinicians should keep in mind that CAD patients undergoing PCI would have other co-morbid vascular manifestations like PAD.
ACKNOWLEDGMENTS
The authors have no conflicts of interest to disclose.
References
- 1.Kennedy M, Solomon C, Manolio TA, Criqui MH, Newman AB, Polak JF. Risk factors for declining ankle-brachial index in men and women 65 years or older: the cardiovascular health study. Arch Intern Med. 2005;165:1896–1902. doi: 10.1001/archinte.165.16.1896. [DOI] [PubMed] [Google Scholar]
- 2.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the strong heart study. Circulation. 2004;109:733–739. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 3.Higgins TL, Estafanous FG, Loop FD, Beck GJ, Blum JM, Paranandi L. Stratification of morbidity and mortality outcome by preoperative risk factors in coronary artery bypass patients. A clinical severity score. JAMA. 1992;267:2344–2348. [PubMed] [Google Scholar]
- 4.Salasidis GC, Latter DA, Steinmetz OK, Blair JF, Graham AM. Carotid artery duplex scanning in preoperative assessment for coronary artery revascularization: the association between peripheral vascular disease, carotid artery stenosis, and stroke. J Vasc Surg. 1995;21:154–160. doi: 10.1016/s0741-5214(95)70254-7. [DOI] [PubMed] [Google Scholar]
- 5.Eagle KA, Rihal CS, Foster ED, Mickel MC, Gersh BJ. Long-term survival in patients with coronary artery disease: importance of peripheral vascular disease. The coronary artery surgery study (cass) investigators. J Am Coll Cardiol. 1994;23:1091–1095. doi: 10.1016/0735-1097(94)90596-7. [DOI] [PubMed] [Google Scholar]
- 6.Jeremias A, Gruberg L, Patel J, Connors G, Brown DL. Effect of peripheral artery disease on in-hospital outcomes after primary percutaneous coronary intervension for acute myocardial infarction. Am J Cardiol. 2010;105:1268–1271. doi: 10.1016/j.amjcard.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 7.Saw J, Bhatt DL, Moliterno DJ, Brener SJ, Steinhubl SR, Lincoff AM, Tcheng JE, Harrington RA, Simoons M, Hu T, et al. The influence of peripheral artery disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol. 2006;48:1567–1572. doi: 10.1016/j.jacc.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 8.McDermott MM, Liu K, Criqui MH, Ruth K, Goff D, Saad MF. Ankle-brachial index and subclinical cardiac and carotid disease: the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2005;162:33–41. doi: 10.1093/aje/kwi167. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW. Peripheral artery disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 10.Atmer B, Jogestrand T, Laska J, Lund F. Peripheral artery disease in patients with coronary artery disease. Int Angiol. 1995;14:89–93. [PubMed] [Google Scholar]
- 11.Girasis C, Garg S, Räber L, Sarno G, Morel MA, Garcia HM, Lüscher TF, Serruys PW, Windecker S. SYNTAX score and Clinical SYNTAX score as predictors of very long-term clinical outcomes in patients undergoing percutaneous coronary interventions: a substudy of SIRolimus-eluting stent compared with pacliTAXel-eluting stent for coronary revascularization (SIRTAX) trial. Eur Heart J. 2011;32:3115–3127. doi: 10.1093/eurheartj/ehr369. [DOI] [PubMed] [Google Scholar]
- 12.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, et al. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 13.Serruys PW, Onuma Y, Garg S, Sarno G, van den Brand M, Kappetein AP, Van Dyck N, Mack M, Holmes D, Feldman T, et al. Assessment of the SYNTAX score in the Syntax study. EuroIntervention. 2009;5:50–56. doi: 10.4244/eijv5i1a9. [DOI] [PubMed] [Google Scholar]
- 14.Ahn S, Park YJ, Min SI, Kim SY, Ha J, Kim SJ, Kim HS, Yoon BW, Min SK. High prevalence of peripheral artery disease in Korean patients with coronary or cerebrovascular disease. J Korean Med Sci. 2012;27:625–629. doi: 10.3346/jkms.2012.27.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dieter RS, Tomasson J, Gudjonsson T, Brown RL, Vitcenda M, Einerson J. Lower extremity peripheral artery disease in hospitalized patients with coronary artery disease. Vasc Med. 2003;8:233–236. doi: 10.1191/1358863x03vm506ra. [DOI] [PubMed] [Google Scholar]
- 16.Huelmos A, Jimenez J, Guijarro C, Belinchon JC, Puras E, Sanchez C. Underrecognized peripheral artery disease in patients with acute coronary syndrome: Prevalence of traditional and emergent cardiovascular risk factors. Rev Esp Cardiol. 2005;58:1403–1410. [PubMed] [Google Scholar]
- 17.Hong YM. Atherosclerotic cardiovascular disease beginning in childhood. Korean Circ J. 2010;40:1–9. doi: 10.4070/kcj.2010.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng GC, Fowkes FG, Lee AJ, Dunbar J, Housley E, Ruckley CV. Use of ankle brachial pressure index to predict cardiovascular events and death: A cohort study. BMJ. 1996;313:1440–1444. doi: 10.1136/bmj.313.7070.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo SH, Gil TY, Lee HW, Lee K, Hong YM. Effects of smoking on the pulse wave velocity and ankle brachial index in adolescents. Korean Circ J. 2007;37:414–418. [Google Scholar]
- 20.Moussa ID, Jaff MR, Mehran R, Gray W, Dangas G, Lazic Z. Prevalence and prediction of previously unrecognized peripheral artery disease in patients with coronary artery disease: the peripheral artery disease in interventional patients study. Catheter Cardiovasc Interv. 2009;73:719–724. doi: 10.1002/ccd.21969. [DOI] [PubMed] [Google Scholar]
- 21.Brooks B, Dean R, Patel S, Wu B, Molyneaux L, Yue DK. TBI or not TBI: that is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients? Diabet Med. 2001;18:528–532. doi: 10.1046/j.1464-5491.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- 22.Aboyans V, Ho E, Denenberg JO, Ho LA, Natarajan L, Criqui MH. The association between elevated ankle systolic pressures and peripheral occlusive artery disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–1203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]