Abstract
Despite surgery and radiotherapy, as many as 50 % of children with ependymomas will suffer from tumor recurrences that will ultimately lead to death. Our group’s initial peptide-based glioma vaccine targeting EphA2, IL-13Rα2, and Survivin, which are overexpressed in pediatric gliomas, has shown promise in its initial phase of testing. We therefore investigated whether EphA2, IL-13Raα2, Survivin, and, additionally, Wilms’ Tumor 1 (WT1), are overexpressed in pediatric ependymomas to determine if a similar immunotherapy approach could be applicable. Immunohistochemistry was performed using antibodies specific for EphA2, IL-13Rα2, Survivin, and WT1 on paraffin-embedded specimens from 19 pediatric and 13 adult ependymomas. Normal brain and ependyma were used for background staining controls. Negative staining was defined as no staining or staining equaling the background intensity in normal brain tissues. In the 19 pediatric cases, 18 (95 %) demonstrated positive staining for EphA2, 16 (84 %) for IL-13Rα2, 18 (95 %) for Survivin, and only 7 (37 %) for WT1. Only 3 of 19 cases were positive for two or fewer tumor-associated antigens (TAAs); 16 of 19 cases were positive for three or more TAAs. In the 13 adult cases, all 13 demonstrated positive staining for EphA2, IL-13Rα2, and Survivin. Only 2 of 13 cases (15 %) demonstrated positive staining for WT1. All adult specimens were positive for three or more TAAs. Some ependymomas showed patchy variability in intensity. Pediatric and adult ependymomas frequently express EphA2, IL-13Rα2, and Survivin. This provides the basis for the utilization of an established multiple peptide vaccine for ependymoma in a clinical trial setting.
Keywords: Tumor-associated antigen, EphA2, Survivin, Interleukin-13 receptor alpha 2, Wilms’ Tumor 1, Ependymoma
Introduction
Ependymomas are the third most common primary brain tumor in children [1]. Despite standard treatment including surgery and radiotherapy, as many as 50 % of children with ependymomas will suffer from tumor recurrences that will ultimately lead to death [2]. The World Health Organization (WHO) classifies ependymomas as grade II or grade III (anaplastic ependymoma), although certain distinct subtypes are classified as grade I (subependymomas and myxopapillary ependymomas) [3]. Ependymomas usually occur in the spinal cord in adults, whereas childhood ependymomas are most commonly infratentorial. Those that are infratentorial or anaplastic may show an increased tendency to metastasize along the neuroaxis [4, 5]. Considering the high rate of overall recurrence and the cumulative morbidity of sequential surgical and conventional adjuvant therapy approaches, new treatment modalities are urgently needed.
Vaccine strategies may be good candidates for treating or preventing recurrences for ependymomas. It is known that tumor-associated immunity plays a crucial role in ependy-momas with non-recurrent phenotypes, as demonstrated by their specific immune-related gene expression [6]. Long-lasting immunity elicited by T cell based vaccination strategies may lower the rate of recurrence. Our group’s peptide-based vaccine for pediatric gliomas has shown promise in initial pilot studies with objective responses as well as prolonged stable disease seen in a number of patients within this treatment cohort [7]. Our pilot trial for pediatric glioma utilized subcutaneous vaccinations with peptides for EphA2, IL-13Rα2, and Survivin epitopes emulsified in Montanide-ISA-51 given every 3 weeks for eight courses along with intramuscular injections of poly-ICLC in HLA-A2+ children [7]. The different strata included newly diagnosed brainstem gliomas, cerebral high-grade gliomas, or recurrent gliomas. Primary end-points were safety and T cell responses against vaccine-targeted tumor-associated antigens (TAAs), assessed by ELISPOT and tetramer analysis. Treatment response was evaluated clinically and by MR imaging. The use of synthetic peptides encoding HLA-A2-restricted T cell epitopes for tumor-associated antigens [TAAs, referred to as glioma-associated antigens (GAAs) in the context of the glioma vaccine study] avoids the need for autologous fresh tumor tissues to generate the vaccine and provides readily available therapy for patients. In light of the initial success of our existing glioma vaccine targeting three TAAs, namely EphA2, IL-13Rα2, and Survivin, we questioned whether this vaccine strategy could be applicable for ependymomas.
We examined whether EphA2, IL-13Rα2, Survivin, and additionally, Wilms’ Tumor 1 (WT1), are overexpressed in ependymomas of various grades in both pediatric and adult cases. If indeed pediatric ependymomas express one or more of these antigens, then it would stand to reason that our existing peptide-based vaccine might benefit these patients as well. We also examined the expression of Human Leukocyte Antigen (HLA) Class I to investigate whether this important molecule for antigen-presentation is intact in ependymoma.
Methods
Tissues
Archival formalin-fixed, paraffin-embedded ependymomas obtained at the time of tumor biopsy or resection were used for this study under approval by the Institutional Review Board at University of Pittsburgh (IRB #PRO07010097). Normal, non-neoplastic brain sections were obtained for negative control from the Brain Tumor Bank, Division of Neuropathology at the University of Pittsburgh. A case of glioblastoma positive for EphA2, IL-13Rα2, Survivin, and WT1 was used as positive control. We also examined normal ependyma for expression of the selected TAAs.
Antibodies
The following primary antibodies were used at indicated dilutions: anti-human EphA2 monoclonal antibody (1:100; H-77, rabbit polyclonal IgG, Santa Cruz), anti-human IL-13Rα2 polyclonal antibody (1:1000, Mouse monoclonal, Abcam), anti-human Survivin polyclonal antibody (1:200, FL-142, rabbit polyclonal, Santa Cruz Biotechnology) and WT1 Protein monoclonal antibody (1:100, 6F-H2, mouse monoclonal, Dako), and HCA2 (1 lg/mL) which recognizes a determinant expressed on b2m-free HLA-A (excluding HLA-A24), HLA-B7301 and HLA-G heavy chains. [8, 9] The secondary antibody was either the per-oxidase-conjugated mouse or rabbit Envision system (Dako). Concentrations of the primary antibodies were optimized to minimize background staining in normal brain tissues.
Immunohistochemistry
Paraffin-embedded tissue sections were deparaffinized in xylene and rehydrated in a series of ethanol/phosphate-buffered saline (PBS) washes. Antigen retrieval was performed with modified citrate solution (Dako) in a pressure cooker for 20 min. Endogenous peroxidase was blocked by incubation in 3.0 % hydrogen peroxide (Dako) in PBS, and the nonspecific binding of antibodies was blocked by incubation in serum-free blocking solution (Dako) for 30 min. The slides were incubated with each of the antigen-specific antibodies at the aforementioned concentrations overnight at 4 °C. After washing, slides were then incubated with corresponding Dako Envision secondary antibody for 30 min at room temperature. Peroxidase labeling was visualized using diaminobenzidine (Dako). The sections were lightly counterstained with Gill’s hematoxylin. The sections were then reviewed by JY and RH.
If disagreement occurred regarding staining intensity, the two reviewers achieved a final consensus after discussion. For TAA expression, negative (0) staining was defined as no staining or staining equaling the background intensity in normal brain tissues; moderate (1) staining was defined by definite staining above background intensity in the tumor; strong (2) staining was defined by intense immunoreactivity. Both moderate and strong staining are considered to be positive for TAA overexpression. When patchy/partial staining was present, the intensity representative of more than 50 % of the tumor cells was used. For WT1, endothelial cells were used as a positive internal control [10]. Results for HLA Class I staining were classified according to a modified version of the criteria established by the HLA and Cancer component of the 12th International Histocompatibility Workshop [11]. Lesions were scored as negative (0), when the percentage of stained tumor cells in a specimen was 0 %, 1 when the percentage of positive staining ranged up to 10 %, and 2 when the staining percentage was >10 %, respectively. We considered grades 1 and 2 immunoreactivity as positive staining. Medulloblastoma, which is known to have down-regulated HLA Class I, was used as negative control for HLA Class I [12].
Results
Patient characteristics
Nineteen pediatric (8 female and 11 male) and 13 adult (5 female and 8 male) ependymomas were evaluated. The median age of the pediatric cases was 7 years [range 7 weeks–14 years] and 52 years [range 36–87 years] for the adult cases. In the pediatric series, there were three myxopapillary, eight WHO grade II, and eight anaplastic ependymomas. In the adult series, there were three myxopapillary, ten WHO grade II, and no anaplastic ependymomas.
Immunohistochemistry results
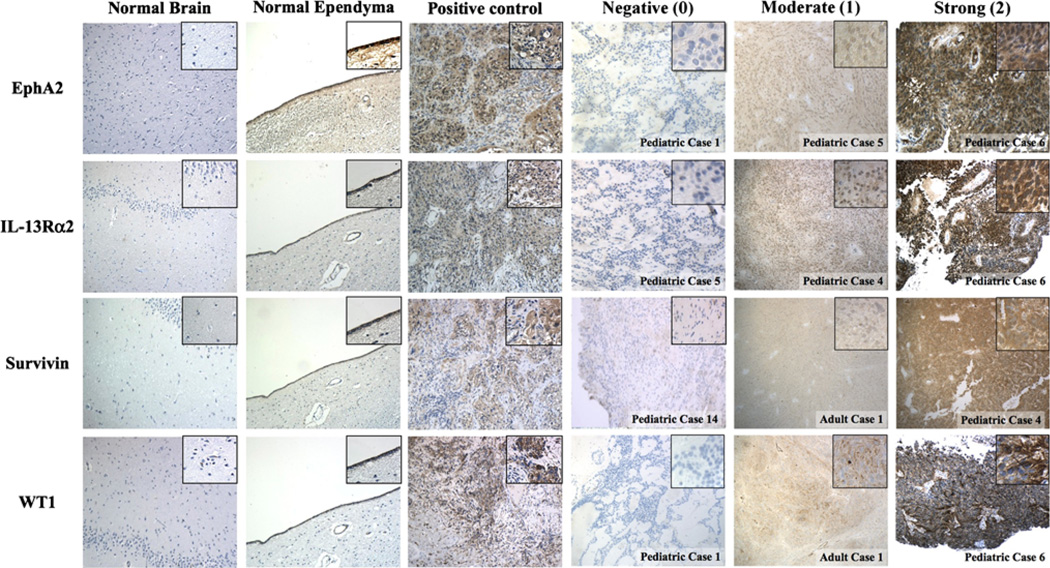
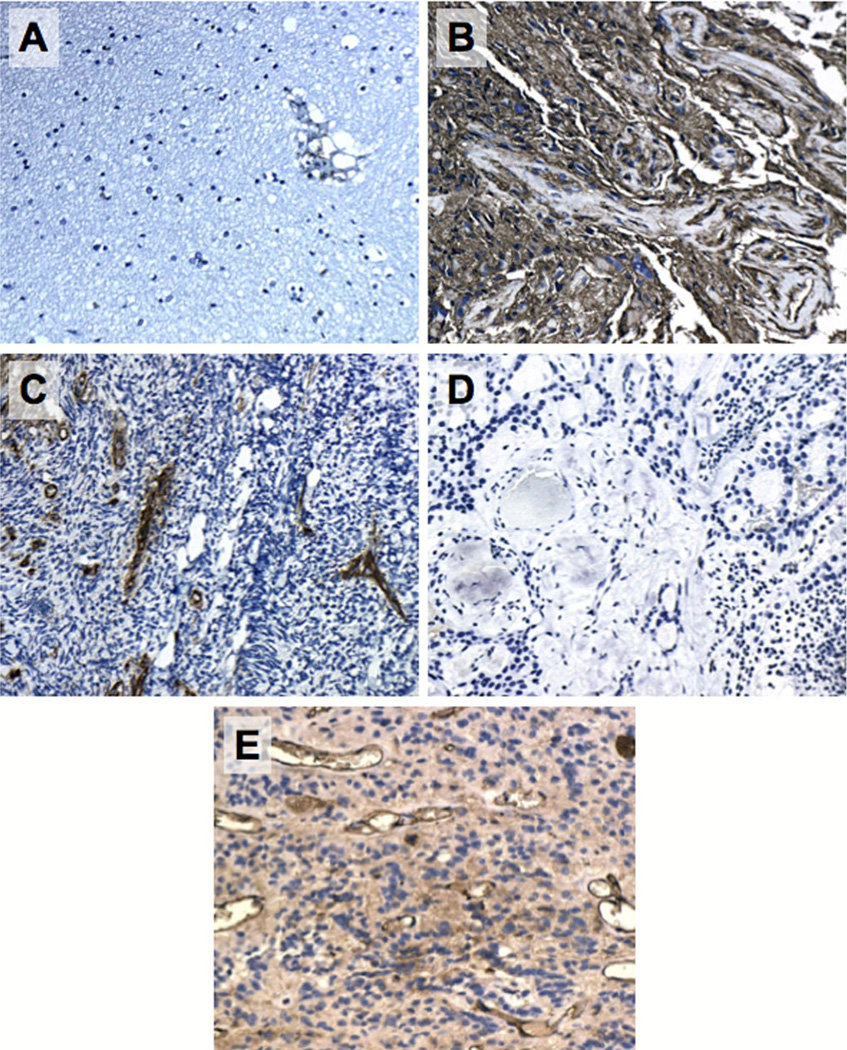
Figure 1 demonstrates representative staining characteristics of the TAAs in normal brain (negative control), normal ependyma, glioblastoma (positive control), and ependymomas. Figure 2 demonstrates representative staining characteristics of HLA Class I in ependymomas.
Fig. 1.
Immunohistochemical analysis of EphA2, IL-13Rα2, Survivin, and WT1 in normal brain (negative control), normal ependyma, glioblastoma (positive control), and ependymoma. Cases representative of different staining intensity for each TAA are shown. The final score was determined by using the intensity representative of more than 50 % of the tumor cells. Images at original magnification (100×) are shown with high power insets (400×) derived from the corresponding low power fields
Fig. 2.
Representative immunohistochemical images of HLA Class I in normal brain (a), glioblastoma with known positive expression (b, positive control), medulloblastoma (c, negative control), ependymoma with negative immunoreactivity (d), ependymoma with positive immunoreactivity (e). Images at original magnification (100×) are shown
Staining results of all evaluated cases are listed in Table 1 and sorted by tumor grade in Table 2. Negative staining is defined by the lack of staining or weak background staining that was not higher than the background signal observed in normal brain samples. Interestingly, normal ependymal lining stain positive for all TAAs. Partial or patchy expression of all four TAAs was noted in a large number of cases.
Table 1.
Expression of tumor-associated antigens (TAA) and HLA Class I in pediatric and adult ependymomas by immunohistochemistry
| Case | WHO grade | EphA2 | IL-13Rα2 | Survivin | WT1 | HLA Class I | |
|---|---|---|---|---|---|---|---|
| Pediatric | 1 | I | 0 | 0a | 1a | 0 | 0 |
| 2 | I | 2 | 2 | 1a | 0 | N/Ab | |
| 3 | I | 2 | 1 | 1 | 0 | 0 | |
| 4 | II | 1 | 1 | 2 | 0a | 1 | |
| 5 | II | 1a | 0 | 1 | 0a | 1 | |
| 6 | II | 2 | 2a | 2a | 2 | N/Ab | |
| 7 | II | 1a | 1a | 1 | 0a | N/Ab | |
| 8 | II | 2a | 1 | 1 | 0a | 2 | |
| 9 | II | 2a | 1a | 1a | 2a | 1 | |
| 10 | II | 1a | 1 | 2 | 1a | 2 | |
| 11 | II | 1 | 1 | 1 | 0 | N/Ab | |
| 12 | III | 2a | 1 | 1a | 0 | N/Ab | |
| 13 | III | 2 | 2 | 2 | 1 | 1 | |
| 14 | III | 1a | 0a | 0a | 0 | 1 | |
| 15 | III | 2 | 1a | 2 | 2 | 0 | |
| 16 | III | 2a | 2a | 2 | 2 | N/Ab | |
| 17 | III | 1a | 1 | 1 | 2a | 1 | |
| 18 | III | 2a | 2a | 2 | 0 | 0 | |
| 19 | III | 1a | 2 | 1 | 0 | N/Ab | |
| Adult | 1 | I | 1 | 2 | 1 | 1a | 1 |
| 2 | I | 2 | 2 | 2 | 0 | 2 | |
| 3 | I | 2 | 2 | 2 | 0 | 1 | |
| 4 | II | 1a | 1 | 1 | 0a | 1 | |
| 5 | II | 2 | 2a | 2 | 2 | 1 | |
| 6 | II | 2 | 2 | 1 | 0 | 0 | |
| 7 | II | 1a | 1 | 1 | 0 | 0 | |
| 8 | II | 1 | 1 | 1 | 0a | 1 | |
| 9 | II | 1 | 2 | 1 | 0 | 1 | |
| 10 | II | 1 | 2 | 1 | 0a | 0 | |
| 11 | II | 2 | 2 | 2 | 0a | 1 | |
| 12 | II | 2 | 1 | 2 | 0 | 2 | |
| 13 | II | 1 | 1 | 1 | 0 | 2 |
Patchy or partial staining
Insufficient tissue for analysis
Table 2.
Tumor-associated antigen (TAA) expression in pediatric and adult ependymoma by tumor grade
| WHO grade | EphA2 (0/1/2) |
IL-13Rα2 (0/1/2) |
Survivin (0/1/2) |
WT1 (0/1/2) |
HLA Class Ia (0/1/2) |
|
|---|---|---|---|---|---|---|
| Pediatric | 1 | 1/0/2 | 1/1/1 | 0/3/0 | 3/0/0 | 2/0/0 |
| 2 | 0/5/3 | 1/6/1 | 0/5/3 | 5/1/2 | 0/3/2 | |
| 3 | 0/3/5 | 1/3/4 | 1/3/4 | 4/1/3 | 2/3/0 | |
| Adult | 1 | 0/1/2 | 0/0/3 | 0/1/2 | 2/1/0 | 0/2/1 |
| 2 | 0/6/4 | 0/5/5 | 0/7/3 | 9/0/1 | 3/5/2 | |
| 3 | N/A | N/A | N/A | N/A | N/A |
Some cases did not have sufficient tissue for analysis
In the 19 pediatric cases, 18 (95 %) demonstrated positive staining for EphA2, 16 (84 %) exhibited positive staining for IL-13Rα2, 18 (95 %) were positive for Survivin, and only 7 (37 %) demonstrated staining for WT1. Overall, 16 of 19 cases were positive for three or more TAAs, one was positive for two, and two showed staining for only one of the antigens.
In the 13 adult cases, all 13 demonstrated positive staining for EphA2, IL-13Rα2, and Survivin. Only 2 of 13 cases (15 %) demonstrated positive staining for WT1. Overall, all adult specimens were positive for three or more TAAs due to the fact that they were all positive for EphA2, IL-13Rα2, and Survivin.
No associations were identified between the levels of TAA expression and age, sex, tumor grade, tumor locations, or Mib-1 proliferation index.
Overall, 8 of 12 pediatric cases available for staining were positive for HLA Class I staining. Five of the eight positive cases were recurrences. Out of 13 adult cases available for staining, 10 had positive had immunoreactivity.
Discussion
In the current study, we investigated the expression of four TAAs (EphA2, IL-13Rα2, Survivin and WT1) in both pediatric and adult ependymomas. We sought to determine whether a cytotoxic T cell based vaccine targeting EphA2, IL-13Rα2, and Survivin, currently used in a pilot trial for newly diagnosed and recurrent gliomas could be utilized in patients with ependymomas. We were also interested in the expression of WT1 as a fourth target that could potentially be added to the aforementioned vaccine cocktail. We found that a majority of pediatric cases and all adult cases of ependymoma overexpressed EphA2, IL-13Rα2, and Survivin relative to normal brain tissue. In contrast, a substantially lower proportion of cases expressed WT1.
EphA2 is a tyrosine kinase receptor that plays a role in angiogenesis and focal adhesion in tumors [13]. An epitope EphA2883–891 was shown by our group to elicit an HLAA2-restricted cytotoxic T cell response against glioma cell lines [14]. In this study, EphA2 was found to be positive in 18 of 19 pediatric cases and all of the adult cases. These results corroborate previous work showing not only that this protein is highly expressed in pediatric [15] and adult gliomas [14, 16], but that mRNA expression for EphA2 was detected in 7 of 7 cases of pediatric ependymoma [17]. Although EphA2 mRNA overexpression was found to correlate inversely with survival in a panel of 21 adult GBM cases [18], the prognostic significance of EphA2 in ependymoma remains unknown. Nevertheless, the overall high expression of EphA2 in ependymoma and the tumor killing efficacy demonstrated in vitro by targeting a specific EphA2 epitope in glioma [14] make this TAA a promising target for immunotherapy in ependymoma.
IL-13Rα2 was similarly highly expressed in 16 of 19 pediatric and all cases of adult ependymoma. This is consistent with a previous study showing that mRNA for this protein was positive in the majority of cases previously evaluated [17]. Kawakami et al. [19] reported that 2 of 3 ependymoma samples expressed IL-13Rα2. All of our adult cases demonstrated positive expression of IL-13Rα2. With the overall high expression of IL-13Rα2 in ependymoma and our previous discovery of an analogue peptide of IL-13Rα2 that is more potent at eliciting an immune response against glioma cells than the native peptide [20], IL-13Rα2 also appears to be a promising target for ependymoma immunotherapy. Furthermore, we have demonstrated the immunogenicity and safety of two peptides (IL-13Rα2- and EphA2-derived peptides) in our previous phase I/II study in adult patients with recurrent high-grade glioma [21].
Survivin is an anti-apoptotic protein widely expressed in fetal tissues and is critical for the development of the normal embryo [22]. Survivin expression has been reported in many adult malignancies [23] and our group previously reported that Survivin was expressed in 16 of 27 pediatric glioma cases [15]. Its expression in intracranial ependymoma has been correlated with tumor cell proliferation and associated with poor patient outcome [24]. Although we were not able to evaluate this in the current study, nuclear expression of Survivin has been found to correlate with morphologic tumor grade in ependymomas and a loss of nuclear expression has been associated with progressive anaplasia [25]. Both positive cytoplasmic and nuclear staining were noted in our cases. In the current series, 18 of 19 pediatric and all the adult cases were positive for Survivin, which is in agreement with the study by Altura et al. [25] showing overall high expression of Survivin in ependymomas of various grades. Survivin, which has had multiple T cell epitopes identified [26, 27], appears to be a third promising vaccine target in ependymomas. Interestingly, although it has been previously reported that normal ependyma can be strongly positive for Survivin, our study suggests that normal ependymal lining is also positive for EphA2, IL-13Rα2, and WT1 [25]. Reasons for positive immunoreactivity of TAAs in normal ependymal lining are still unclear. In the setting of normal ependymal tissue, Altura et al. reported positive expression of Survivin and theorized that its expression within the ependyma of normal human brain might be critical for the support of neural stem cell growth and contribute to cell–cell interactions within the subventricular zone and the ependymal layer [25, 28]. Expressions of these TAAs that are generally supportive of cellular survival may contribute in a similar manner to the ependyma’s role in stem cell maintenance. In the context of immunotherapy, the majority of TAAs identified have been self-proteins and chemokine expression within tumors is necessary to induce the migration of T cells and antigen presenting cells to those sites and mediate anti-tumor responses (reviewed by Kaufmann et al. [29]). The expression of TAAs in normal tissue, which lacks an inflammatory environment, would therefore be unlikely to attract tumor-specific effector cells. Additionally, within the limit of our current experience in our glioma trial that utilizes EphA2, IL-13Rα2, and Survivin peptides, no immune responses outside of the target tissue have been noted.
Wilms’ Tumor 1 is a zinc finger transcription factor that binds to GC-rich sequences and controls the expression of various growth factor genes [30]. It is expressed in many solid tumors [31] and various normal adult tissues [32]. In our series of ependymomas, WT1 was only positive in 7 of 19 pediatric and 2 of 13 adults cases, congruent with a report by Idowu et al. [33] showing WT1 positive staining in 4 of 10 cases and another report by Schittenhelm et al. [34] showing WT1 positivity in 6 of 14 adult myxopapillary ependymomas (grade I), 10 of 24 grade II ependymomas, and 8 of 9 anaplastic ependymomas (grade III). We had no anaplastic cases in our adult series, so we were unable to corroborate this finding. Hashiba et al. [35] also showed strong WT1 expression by immunohistochemistry in 3 of 4 anaplastic ependymomas (age of cases unknown). We did find a high percentage of pediatric anaplastic ependymomas (4 of 8, 50 %) positive for WT1 compared to grade I/II ependymomas (3/11, 27 %), although this was not statistically significant. Further studies are needed to determine whether a vaccine strategy targeting WT1 may be more applicable to high-grade ependymomas.
Out of all 32 cases of ependymomas, patchy or partial expression was noted in 14 for EphA2, 9 for IL-13Rα2, 6 for Survivin, and 13 for WT1. This differential expression of TAAs may be due to the heterogeneous nature of the tumors or due to “immuno-editing”, in which progressive lesions exhibit loss of specific antigens [36]. This provides further support for the strategy of targeting multiple TAAs for immunotherapy in ependymomas.
The recognition of tumor cells by cytotoxic T cells requires adequate antigen presentation via the peptide-MHC Class I complex. To investigate this, we further performed immunohistochemistry of the tumor tissues using a monoclonal antibody recognizing most of HLA-A. We chose this antibody because our vaccine strategy utilizes HLA-A2 restricted peptides. Similar to other brain tumors, such as glioblastoma and medulloblastoma, ependymomas can demonstrate a range of expression of HLA Class I molecules as determined by immunohistochemistry. This provides support for the use of an immunoadjuvant, such as imiquimod or poly-ICLC, which might act indirectly through interferon [37] to up-regulate the expression of HLA Class I [38, 39] and enhance cytotoxic T cell activity [40]. Encouragingly, in our pediatric glioma vaccine trial, which also utilizes a peptide plus adjuvant approach, we have observed immunological and radiological responses in some patients.
While immunohistochemistry provides relative expression of a specific protein, which may be subject to the binding specificities of particular antibodies, we chose antibodies reported in prior publications, to facilitate cross-study comparisons [10, 15, 25]. Additionally, we have previously conducted a similar study in pediatric gliomas to translate findings from adult specimens, which became the basis of the ongoing pediatric trial [15].
In conclusion, our study demonstrates frequent overexpression of EphA2, IL-13Rα2, and Survivin in both pediatric and adult ependymomas of all grades. Positive WT1 expression is detected in a minority of cases. A vaccine targeting EphA2, IL-13Rα2, and Survivin is currently being used in a clinical trial for astrocytomas, which incorporates immune response monitoring. Our findings in this study suggest that EphA2, IL-13Rα2, and Survivin may also be reasonable targets in peptide-based immunotherapy for ependymomas.
Acknowledgments
We thank Maki Ikeura, BS for providing support with immunohistochemistry. This work was supported by a grant from the Doris Duke Charitable Foundation to University of Pittsburgh to J.Y. and a grant from National Institute of Health (R21 CA149872-02) to R.H. and I.P.
Footnotes
Ethical standards This study complies with the current laws of the country in which they were performed.
Conflict of interest The authors do not have any conflict of interest to declare.
Contributor Information
Jacky T. Yeung, Department of Neurological Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Ronald L. Hamilton, Department of Pathology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Hideho Okada, Department of Neurological Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Regina I. Jakacki, Departments of Pediatrics, Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA
Ian F. Pollack, Department of Neurological Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA Children’s Hospital of Pittsburgh, 4401 Penn Avenue, Pittsburgh, PA 15224, USA, ian.pollack@chp.edu.
References
- 1.Hamilton RL, Pollack IF. The molecular biology of ependymomas. Brain Pathol. 1997;7:807–822. doi: 10.1111/j.1750-3639.1997.tb01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agaoglu FY, Ayan I, Dizdar Y, Kebudi R, Gorgun O, Darendeliler E. Ependymal tumors in childhood. Pediatr Blood Cancer. 2005;45:298–303. doi: 10.1002/pbc.20212. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poltinnikov IM, Merchant TE. CSF cytology has limited value in the evaluation of patients with ependymoma who have MRI evidence of metastasis. Pediatr Blood Cancer. 2006;47:169–173. doi: 10.1002/pbc.20587. [DOI] [PubMed] [Google Scholar]
- 5.Kawabata Y, Takahashi JA, Arakawa Y, Hashimoto N. Long-term outcome in patients harboring intracranial ependymoma. J Neurosurg. 2005;103:31–37. doi: 10.3171/jns.2005.103.1.0031. [DOI] [PubMed] [Google Scholar]
- 6.Donson AM, Birks DK, Barton VN, Wei Q, Kleinschmidt-Demasters BK, Handler MH, Waziri AE, Wang M, Foreman NK. Immune gene and cell enrichment is associated with a good prognosis in ependymoma. J Immunol. 2009;183:7428–7440. doi: 10.4049/jimmunol.0902811. [DOI] [PubMed] [Google Scholar]
- 7.Pollack I, Jakacki R, Butterfield L, Okada H. Peptide vaccine therapy for childhood gliomas. J Neurosurg Pediatr. 2012 doi: 10.1227/01.neu.0000430769.33467.68. A341 (abstract) [DOI] [PubMed] [Google Scholar]
- 8.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177–188. doi: 10.1016/s0161-5890(98)00026-1. [DOI] [PubMed] [Google Scholar]
- 9.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 10.Bourne TD, Elias WJ, Lopes MB, Mandell JW. WT1 is not a reliable marker to distinguish reactive from neoplastic astrocyte populations in the central nervous system. Brain Pathol. 2010;20:1090–1095. doi: 10.1111/j.1750-3639.2010.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dominique Charron. 12th International Histocompatibility Conference; Genetic diversity of HLA: functional and medical implications; June 9–12, 1996; Paris, France. New York: Elsevier; 1996. pp. 1–184. Abstracts. Human immunology. [PubMed] [Google Scholar]
- 12.Raffaghello L, Nozza P, Morandi F, Camoriano M, Wang X, Garre ML, Cama A, Basso G, Ferrone S, Gambini C, Pistoia V. Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res. 2007;67:5471–5478. doi: 10.1158/0008-5472.CAN-06-4735. [DOI] [PubMed] [Google Scholar]
- 13.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614–5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 14.Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, Pollack IF, Hamilton RL, Storkus WJ, Okada H. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okada H, Low KL, Kohanbash G, McDonald HA, Hamilton RL, Pollack IF. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol. 2008;88:245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 17.Zhang JG, Kruse CA, Driggers L, Hoa N, Wisoff J, Allen JC, Zagzag D, Newcomb EW, Jadus MR. Tumor antigen precursor protein profiles of adult and pediatric brain tumors identify potential targets for immunotherapy. J Neurooncol. 2008;88:65–76. doi: 10.1007/s11060-008-9534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, Jiang X, Yu Y, Brosius A, Thomas M, Chin L, Brennan C, DePinho RA, Kohane I, Carroll RS, Black PM, Johnson MD. A genomewide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815–10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami M, Kawakami K, Takahashi S, Abe M, Puri RK. Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer. 2004;101:1036–1042. doi: 10.1002/cncr.20470. [DOI] [PubMed] [Google Scholar]
- 20.Eguchi J, Hatano M, Nishimura F, Zhu X, Dusak JE, Sato H, Pollack IF, Storkus WJ, Okada H. Identification of interleukin-13 receptor alpha2 peptide analogues capable of inducing improved antiglioma CTL responses. Cancer Res. 2006;66:5883–5891. doi: 10.1158/0008-5472.CAN-06-0363. [DOI] [PubMed] [Google Scholar]
- 21.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29:330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uren AG, Wong L, Pakusch M, Fowler KJ, Burrows FJ, Vaux DL, Choo KH. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–1328. doi: 10.1016/s0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- 23.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 24.Preusser M, Wolfsberger S, Czech T, Slavc I, Budka H, Hainfellner JA. Survivin expression in intracranial ependymomas and its correlation with tumor cell proliferation and patient outcome. Am J Clin Pathol. 2005;124:543–549. doi: 10.1309/PP2G5GAAFKV82DTG. [DOI] [PubMed] [Google Scholar]
- 25.Altura RA, Olshefski RS, Jiang Y, Boue DR. Nuclear expression of Survivin in paediatric ependymomas and choroid plexus tumours correlates with morphologic tumour grade. Br J Cancer. 2003;89:1743–1749. doi: 10.1038/sj.bjc.6601334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, thor Straten P. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 27.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman HL, Disis ML. Immune system versus tumor: shifting the balance in favor of DCs and effective immunity. J Clin Investig. 2004;113:664–667. doi: 10.1172/JCI21148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakatsuka S, Oji Y, Horiuchi T, Kanda T, Kitagawa M, Takeuchi T, Kawano K, Kuwae Y, Yamauchi A, Okumura M, Kitamura Y, Oka Y, Kawase I, Sugiyama H, Aozasa K. Immunohistochemical detection of WT1 protein in a variety of cancer cells. Mod Pathol. 2006;19:804–814. doi: 10.1038/modpathol.3800588. [DOI] [PubMed] [Google Scholar]
- 31.Oji Y, Ogawa H, Tamaki H, Oka Y, Tsuboi A, Kim EH, Soma T, Tatekawa T, Kawakami M, Asada M, Kishimoto T, Sugiyama H. Expression of the Wilms’ tumor gene WT1 in solid tumors and its involvement in tumor cell growth. Jpn J Cancer Res. 1999;90:194–204. doi: 10.1111/j.1349-7006.1999.tb00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharnhorst V, van der EbAJ, Jochemsen AG. WT1 proteins: functions in growth and differentiation. Gene. 2001;273:141–161. doi: 10.1016/s0378-1119(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 33.Idowu MO, Rosenblum MK, Wei XJ, Edgar MA, Soslow RA. Ependymomas of the central nervous system and adult extra-axial ependymomas are morphologically and immunohistochemically distinct—a comparative study with assessment of ovarian carcinomas for expression of glial fibrillary acidic protein. Am J Surg Pathol. 2008;32:710–718. doi: 10.1097/PAS.0b013e318159a2b4. [DOI] [PubMed] [Google Scholar]
- 34.Schittenhelm J, Beschorner R, Simon P, Tabatabai G, Herrmann C, Schlaszus H, Capper D, Weller M, Meyermann R, Mittelbronn M. Diagnostic value of WT1 in neuroepithelial tumours. Neuropathol Appl Neurobiol. 2009;35:69–81. doi: 10.1111/j.1365-2990.2008.00957.x. [DOI] [PubMed] [Google Scholar]
- 35.Hashiba T, Izumoto S, Kagawa N, Suzuki T, Hashimoto N, Maruno M, Yoshimine T. Expression of WT1 protein and correlation with cellular proliferation in glial tumors. Neurol Med-Chir. 2007;47:165–170. doi: 10.2176/nmc.47.165. discussion 170. [DOI] [PubMed] [Google Scholar]
- 36.Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. Int J Cancer. 1996;66:470–476. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 37.Gibson SJ, Imbertson LM, Wagner TL, Testerman TL, Reiter MJ, Miller RL, Tomai MA. Cellular requirements for cytokine production in response to the immunomodulators imiquimod and S-27609. J Interferon Cytokine Res. 1995;15:537–545. doi: 10.1089/jir.1995.15.537. [DOI] [PubMed] [Google Scholar]
- 38.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res. 2001;61:1095–1099. [PubMed] [Google Scholar]
- 39.Yang I, Kremen TJ, Giovannone AJ, Paik E, Odesa SK, Prins RM, Liau LM. Modulation of major histocompatibility complex Class I molecules and major histocompatibility complex- bound immunogenic peptides induced by interferon-alpha and interferon-gamma treatment of human glioblastoma multiforme. J Neurosurg. 2004;100:310–319. doi: 10.3171/jns.2004.100.2.0310. [DOI] [PubMed] [Google Scholar]
- 40.Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, Miller JF, Liau LM. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. J Immunol. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]