Abstract
BACKGROUND
Very preterm infants commonly develop anemia requiring multiple red blood cell transfusions (RBCTx). This is in part attributable to heavy laboratory phlebotomy loss. Quantification of the extent to which laboratory blood loss contributes to anemia sufficient to prompt RBCTx has not been examined.
STUDY DESIGN AND METHODS
Twenty-six preterm infants weighing <1500 g at birth requiring ventilator support who received one or more RBCTx were intensively studied during the first month of life. Hemoglobin (Hb) loss via laboratory blood loss and RBC senescence, and Hb gain from RBCTx were precisely accounted for in a Hb mass balance mathematical model developed to assess the impact of phlebotomy on RBCTx when a restrictive RBCTx criteria were applied.
RESULTS
Study subjects had a birth weight 880±240 g (mean±SD), a Hb level of 14.4±2.4 g/dl at birth and received 3.81±2.15 RBCTx during the study period. Modeling indicated that even with the total elimination of laboratory phlebotomy loss, a reduction of 41–48% in RBCTx was achievable.
CONCLUSION
The present modeling results indicate that while phlebotomy reduction can significantly decrease the number of RBCTx administered to preterm infants, total elimination of all RBCTx will likely require other approaches, e.g., stimulation of erythropoiesis with erythropoiesis stimulating agents.
Keywords: Hematology–Red Cells; RBC Transfusion; Transfusion Practices (Neonatal, Pediatrics)
INTRODUCTION
All extremely premature infants develop anemia in the early weeks of life. This is the result of heavy laboratory blood loss, shortened red blood cell (RBC) lifespan, low plasma erythropoietin levels, inadequate erythropoiesis, and perhaps other factors.1 As treatment for clinically significant anemia, approximately 80% of very low birth weight infants (VLBW) weighing less than 1,500 g at birth and 95% of extremely low birth weight infants (ELBW) weighing less than 1,000 g at birth receive one or more red blood cell transfusions (RBCTx).2 RBCTx are important because they are expensive and associated with complications including infection, fluid overload, electrolyte imbalance, and exposure to plasticizers, lead, and other toxins.3 Thus, therapies for neonatal anemia resulting in fewer RBCTx are highly desirable.
Three therapies shown to reduce RBCTx in VLBW infants include treatment with recombinant human erythropoietin, institution of restrictive RBCTx criteria, and reduction in laboratory phlebotomy loss. While erythropoietin administration has been shown to be effective in reducing the number of RBCTx in some preterm infants, its routine use is controversial because of its association with retinopathy of prematurity and its modest effect on decreasing RBCTx.3 Similarly, in the two largest randomized trials of liberal versus restrictive RBCTx criteria, restrictive criteria have demonstrated only a slight reduction in RBCTx when applied as intended.4,5
Reduction in the extensive laboratory phlebotomy losses experienced by very premature infants is the third, and the most promising clinical intervention associated with a reduction in RBCTx. Phlebotomy loss is particularly important among this patient group because even seemingly small volumes of laboratory blood loss are large relative to their weight. Numerous studies have reported that laboratory phlebotomy loss in preterm infants is a major contributor to neonatal anemia and to RBCTx.1,6–8 For example, drawing 6.5 mL of blood from a 1 kg infant whose total blood volume is approximately 80 mL/kg is equivalent to drawing a 450 mL donor blood unit.1 Although there have been successful efforts in reducing iatrogenic laboratory phlebotomy loss in preterm infants 9,10, quantification of the relationship of phlebotomy loss and RBCTx has not been reported.
Thus, our objective was to quantitatively explore the relationship of phlebotomy loss and RBCTx by applying mathematical modeling when uniformly restrictive RBCTx criteria were applied.5 In doing so, we relied on accurate phlebotomy and RBCTx data gathered previously 11, on modeled blood volume, and on RBC lifespan data in the literature.12 The mathematical modeling was used to conduct simulations based on reductions in phlebotomy loss.
MATERIALS AND METHODS
Subjects
Pregnant women delivering and infants born at <29 weeks gestation who were intubated were eligible. Infants excluded were those presenting with hematological diseases (except for anemia of prematurity), receiving RBCTx prior to enrollment, or receiving erythropoiesis stimulating agents. A total of 162 subjects met study eligibility criteria. Of those eligible for study, 119 were not approached because: 1) they had already been approached for another clinical study with similar eligibility criteria and not approached for this protocol (n=39); 2) only two research subjects could be studied at one time due to clinical laboratory workload difficulties in weighing all blood samples (n=62); 3) the infant having received a RBCTx before consent was obtained (n=13); and 4) staff availability (n=5).
Forty-three families were approached between January 2007 and October 2009, 11 before and 32 after delivery. Consent was obtained from 33 families while 10 families refused. Women who were consented antenatally delivered at >29 weeks and became ineligible (n=6). Twenty-seven infants were enrolled and studied over a one-month period (31.6±2.2 days). One of the 27 infants was excluded because the infant was not transfused during the study.
Study Procedures
Laboratory phlebotomy and RBCTx data were obtained from the subjects’ electronic medical record. Accurate weights for 97% of all 2,656 laboratory blood samples drawn in the first month of life were recorded to 0.1 mg.12 Following clinically ordered laboratory testing, leftover anticoagulated blood less than 3 days old was analyzed for hematological parameters using the Sysmex XE-2100 automatic hematology analyzer (Sysmex Corporation, Kobe, Japan).
Intravascular Blood Volume Determination
The blood volume for each infant was calculated from the RBCTx volume, the Hb concentration of the transfused donor blood, and the increase in Hb concentration as previously described 13:
| (1) |
where BV/kg is the total intravascular blood volume per kg body weight; TRXV is the volume of packed RBCs transfused; HCTtrx is the hematocrit of the transfused donor RBCs and HCTtrx/3 is the Hb concentration equivalent 12; ΔHb is the change in Hb concentration after the RBCTx; W is the weight of the infant at the time of transfusion. Blood volumes were calculated for all RBCTx in all infants. For the modeling in this study, individual blood volumes were used for each infant. For example, if an infant received 7 RBCTx, the blood volume used in the model was the average calculated BV for the 7 RBCTx based on Equation 1, i.e., each individual infant’s calculated BV/kg was assumed not to change during the course of the study.
In 40 of 101 of the RBCTx, the HCT aliquots of the packed donor RBCTx were measured. For packed RBCTx in which HCT was not determined, the average HCT for all RBCTx was applied, i.e., 83.1%.
Modeling Phlebotomy Reduction
The number of RBCTx each individual infant was predicted to receive following simulated reductions in phlebotomy was determined. The amount of Hb remaining from the transfused blood was subtracted from each infant’s total Hb to determine the quantity of Hb present in the endogenous blood. The amount of Hb each RBCTx contributed to the infant’s total body Hb at different time points was determined as follows:
| (2) |
where Hb(t)trx is the amount of Hb remaining from a donor RBCTx at time t; Hb(t0)trx is the Hb amount in the RBCTx occurring at time t0; t0 is the time when the most recent RBCTx occurred; τA is the lifespan of transfused adult donor RBCs, i.e., 120 days 14; k is the number of phlebotomies after the RBCTx time t0 but before time t; j is the given phlebotomy number; Fj is the fraction of the total blood volume removed in phlebotomy j. Equation 2 was used to calculate the Hb contribution from each simulated RBCTx at time t. As an illustration of Equation 2, if 10 g of Hb (Hb(t0)trx=10) are administered in a RBCTx given at time 0 (t0=0) and 3 phlebotomies have occurred since the RBCTx, each corresponding to a 5% removal of the infant’s total blood volume (k=3, F1=F2=F3=0.05), the Hb(t)trx can be calculated 5 days after the RBCTx as follows:
To determine the amount of Hb administered in each RBCTx, Hb(t0)trx, the following equation was used:
| (3) |
, where TRXv is the volume of blood transfused and HCTtrx is the HCT of a given RBCTx.
Phlebotomy reduction simulation analysis was performed by adding a fraction of the amount of the Hb removed by phlebotomy to the total amount of Hb in the body of each infant. The amount of Hb added to achieve specified percent phlebotomy reductions was determined as follows:
| (4) |
where PHLEV is the volume of a single phlebotomy; Hbphle is the Hb concentration immediately before the phlebotomy; PR is the percentage by which the phlebotomy removal was reduced (i.e., PR=0.0 indicates no change from what was observed and PR=1.0 is the simulation value if no phlebotomies occurred); Hb(T)phle is the amount of Hb added for a given PR occurring at time T.
To determine the endogenous Hb contribution of phlebotomy reduction at different time points following birth, equation 2 is modified such that:
| (5) |
, where Hb(t)phle is the Hb amount remaining in the body at time t after the phlebotomy; τI is the lifespan of preterm infant RBCs fixed at 65.8 days;12 and m is the number of phlebotomies that occurred after the phlebotomy at time T but before t. Equation 5 was used to calculate the Hb contribution of individual simulated phlebotomy reductions (PR) at time t following which each infant’s Hb concentration was in response adjusted accordingly. Equation 5 determines the Hb contribution from the infant’s own blood while Equation 2 determines the Hb contribution of transfused donor blood. It was assumed for this study that the rate of endogenous Hb production did not change at different Hb levels.
Transfusion Simulations
Prediction of the extent to which the number of each infant’s RBCTx were hypothetically reduced as a result of phlebotomy reduction was determined in two steps. It was first determined what the intravascular Hb concentration versus postnatal age profile would be for each infant in the absence of both RBCTx and phlebotomies. Second, it was determined what the “no intervention profile” would be if each infant was phlebotomized as was done clinically, but with the volume of each phlebotomy reduced by a specified percentage. This resulted in a Hb versus time profile that could be evaluated according to the PINT RBCTx trigger criteria to determine if one or more RBCTx were indicated. The effect of the phlebotomy reduction was expressed as the percent RBCTx reduction calculated from predicted RBCTx relative to the actual number of RBCTx administered. The three RBCTx criteria applied were: 1) the PINT restrictive Hb threshold criteria for infants receiving respiratory support with capillary Hb correction 5 (Figure 1); 2) the PINT restrictive threshold criteria for infants receiving respiratory support with no capillary Hb correction; and 3) the Hb level and day of life when infants were transfused clinically. For the PINT RBCTx criteria with Hb correction, a 10% reduction was made for capillary blood sampling.15
Figure 1.
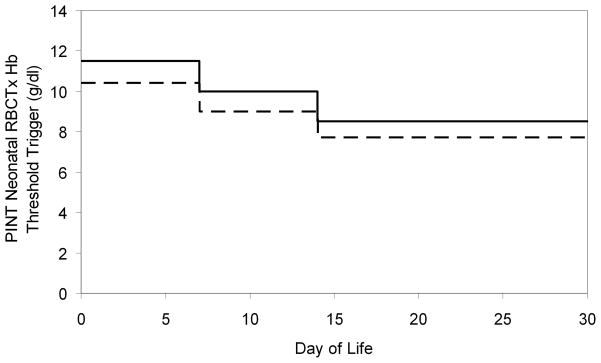
PINT restrictive threshold Hb RBCTx criteria for ventilated VLBW infants. Age versus Hb concentration for administration of RBCTx to preterm infants in PINT trial 5. Capillary Hb levels (solid line) are 10% higher than central Hb levels (Dashed line).
To determine whole blood Hb concentration for the above analysis, the Hb amounts derived from Equations 2 and 5 were converted to Hb concentrations as follows:
| (6) |
, where HbC is the Hb concentration and HbA is the Hb amount. The blood volume was determined in Equation 1. In the current work, many of the results are presented in Hb amount (g) which is determined by multiplying the concentration of Hb by the model determined total blood volume.
When an individual infant’s Hb concentration reached the specific RBCTx criteria, a simulated RBCTx was administered. All simulated RBCTx were 15 mL/kg of packed RBCs with an assumed Hct of 83.1%. The resulting Hb amount was estimated for each simulated RBCTx according to Equations 2 and 3. The simulations were programmed in FORTRAN and graphical output was done using WINFUNFIT 16 or Microsoft Excel (Microsoft Redmond, WA).
RESULTS
Study Subjects
The 26 ventilated study infants had a gestational age of 26.6±1.3 weeks (mean±SD) and a mean birth weight of 880±240 g. Infants received an average of 3.81±2.15 RBCTx and underwent 138±21.2 clinical phlebotomies for laboratory blood testing during the month long study period. Ninety-seven percent of all laboratory blood samples drawn were weighed. The average volume of transfused donor packed RBCs was 14.4±1.8 mL/kg with 85% of the transfusions being between 13 and 17 mL/kg of packed RBCs. At birth infants had a mean amount of total Hb of 12.1±3.29 g. During the one-month study period, 14.4±5.27 g of Hb was transfused and 6.28±2.07 g of Hb was drawn for laboratory tests. When expressed relative to birth weight, this corresponded to a mean 14.1±3.05 g/kg of Hb at birth, 18.6±10.3 g/kg of Hb transfused, and 8.03±4.06 g/kg of Hb phlebotomized. Of the 2,656 laboratory samples in which Hb concentration was determined, 36.2% of the samples were drawn centrally (arterial or venous) and 63.8% were drawn as a capillary sample. The Hb concentration was determined in 40 of the 101 RBCTx administered; the mean Hb concentration of transfused blood was 27.7±1.4 g/dl. The mean estimated infant blood volume based on Equation 1 was 93.2±24.9 mL/kg.
Hb levels at time of clinical transfusion versus PINT RBCTx criteria
The PINT RBCTx criteria become increasingly restrictive with increasing postnatal age (Figure 1). Comparison of the Hb levels at which infants actually received clinical transfusions relative to PINT RBCTx criteria indicate that infants were liberally transfused 4. Of the 101 RBCTx administered, 88 (87%) were administered at Hb levels greater than that required had PINT RBCTx criteria been applied (Figure 2).
Figure 2.
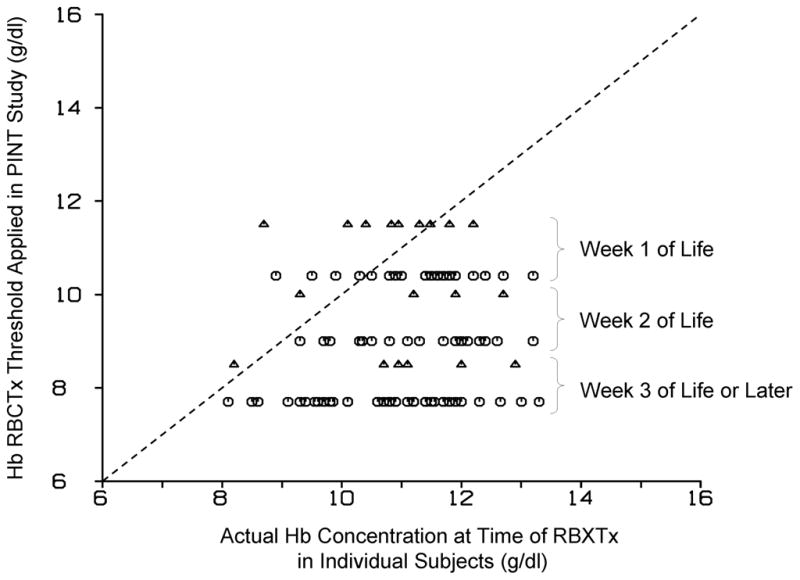
Iowa clinical RBCTx practice is more liberal than PINT restrictive threshold Hb criteria. Hb concentration when individual study infants were transfused is shown compared to the successive postnatal decline in Hb for the PINT restrictive threshold Hb RBCTx criteria for infants requiring respiratory support. Capillary Hb levels (triangles) are 10% higher than central Hb levels (circles). The dashed line represents the line of identity.
Fate of Phlebotomized Blood
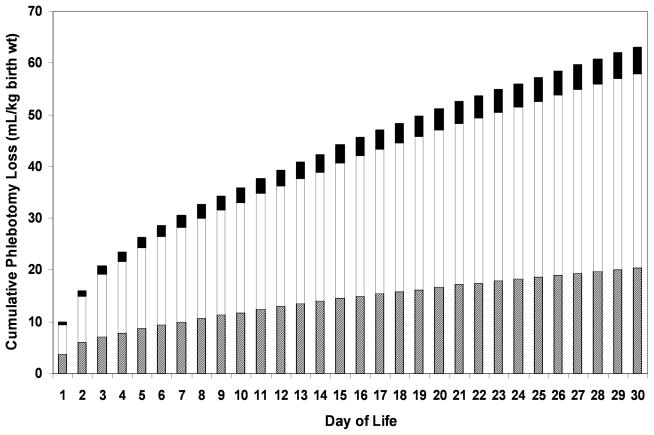
The cumulative amount of laboratory blood drawn for the 26 individual study infants over the first 30 days of life was determined based on weighed blood samples (Figure 3). Notably, only 33% of the blood withdrawn was required by the laboratory instruments, while 59% was discarded as waste and 8% was attributable to hidden blood loss in syringes or on to gauze pads, bandages, etc. 12.
Figure 3.
Most laboratory blood loss in VLBW preterm infants is discarded. Average cumulative infant laboratory testing blood loss during the first month. Of all blood taken from the 26 VLBW study infants, 33% is required for laboratory analysis (hashed bars), 59% of is discarded as waste (white bars), and 8% represents hidden blood loss (black bars) (i.e., blood left in syringes or on gauze pads, bandages, etc.)
Blood Removed and Blood Transfused
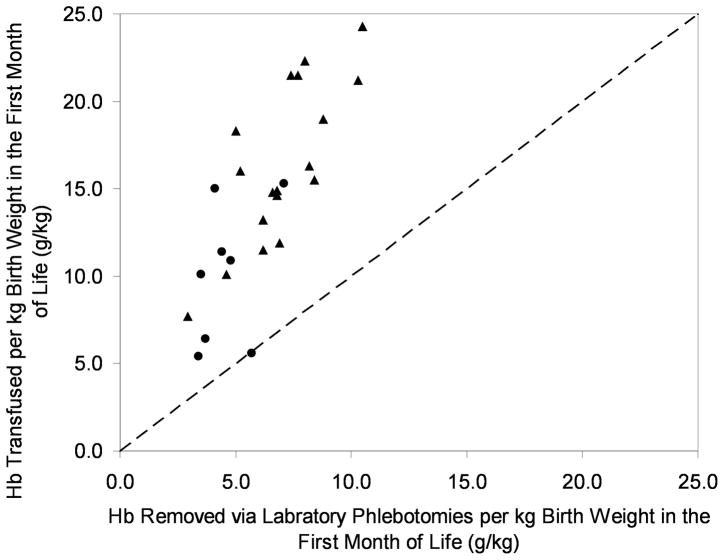
Based on precisely determined phlebotomy and RBCTx data, it was possible to determine the cumulative amounts of blood transfused and removed for individual infants. When all infants were combined, there was a significant association of phlebotomy loss (per kg birth weight) and birth weight (r2=0.6329, p<0.05). When adjusted for birth weight, Hb removed and Hb transfused during the first month of life demonstrated that study subjects had 2.3 times as much Hb transfused as was removed (Figure 4). ELBW infants had more Hb transfused compared to VLBW infants.
Figure 4.
Preterm infants receive more Hb from RBCTx than is removed for laboratory testing. For 26 preterm study infants, the Hb removed and transfused is shown by triangles for ELBW (birth weight <1.0 kg) and by circles for VLBW infants (birth weight 1.0–1.5 kg). Approximately 2.3 times more Hb was transfused as was removed. The dashed line represents the line of identity.
Modeling of Phlebotomy Reduction
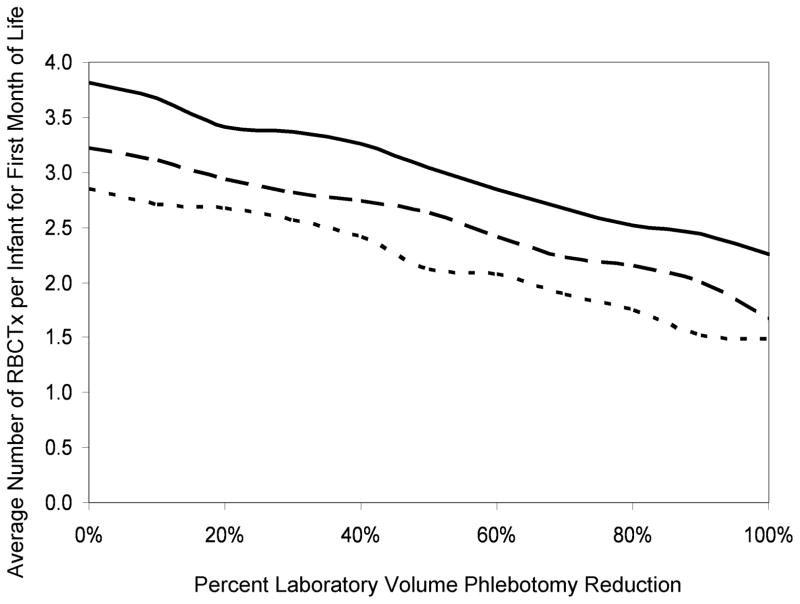
In modeling simulated reductions of laboratory phlebotomy loss in the 26 study subjects according to Equation 5, resulting reductions in RBCTx (Figure 5) could be determined for the three separate transfusion criteria applied. Although the average number of RBCTx per infant demonstrated a progressive decline as the phlebotomy reduction increased, the mean modeled RBCTx could not be eliminated, even with elimination of all phlebotomy loss. When applying the actual RBCTx practices and laboratory blood loss was totally eliminated in the model, mean RBCTx were reduced by only 41%, i.e., from 3.81 to 2.26 RBCTx per infant. For the PINT low Hb threshold with and without adjustment for capillary sampling, the decline was 48% (3.22 to 1.66 RBCTx per infant) and 48% (2.85 to 1.48 RBCTx per infant), respectively. When individual infants were modeled using the PINT RBCTx criteria with adjustment for capillary blood samples, elimination of all laboratory phlebotomy loss resulted in 4 infants who were predicted to require no RBCTx.
Figure 5.
Simulation of phlebotomy reduction among VLBW preterm infants showing a reduction in the number of RBCTx. The average number of RBCTx during the first month of life determined by modeling of all 26 infant study subjects versus the percentage phlebotomy reduction. The actual RBCTx practice (solid line), the PINT restrictive RBCTx criteria (slightly broken line) and the PINT restrictive RBCTx without capillary Hb adjustment (more broken line) are shown.
Modeled decline in infant Hb level with no laboratory phlebotomy loss
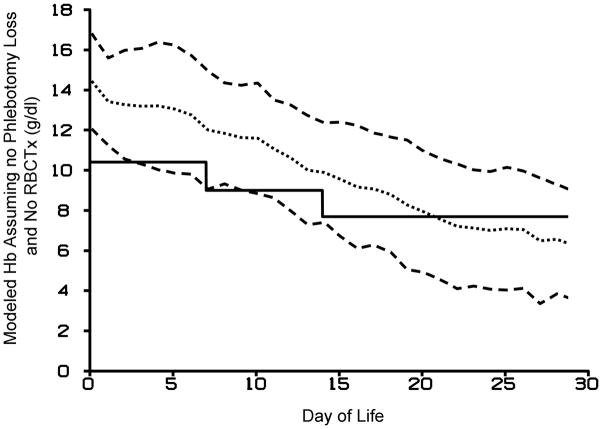
The modeling developed in this study was able to predict changes in infant Hb levels with specified reductions or increases in laboratory phlebotomy loss. With no phlebotomy loss and no RBCTx, the average Hb concentration for all 26 subjects at birth and day 30 fell from 14.4±2.4 g/dl to 6.3±2.7 g/dl, respectively (Figure 6). These results are based on the assumption that low Hb levels do not result in an increase in erythropoietin production leading to increased RBC production and Hb levels.
Figure 6.
Modeled infant Hb concentration (dotted line) ± 1 SD (dashed lines) assuming no RBCTx and no phlebotomy blood loss relative to PINT Hb RBCTx criteria (solid line). The postnatal decline in modeled Hb levels is likely an under estimate because increased in endogenous erythropoietin production would be expected to reverse the decline in Hb.
DISCUSSION
In the current report we demonstrate for the first time the ability to estimate the number of RBCTx received by critically ill, preterm VLBW infants by simulating reductions in laboratory phlebotomy loss when uniform RBCTx criteria are applied. We observed that with the hypothetical elimination of all laboratory phlebotomy loss and the use of the actual RBCTx criteria, the average number of RBCTx per infant decreased from 3.8±2.2 to 2.3±1.5, a 41% reduction (Figure 5). While this represents a highly significant decrease in RBCTx, the more desirable goal would be to completely eliminate all RBCTx received by all infants. An additional advantage of the simulation model approach is that in the examples provided in the present study it offers insight as to which factors other than laboratory blood loss may contribute to neonatal anemia and the need for RBCTx. Based on the present data, we speculate that other factors may include the inability of premature infants—particularly the smallest and least mature—to mount an effective erythropoietic response to anemia and to produce RBCs that survive sufficiently long enough in the circulation to avoid severe anemia.
Advantages of reducing laboratory phlebotomy loss in preterm infants
Research has shown that RBCTx are expensive and cost between $522 and $1,183 per unit.17 In addition to the cost of administering RBCTx, the complications associated with RBCTx include infection, fluid overload, electrolyte imbalance, and exposure to plasticizers, lead and other toxins.3 Baer et al. demonstrated a significant correlation between RBC transfusions and intraventricular hemorrhage.18 Additionally, it has been suggested that necrotizing enterocolitis, a potentially fatal condition among preterm infants, is temporally related to RBCTx.19 With the multiple risks associated with RBCTx in preterm infants, one could hypothesize that a decrease in RBCTx would be associated with a decrease in morbidities.
Estimates of blood volume and survival of autologous and transfused erythrocytes
In addition to requiring direct measurement of Hb removed for laboratory blood testing, Hb added via RBCTx, the simulation model requires estimates of circulating RBC survival. Estimates of RBC survival used in the present study taken from the literature included using 65.8 d for the infants’ own RBCs 12, and 120 d for adult donor RBCs.14 Previous work in adults has shown that the percent of transfused cells surviving 24 hours after a RBCTx is 74–86%.20 This was not included in the current model, because of our group’s previous work documenting that the percent of transfused cells surviving 24 hours in preterm infants is close to 100%.21 In addition, it is possible that transfused RBCs have a shorter lifespan than 120 days as a result of environmental factors, e.g., hyper- or hypoxic stress.22 In support of the 120 day lifespan used in this study, a study published in adults showed that transfused cells survive for 114–116 days after the initial removal of cells from the circulation.20 Additional studies in preterm infants are needed in which the survival of transfused donor RBCs is directly measured,. When such data become available, the model can be easily modified to accommodate these — and other — new findings.
Based on the present study’s observed 2.3-fold greater amount of Hb transfused relative to that phlebotomized (Figure 4) and similar report from the literature 23, the RBC survival estimates applied in the present study are likely overestimates of the infant’s actual RBC survival. Our research group anticipates soon being able to overcome this impediment with the ability to directly measure in vivo RBC survival in all critically ill VLBW infants using a safe, accurate method of labeling RBCs with biotin using minute quantities of blood left over from laboratory sampling.24
Laboratory Phlebotomy Loss Does Not Account for All RBCTx in Infants
Quantitative assessment of the contribution of Hb removed as part of laboratory phlebotomy loss and the amount of Hb added in RBCTx can elucidate the contribution of phlebotomy loss to anemia of prematurity. Based on the modeled results of the present study it appears that laboratory phlebotomy loss, although important, only accounts for approximately half of the RBCTx administered to critically ill VLBW infants. The present results also indicate that the greatest relative per kg phlebotomy loss and need for RBCTx is most pronounced in the smallest, least mature infants who manifest the most severe cardiorespiratory illness in the early weeks of life (Figure 4). This could be a consequence of two other potentially important factors—but as yet unstudied—associated with increasing immaturity: inability to maximally increase erythropoiesis for reasons other than phlebotomy loss, and accelerated RBC senescence.
Feasibility of Reducing Laboratory Phlebotomy Loss
The present results suggest that the amount of phlebotomized blood drawn from VLBW preterm infants for laboratory testing can be reduced by relatively simple changes in practice. In the current study, 59% of blood obtained from infants was discarded (Figure 3). This indicates the possibility of reducing laboratory phlebotomy by sampling less volume. Justification by the laboratory for the large excess volumes of blood drawn from neonates is that the volume taken allows the sample to be reanalyzed if necessary should the results be questionable due to instrument error or malfunction, or result in critical outlier values. Other studies utilizing point-of-care bedside analyzers and in-line monitors can also lead to significant decreases in laboratory phlebotomy loss and resultant RBCTx.9,10 In a small, single-center study, Rabe et. al. demonstrated a decrease in RBCTx in preterm infants by half when using delayed cord clamping, early protein and iron administration and a restrictive RBCTx threshold.25
Limitations in Modeling Phlebotomy Loss to Determine Reductions in RBCTx
The accuracy in modeling the number of RBCTx with reductions in laboratory phlebotomy relies on the accuracy with which all other measurements can be made, or estimated. Blood volume determinations were estimated from the pre and post-transfusion Hb level based on the dilution principle 13, along with estimates of RBC lifespan. The blood volume determined for this study, 93.2±24.9 mL/kg, is within the reported range of blood volumes for preterm infants.13,26,27 The number of simulated RBCTx is based on the anticipated volume of packed RBCs transfused. For our study subjects, 85% received RBCTx in which the Hct measured was between 13–17 mL of packed RBCs per kg. The current study also has a small total enrollment (n=26) relative to the number of eligible infants (n=162). This was due to unavoidable circumstances the most common were the time limitations of laboratory staff in weighing all blood samples and in competing enrollment from other studies. Future studies enrolling a larger number of preterm infants at multiple sites will be needed to address these and other limitations.
An additional weakness of the phlebotomy reduction analysis done is that the endogenous Hb production of each infant was assumed not to change based on the Hb concentration. This assumption may be acceptable because each infant’s Hb was not allowed to fall below the RBCTx criteria according to the model. In addition, our previous work has shown that the Epo concentrations in preterm infants start at an elevated level and reach a baseline concentration 1–2 days after birth.12 This baseline level is reached regardless of the infant’s endogenous Hb concentration. Finally, infants may in fact produce more Epo when there Hb reaches a level below what is allowed clinically, however, this can never be tested due to ethical concerns.
Summary and Conclusions
The present study demonstrates that when uniform, restrictive RBCTx criteria together with modeled phlebotomy reduction are applied, a predictable reduction in RBCTx administered to anemic preterm VLBW infants is observed. Under modeling conditions in which 100% of laboratory phlebotomy loss was assumed, the average number of RBCTx could be reduced by 41–48%. This finding is due in part to study infants having 2.3 times more Hb transfused as that phlebotomized. This discrepancy suggests that other factors—and not merely laboratory blood loss alone—are major contributors to neonatal anemia among critically ill VLBW premature infants. The present study demonstrated complete elimination of modeled RBCTx in four of the 26 (15%) study subjects suggesting the goal of completely eliminating RBCTx in critically ill preterm infants will likely require a multi-prong approach. Based on our prior model-based simulation employing optimized r-HuEpo dosing in which we demonstrated that 13 (46%) of the same study infants would have avoided RBCTx entirely 11, the combination of phlebotomy reduction together with optimized erythropoietin administration may well achieve the goal of completely eliminating RBCTx in critically ill preterm infants. An important advantage of the simulation model in the present study is its versatility in being modifiable to accommodate to new changes taking place in RBCTx practice, direct measurements of RBC survival or blood volume measurements, or changes in volumes of RBCTx administered.
Acknowledgments
We appreciate the outstanding contributions of the clinical laboratory staff led by Mitchell J. Owen and overseen by Matthew D. Krasowski, M.D., Ph.D. We also appreciate the many contributions of our laboratory research team (Robert L. Schmidt, Earl L. Gingerich and Jessica A. Goehring) and of our research nursing team (Gretchen A. Cress, Karen J. Johnson, Nancy E. Krutzfield, Sara K.B. Scott and Ruthann Schrock). We are thankful for the support from Sysmex America, Inc. for the loan of the Sysmex XE-2100 automatic hematology analyzer. We are also thankful for the advice and suggestions of Ronald G. Strauss, M.D. and Edward F. Bell, M.D. on the manuscript.
Statement of Financial Support: This study was supported by NIH P01 HL046925.
Abbreviations
- BV
blood volume
- ELBW
extremely low birth weight (<1,000g)
- Fj
fraction of blood removed for phlebotomy j
- HbA
hemoglobin amount
- HbC
hemoglobin concentration
- Hbphle
hemoglobin concentration of the phlebotomy
- Hb(t)phle
amount of Hb removed by a phlebotomy at time t
- Hb(t)trx
amount of Hb transfused at time t
- ΔHb
change in Hb concentration
- HCT
Hematocrit
- HCTtrx
HCT percent of transfused RBCs
- j
phlebotomy number
- k
number of phlebotomies occurring after t0 but before t
- m
number of phlebotomies occurring after T but before t
- NICU
neonatal intensive care unit
- PINT
Preterm Infants in Need of Transfusion
- PHLEV
volume of phlebotomized blood
- PR
percentage reduction in phlebotomy
- RBC
red blood cell
- RBCTx
RBC transfusion
- τA
lifespan of transfused adult donor RBCs
- τI
lifespan of infant’s endogenous RBCs
- t
time
- T
time phlebotomy occurred
- t0
time a given RBCTx occurred
- TRXV
volume of packed RBCs transfused
- VLBW
very low birth weight (<1,500g)
- W
weight of infant
Footnotes
Conflict of Interest: There are no conflicts of interest
References
- 1.Widness JA. Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. Neoreviews. 2008;9:e520. doi: 10.1542/neo.9-11-e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carroll PD, Widness JA. Non-Pharmacological, Blood Conservation Techniques for Preventing Neonatal Anemia – Effective and Promising Strategies for Reducing Transfusion. Seminars in Perinatology. 2012;36:232–43. doi: 10.1053/j.semperi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;3:CD004863. doi: 10.1002/14651858.CD004863.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–7. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Strauss RG. Red blood cell transfusion practices in the neonate. Clin Perinatol. 1995;22:641–55. [PubMed] [Google Scholar]
- 7.Kling PJ, Sullivan TM, Leftwich ME, Roe DJ. Score for neonatal acute physiology and phlebotomy blood loss predict erythrocyte transfusions in premature infants. Arch Pediatr Adolesc Med. 1997;151:27–31. doi: 10.1001/archpedi.1997.02170380031005. [DOI] [PubMed] [Google Scholar]
- 8.Ohls RK. Erythropoietin treatment in extremely low birth weight infants: blood in versus blood out. J Pediatr. 2002;141:3–6. doi: 10.1067/mpd.2002.125853. [DOI] [PubMed] [Google Scholar]
- 9.Madan A, Kumar R, Adams MM, et al. Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol. 2005;25:21–5. doi: 10.1038/sj.jp.7211201. [DOI] [PubMed] [Google Scholar]
- 10.Widness JA, Madan A, Grindeanu LA, et al. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005;115:1299–306. doi: 10.1542/peds.2004-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosebraugh MR, Widness JA, Veng-Pedersen P. Multidose optimization simulation of erythropoietin treatment in preterm infants. Pediatr Res. 2012;71:332–7. doi: 10.1038/pr.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freise KJ, Widness JA, Veng-Pedersen P. Erythropoietic response to endogenous erythropoietin in premature very low birth weight infants. J Pharmacol Exp Ther. 2010;332:229–37. doi: 10.1124/jpet.109.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawlings JS, Pettett G, Wiswell TE, Clapper J. Estimated blood volumes in polycythemic neonates as a function of birth weight. J Pediatr. 1982;101:594–9. doi: 10.1016/s0022-3476(82)80716-6. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg MH, Benz EJ, Jr, Adewoye HA, Ebert B. Pathobiology of the human erythrocyte and its hemoglobins. In: Hoffman R, Benz EJ Jr, Shattil SJ, et al., editors. Hematology: Basic Principles and Applications. United States of America: Elsevier Inc; 2005. pp. 442–54. [Google Scholar]
- 15.Blanchette VS, Zipursky A. Assessment of anemia in newborn infants. Clin Perinatol. 1984;11:489–510. [PubMed] [Google Scholar]
- 16.Veng-Pedersen P. Curve fitting and modeling in pharmacokinetics and some practical experiences with NONLIN and a new program FUNFIT. J Pharmacokinet Biopharm. 1977;5:513–31. doi: 10.1007/BF01061732. [DOI] [PubMed] [Google Scholar]
- 17.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 18.Baer VL, Lambert DK, Henry E, et al. Among very-low-birth-weight neonates is red blood cell transfusion an independent risk factor for subsequently developing a severe intraventricular hemorrhage? Transfusion. 2011;51:1170–8. doi: 10.1111/j.1537-2995.2010.02980.x. [DOI] [PubMed] [Google Scholar]
- 19.LaGamma EF, Blau J. Transfusion Related Acute Gut Injury (TRAGI): Feeding, Flora, Flow and Barrier Defense. Semin Perinatol. 2012;36:294–305. doi: 10.1053/j.semperi.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, et al. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 21.Strauss RG, Mock DM, Widness JA, et al. Posttransfusion 24-hour recovery and subsequent survival of allogeneic red blood cells in the bloodstream of newborn infants. Transfusion. 2004;44:871–6. doi: 10.1111/j.1537-2995.2004.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landaw SA. Factors that accelerate or retard red blood cell senescence. Blood Cells. 1988;14:47–67. [PubMed] [Google Scholar]
- 23.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of transfusions in extremely low birth weight infants: a retrospective study. J Pediatr. 2009;155:331–37. e1. doi: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mock DM, Matthews NI, Zhu S, et al. Red blood cell (RBC) survival determined in humans using RBCs labeled at multiple biotin densities. Transfusion. 2011;51:1047–57. doi: 10.1111/j.1537-2995.2010.02926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabe H, Alvarez JR, Lawn C, et al. A management guideline to reduce the frequency of blood transfusion in very-low-birth-weight infants. Am J Perinatol. 2009;26:179–83. doi: 10.1055/s-0028-1103024. [DOI] [PubMed] [Google Scholar]
- 26.Schulman I, Smith CH, Stern GS. Studies on the anemia of prematurity. AMA Am J Dis Child. 1954;88:567–95. doi: 10.1001/archpedi.1954.02050100569001. [DOI] [PubMed] [Google Scholar]
- 27.Usher R, Lind J. Blood Volume of the Newborn Premature Infant. Acta Paediatr Scand. 1965;54:419–31. doi: 10.1111/j.1651-2227.1965.tb06397.x. [DOI] [PubMed] [Google Scholar]