Abstract
Background
Antegrade continence enemas (ACE) have been used in the treatment of defecation disorders in children; little is known on their effect on colon motility and the utility of the colon manometry (CM) predicting long term ACE outcomes.
Methods
Retrospective review of children with constipation undergoing CM before and after ACE to evaluate CM changes and their utility on predicting ACE outcome.
Results
40 patients (mean age 8.8 SD 3y and 53% female) were included; 39/40 responded to the ACE. Of these 39, 14 (36%) were dependent and 25 (64%) had decreased it (11 of those or 28% discontinued it). On repeat CM we found a significant increase in the fasting (p<0.01) and post-prandial (p=0.03) motility index, number of bisacodyl-induced high amplitude propagating contractions (HAPC’s) (p=0.03) and total HAPC’s (p=0.02). Gastrocolonic response to a meal, propagation and normalization of HAPC’s improved in 28%, 58% and 33%, respectively with CM normalizing in 33% of patients. The baseline CM did not predict ACE outcome. The presence of normal HAPC’s on the repeat CM was associated with ACE decrease. Progression and normalization of HAPC’s (p=0.01 and 0.02 respectively) and CM normalization (p=0.01) on repeat CM were individually associated with ACE decrease. No CM change was associated with ACE discontinuation. Multivariate analysis showed that older age and HAPC normalization on CM predict ACE decrease and older age is the only predictor for ACE discontinuation.
Conclusions
Colon motility improves after ACE and the changes on the repeat CM may assist in predicting ACE outcome.
Keywords: constipation, colon motility, antegrade colonic enemas
INTRODUCTION
The antegrade continence enema (ACE) has been widely used in the management of pediatric patients with defecatory disorders ranging from idiopathic constipation to anorectal malformations, Hirschsprung’s disease, spine abnormalities, perineal trauma and cerebral palsy.[1–4] It is a very effective way to treat intractable defecation disorders and it has been shown to improve quality of life. Its effectiveness over time varies, with some patients showing a lack of response, others becoming dependent on its use,[5] some having a relapse[3] and some able to wean and even stop using it.[3, 5] At the present time there is no way to predict how the patients will respond, or to decide if the irrigations can be weaned. It is possible that the underlying colonic function may be predictive of response to the ACE, and that changes in colonic function that occur over time may allow some patients to respond better. The aims of the present study were to evaluate the relationship between baseline colonic motility and response to the ACE, to evaluate changes in colonic motility after the ACE procedure, and to correlate colon motility parameters and their changes with the ability to decrease and eventually discontinue the ACE.
METHODS
We present our experience in patients with defecation abnormalities that underwent evaluation with a colon manometry before and after an ACE procedure at two tertiary care referral motility centers. Institutional review board approval at both institutions was obtained.
Patient population
Records of all children with constipation refractory to maximal medical therapy that required an ACE procedure, and that underwent a baseline colonic motility evaluation before surgery (CM1) were reviewed. Only patients in whom a repeat colonic motility was performed after the ACE (CM2) were included.
Colonic manometry
Colon manometry catheter placement was performed according to previously reported protocol.[6] All patients underwent a bowel cleanout with electrolyte solutions the day before the colonoscopy. A catheter with eight ports with longitudinal staggered sensors spaced by 10–15 cm (according to patient’s size) was used and placed during colonoscopy while the children were under anesthesia. All patients underwent an abdominal radiography the day of the motility study to ascertain correct catheter placement. The study was divided in three segments: 60 minutes of fasting, 60 minutes of post-prandial evaluation and 60 minutes after bisacodyl challenge with 0.25 mg/kg. The CM was performed with a continuously non-complaint perfused catheter (Medical Measurement Systems, New Hampshire, US).
Data
The interpretation of the colon motility studies for the present study was done blindly by LR without any knowledge of the outcome. The variables obtained from the colon motility study included:
Fasting and post-prandial motility index (MI) measured by the median area under the pressure curve and calculated by the proprietary motility software on all ports
Gastrocolonic (GC) response to a meal (increase in motility index >15%[7] and observed visually) was classified as being present or absent
High-amplitude propagating contractions (HAPCs) were defined by an amplitude of at least 60 mmHg, a duration of 10 seconds or more, and an antegrade propagation over at least 30 cm.
Number, quality (absent, partially or fully propagated) of spontaneous (fasting), post-prandial and bisacodyl-induced HAPCs. Fully propagated HAPC’s were defined as those migrating down to the rectosigmoid colon and partially propagated as those stopping before the rectosigmoid colon. Absent HAPC’s were scored when there were no HAPC’s observed.
Outcome
Successful decrease of ACE use was defined as 50% decrease on irrigation frequency. Successful discontinuation was defined as no irrigations given for >6 months and ACE removed if cecostomy or discontinued and allow natural closure of ostomy if appendicostomy. All patients in this group are also included in the group of successful decrease of the ACE use.
Statistical analysis
Continuous variables are presented as medians and comparisons were performed using the non-parametric testing Wilcoxon signed ranks test. Proportions were compared using X2. Joint effect of age, gender, idiopathic etiology, GC normalization, HAPC normalization and time in months of CM2 after ACE procedure on ACE decrease and discontinuation was evaluated with binary logistic regression.
The following are the main comparisons obtained:
To evaluate the effect of antegrade colonic enemas on colonic motility we compared the fasting MI, post-prandial MI, number of HAPC’s (fasting, post-prandial and bisacodyl-induced), quality (absent, partially propagated and fully propagated) and proportion of normalization of HAPC’s between CM1 and CM2. We compared the proportions of presence of normal GC, normal HAPC’s and overall normal CM between CM1 and CM2.
To evaluate the successful response to ACE (defined as bowel movements with irrigations at least 3 times per week and soiling <1 episode per week) we compared the colon motility parameters at baseline (CM1) and on repeat CM (CM2) with the ACE response.
To evaluate predicting factors of successful decrease on ACE use and successful discontinuation we evaluated demographic factors (age, gender, follow up time and time between ACE procedure and CM2) and CM parameters.
RESULTS
A total of 40 patients were included. Mean age was 8.8 SD 3 years (median 8 years with a range 3–16 years) and 21 (53%) were female. The diagnosis for the ACE included idiopathic intractable constipation in 36 (90%), internal anal sphincter achalasia refractory to botulinum toxin A injections in 2 (5%) and Hirschsprung’s disease s/p surgical correction in 2 (5%). These last 4 patients were treated with internal anal sphincter (IAS) botulinum toxin injections and the subsequent anorectal manometry demonstrated normal IAS resting pressures so the ACE was performed due to colonic dysfunction (all 4 demonstrated abnormal baseline colon motility). ACE procedure included 35 cecostomy tube placement and 5 appendicostomies. The median time for repeating the colon manometry after the ACE procedure was 19 months (range 10–60 months) and the median follow up was 48 months (range 14–74 months).
From the 40 patients, 39 responded well to the ACE. Of the 39 ACE responders, 14 (36%) were unable to decrease its use by the end of the follow up of the study, 14 (36%) were decreasing the ACE but were still using it and 11 (28%) successfully discontinued the ACE. (See Figure 1) One patient responded successfully to the ACE but was unable to discontinue it and due to persistent sigmoid dysfunction on both CM studies he underwent a sigmoid colon resection and ACE removal with subsequent improvement. This patient is included in the study as unable to decrease ACE use, not in the successful discontinuation of ACE group.
Figure 1.
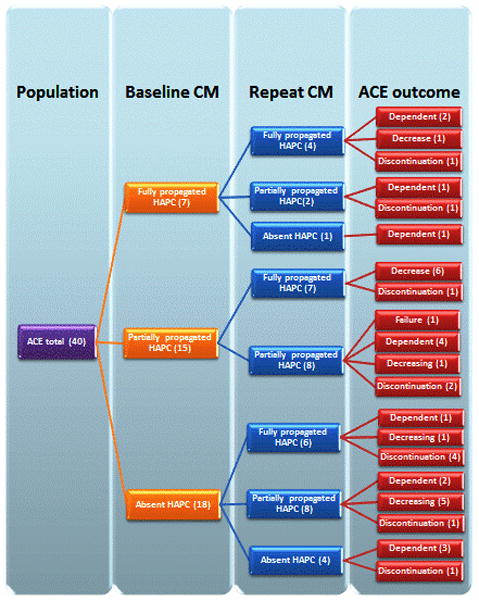
Flow diagram of the association between colon motility (quality of HAPC’s) and ACE outcome. Note that close to half of the patients with partially propagated HAPC’s on the baseline improved to full propagation after ACE and none of them is ACE dependent. Also note that 14 from the 18 with absent HAPC’s on baseline CM showed an improvement on HAPC’s and importantly; from those 14 the ACE was decreased in 6 and discontinued on 5. From the 4 with persistent absence of HAPC’s on repeat CM, 3 became dependent on the ACE but on 1 it was successfully discontinued.
Baseline colonic manometry (CM1) findings and long term outcome
The findings of the baseline CM are shown in Table 1, with 6 (15%) patients showing a normal CM at baseline. The relation between CM1 and long term outcome is shown in Figure 1. All six patients with a normal baseline CM1, and 33/34 (97%) patients with an abnormal CM1 responded successfully to the ACE treatments. Only one patient did not respond to the ACE and that patient had an abnormal CM1 showing presence of GC and partially propagated HAPC’s. After long term follow up of those 6 with normal CM1, 4 were dependent on the ACE, one was able to decrease its use and 1 eventually discontinued the ACE. Of the 33 with abnormal CM1 that responded to ACE, 18 had absent HAPC’s (6 were unable to decrease the ACE use, 6 weaned down the ACE use and 6 were able to successfully discontinue use of the ACE). Overall, the HAPC’s characteristics in CM1 demonstrated the following association with ACE outcome: 4/14 (29%) that were ACE dependent had normal HAPC’s, 3/25 (12%) that were able to decrease the ACE had normal HAPC; of those 25, 1/14 (7%) that were able to decrease the ACE without discontinuing its use had normal HAPC’s and 2/11 (18%) that successfully discontinued the ACE use had normal HAPC’s. The patient that failed the ACE treatment had abnormal HAPC’s. (See Table 2)
Table 1.
Baseline and repeat Colon Manometry results
| HAPC quality | Gastrocolonic meal response | CM interpretation | ||||
|---|---|---|---|---|---|---|
| Absent | Partially propagated | Fully propagated | Present | Absent | Normal (%) | |
| CM1 | 18 | 15 | 7 | 25 | 15 | 6 (15%) |
| CM2 | 6 | 17 | 17 | 34 | 6 | 16 (40%) |
HAPC’s, high amplitude propagating contractions; CM, colon motility interpretation; CM1, baseline colon manometry; CM2, repeat colon manometry
Table 2.
Univariate analysis of effect of quality of HAPC’s in CM1 and CM2 and colon motility changes on decrease and discontinuation of ACE use
| ACE decrease* | ACE discontinuation | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Yes | No | p-value | Yes | No | p-value | |
| CM1 | ||||||
|
| ||||||
| HAPC normal | 3/25 (12) | 4/14 (29) | 0.24 | 2/11 (18) | 5/28 (18) | 0.94 |
|
| ||||||
| CM2 | ||||||
|
| ||||||
| HAPC normal | 14/25 (56) | 3/14 (21) | 0.02 | 6/11 (55) | 11/28 (39) | 0.34 |
|
| ||||||
| CM changes | ||||||
|
| ||||||
| GC improvement | 6/25 (24) | 5/14 (36) | 0.52 | 3/11 (27) | 8/28 (29) | 0.80 |
| HAPC improvement | 18/25 (72) | 5/14 (36) | 0.01 | 6/11 (55) | 17/28 (61) | 0.76 |
| HAPC normalizing | 12/25 (48) | 1/14 (7) | <0.01 | 5/11 (45) | 8/28 (29) | 0.28 |
| CM normalizing | 13/25 (52) | 1/14 (7) | <0.01 | 7/11 (64) | 9/28 (32) | 0.16 |
Includes those that discontinued ACE use. All values are expressed as proportion (%).
CM1, baseline colon manometry; CM2, repeat colon manometry; HAPC’s, high amplitude propagating contractions; GC, gastrocolonic response; ACEantegrade continence enemas
Repeat colon manometry parameters after ACE use (CM2) and their relationship to long term outcome
Findings of CM2 are shown in Table 1. A total of 16 subjects had a normal CM2. Overall, HAPC’s characteristics in CM2 demonstrated the following association with ACE outcome: 3/14 (21%) that were ACE dependent had normal HAPC’s and 14/25 (56%) that were able to decrease the ACE had normal HAPC’s; of those 25, 8/14 (57%) that were able to decrease the ACE without discontinuing its use had normal HAPC’s, 6/11 (55%) that successfully discontinued the ACE use had normal HAPC’s. The patient that failed the ACE had abnormal HAPC’s. (See Table 2)
Changes in colon motility after long term use of ACE (CM1 vs. CM2)
We noticed an improvement on the gastrocolonic response to a meal in 11/40 (28%) of patients, and also on the quality and normalization of HAPC’s in 23/40 (58%) and 13/40 (33%) patients, respectively. (See Figures 2A and 2B for examples of HAPC normalization) The overall normalization of the colon motility was seen in 13/40 (33%) of patients. Although we noticed an improvement in GC between both studies, from 24 to 33, this was not statistically significant (p=0.11). The quality and normalization of HAPC’s also improved but did not reach statistical significance (p=0.34 and 0.69, respectively).
Figure 2.
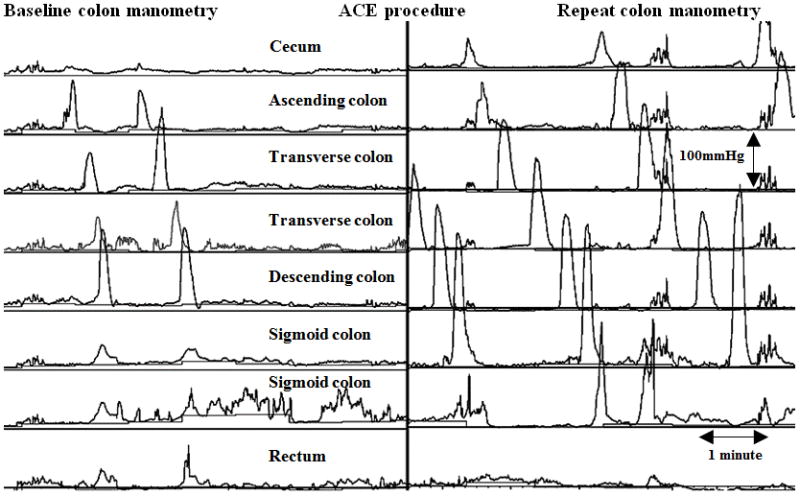
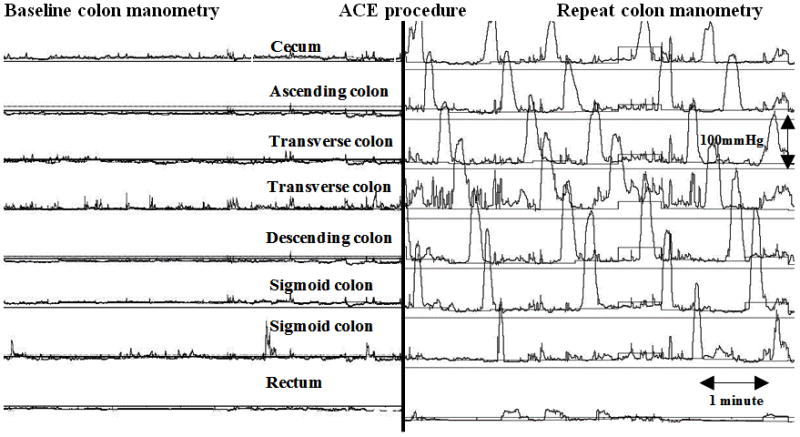
Colon motility tracing demonstrating improvement of HAPC’s. A. Normalization of HAPC’s from partial to full propagation. Note the HAPC’s on the baseline manometry propagate down to the descending colon then becoming simultaneous and on the repeat colon manometry after ACE the propagation is reaching the rectosigmoid colon. B. Normalization of HAPC’s from absent to full propagation. Note the significant improvement from absent HAPC’s on baseline colon manometry to fully propagated on the repeat colon manometry after ACE.
A total of 16 subjects had a normal CM2: 4 that were already normal before the ACE and 12 that had either partially propagated (n=7) or absent (n=5) HAPC’s before the ACE. Therefore 2/6 (33%) patients with a normal CM1 showed deterioration of the colon motility on the CM2. We observed that from 18 patients with absent HAPC’s on baseline, 6 developed fully propagated HAPC’s on follow up. All of those had the repeat CM >12 months after the ACE procedure was performed, as compared with 6/12 (50%) that did not (p=0.017).
We found a significant improvement on the motility index during fasting (p<0.01) and post-prandial period (p=0.03) after the use of the ACE. We found no significant difference in the number of HAPC’s after a meal (p=0.58) but we found a tendency towards significance in the number of fasting HAPC’s (p=0.06) and a significant increase in the number of HAPC’s after bisacodyl (p=0.03) and the total number of HAPC’s (p=0.02). (See Table 3)
Table 3.
Evaluation of improvement on colon motility after use of ACE
| CM 1 | CM 2 | ||
|---|---|---|---|
| CM Parameter | Median (range) | p value | |
| Fasting MI | 4.70 (3.5–6.9) | 5.55 (2.6–9.0) | <0.01 |
| Post-prandial MI | 5.69 (4.6–9.6) | 6.09 (4.7–9.9) | 0.03 |
| Number of Fasting HAPC’s | (0) | (0–2) | 0.10 |
| Number of Post-prandial HAPC’s | (0–3) | (0–5) | 0.59 |
| Number of bisacodyl-induced HAPC’s | 3 (0–15) | 5 (0–13) | 0.04 |
| Total number of HAPC’s | 3 (0–16) | 5 (0–18) | 0.04 |
CM1, baseline colon manometry; CM2, repeat colon manometry; MI, motility index; HAPC’s, high amplitude propagating contractions; GC, gastrocolonic response
Predictive factors for ACE decrease or discontinuation
Baseline CM (CM1)
We found no association between the CM1 parameters (presence of GC, presence and quality of HAPC’s and CM final interpretation) and the ability to successfully decrease or discontinue the ACE use. (See Table 2)
Repeat CM (CM2)
We found an association between the ability to decrease the use of ACE and the proportion of normal HAPC’s in CM2 and no association with GC response. We found no association between CM2 parameters and the discontinuation of the ACE use. (See Table 2)
Changes in CM parameters after long term use of ACE
Factors individually associated with a successful decrease in ACE use included the progression and normalization of HAPC’s and the overall normalization of the colon motility. (See Table 2) We did not find an association between decrease use of ACE and follow up time (p=0.24) and time in months of CM2 after ACE (62% <12 months and 64% >12 months, p=0.86). We did not find any factor individually associated with successful ACE discontinuation. (See Table 2) Multivariate analysis showed that older age and normalization of HAPC were predictors of successful decrease in ACE use and older age was the only predicting factor for successful ACE discontinuation. (See Table 4)
Table 4.
Multivariate analysis for ACE decrease and discontinuation
| ACE decrease* | ACE discontinuation | |||||||
|---|---|---|---|---|---|---|---|---|
| OR 95% CI | OR 95% CI | |||||||
| Sig | OR | Lower | Upper | Sig | OR | Lower | Upper | |
| Age | 0.01 | 1.80 | 1.08 | 2.99 | 0.03 | 1.45 | 1.03 | 2.04 |
| Gender | 0.34 | 4.05 | 0.28 | 56.8 | 0.79 | 0.73 | 0.08 | 6.99 |
| Idiopathic constipation | 0.99 | 9.73 | 0.54 | 175 | 0.05 | 16.8 | 0.97 | 280.6 |
| Months CM2 after ACE | 0.07 | 0.83 | 0.68 | 1.02 | 0.28 | 1.03 | 0.94 | 1.12 |
| GC Normalization | 0.61 | 2.63 | 0.20 | 34.4 | 0.30 | 1.96 | 0.22 | 17.73 |
| HAPC Normalization | 0.02 | 59.0 | 1.72 | 2025 | 0.29 | 0.33 | 0.04 | 2.62 |
Includes those that discontinued ACE use. HAPC’s, high amplitude propagating contractions; GC, gastrocolonic response; CM2, repeat colon manometry
DISCUSSION
The goal of the ACE procedure is to allow the patient with intractable constipation and their family easy and comfortable access to the colon in order to permit effective antegrade colonic irrigations. We believe that this approach can be temporary in otherwise healthy patients, as it may allow recovery of the colonic dysfunction and eventually discontinue its use, although this has not been studied. The colon manometry has been reported as useful in clinical practice to typify the colonic physiology in defecations disorders in patients with colorectal disorders like Hirschsprung’s disease [8, 9] and imperforate anus[10], to predict ACE outcome [11] and to guide surgical therapy in refractory constipation.[12, 13] However, the diagnostic value of the CM to predict outcomes has not been properly evaluated. Our aims were to evaluate the improvement on colon manometry after use of ACE and to predict ACE outcome using the colon manometry to measure colon motility changes/improvement.
The present study confirms that the ACE is very effective in the treatment of children with intractable constipation. While most responded to therapy, we found that 64% were able to wean the irrigations, but only 28% of responders were able to discontinue completely the use of the ACE. The other 36% became dependent on it use and were unable to wean it.
We have also demonstrated a significant improvement on the colonic motility and in some cases a complete recovery of the colonic function with the use of ACE. We have also showed that the improvement on the colonic motility after treatment with ACE can be used to predict which patients will be able to successfully decrease the use of the ACE, although we did not find any manometry patterns that predict the successful discontinuation of the ACE treatments.
The reason for the colonic function improvement is probably multifactorial. A successful bowel regimen probably allows for decompression of the colon, which may have a positive effect on colonic function. Similar findings in changes in colonic motility with successful treatment were previously shown in a small case series[14] and have been also described after colonic diversion by Villarreal at al who demonstrated normalization of colonic motility in 4/12 patients.[13] It is possible that decreasing colonic distention has a positive effect on healing of colonic injury, as distention has shown experimentally to affect the colonic enteric nervous system.[15] Even though it is not possible to establish if the initial colonic dysfunction seen could have been the result of colonic distention potentially impairing the measurement of the baseline colon motility, the fact that there were 6 patients that had a normal colonic motility at baseline, and that even after successful treatment and decompression, 66% of the patients still had an abnormal colonic motility, suggests the baseline and follow manometry findings reflect true colonic function.
We did not find that a baseline CM can predict patients that will successfully respond to the ACE. In our population 89% of patients had baseline abnormal CM, and after long term follow up 39/40 responded well to the ACE. In the present series even all 18 patients with no HAPCs at baseline had a satisfactory long term response and of those 6 were able to completely discontinue the use of ACE. Therefore our findings do not support what others have previously reported, that absence of HAPC’s on the colon manometry before ACE is associated with ACE therapeutic failure.[11] We do not have an explanation for this discrepancy. The sensitivity or specificity of the CM has not been established. It has also been suggested that depending on the placement of the transducers the motility patterns may change, as the present use of perfused catheters allows for large segments of colon that are not being studied. Our studies were performed with an 8 port catheter, and it is possible that when high resolution or high definition catheters are used, the sensitivity or specificity of colonic motility findings will greatly improve.
We found that the most important colonic motility factor individually associated with successful decrease on the ACE use was the improvement and normalization of the HAPC’s, an observation that emphasizes the importance of HAPC’s in normal colonic function and defecation. We also observed an association between time from ACE to CM2 >12 months and HAPC progression from absent to fully propagated but not with partial to fully propagated, suggesting that colonic motility improvement may take time, with a transition from absent to partial and then to normal motility. Prospective studies to address this question will be needed.
We also found that another predictor of weaning of the ACE was an older age of the patient. This may reflect developmental maturation may play a role. The fact that the colon seems to be more susceptible to distention and subsequent dysfunction at younger age, may also be a factor, as chronic constipation resulting in significant distention is rarely seen and reported in adults. It is unlikely that this effect of age was related to the fact that those patients had been using the ACE for longer time as we did not see any difference on follow up or time from the ACE to CM2 between patients able to decrease ACE use and those who became dependent on it.
Another possible use of a colonic manometry in these patients could be to evaluate the effect of other interventions (including surgical) on the ability to successfully discontinue the ACE. It has been suggested that colonic manometry detects regional abnormalities that may guide the surgeon in resection the segment that is malfunctioning.[12] In our series one patient that responded well to the ACE but was dependent on the irrigations opted for a segmental colonic resection (as suggested by the abnormal segment in CM2). The ACE was removed and he is doing well so far 2 years after surgery. These observations need to be studied prospectively, as the exact role of segmental colonic resections in the treatment of children with constipation is not clear.
There are some limitations for the study. It is a retrospective study of children that underwent repeated colonic manometry in an attempt to decide if the ACE could be discontinued or to understand those that were not responding. Therefore some ACE patients managed in our institutions have not undergone repeated procedures, so our findings represent a selected population. Also not all repeat manometries were done at the same time interval, which makes difficult to establish the time it takes for colon motility to recover after successful treatment. Prospective studies in these patients are needed.
In conclusion we have demonstrated an improvement on colon motility function after using the ACE in children with defecation disorders. Even though baseline colonic motility does not predict the initial response to an ACE, changes in motility may predict those patients that may be successful in decreasing and eventually discontinuing its use and it can be used as an adjunct to clinical assessment.
Acknowledgments
Funding: Dr Nurko is supported by NIH grant K24DK082792A
References
- 1.Graf JL, et al. The antegrade continence enema procedure: a review of the literature. J Pediatr Surg. 1998;33(8):1294–6. doi: 10.1016/s0022-3468(98)90172-5. [DOI] [PubMed] [Google Scholar]
- 2.Squire R, et al. The clinical application of the Malone antegrade colonic enema. J Pediatr Surg. 1993;28(8):1012–5. doi: 10.1016/0022-3468(93)90505-f. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui AA, et al. Long-term follow-up of patients after antegrade continence enema procedure. J Pediatr Gastroenterol Nutr. 2011;52(5):574–80. doi: 10.1097/MPG.0b013e3181ff6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez L, et al. Laparoscopic-assisted percutaneous endoscopic cecostomy in children with defecation disorders (with video) Gastrointest Endosc. 2011;73(1):98–102. doi: 10.1016/j.gie.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Jaffray B. What happens to children with idiopathic constipation who receive an antegrade continent enema? An actuarial analysis of 80 consecutive cases. J Pediatr Surg. 2009;44(2):404–7. doi: 10.1016/j.jpedsurg.2008.10.097. [DOI] [PubMed] [Google Scholar]
- 6.Di Lorenzo C, et al. Use of colonic manometry to differentiate causes of intractable constipation in children. J Pediatr. 1992;120(5):690–5. doi: 10.1016/s0022-3476(05)80229-x. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien MD, et al. Motility and tone of the left colon in constipation: a role in clinical practice? Am J Gastroenterol. 1996;91(12):2532–8. [PubMed] [Google Scholar]
- 8.Kaul A, et al. Colonic hyperactivity results in frequent fecal soiling in a subset of children after surgery for Hirschsprung disease. J Pediatr Gastroenterol Nutr. 2011;52(4):433–6. doi: 10.1097/MPG.0b013e3181efe551. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo C, et al. Colonic motility after surgery for Hirschsprung’s disease. Am J Gastroenterol. 2000;95(7):1759–64. doi: 10.1111/j.1572-0241.2000.02183.x. [DOI] [PubMed] [Google Scholar]
- 10.Heikenen JB, et al. Colonic motility in children with repaired imperforate anus. Dig Dis Sci. 1999;44(7):1288–92. doi: 10.1023/a:1026614726976. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg MM, et al. Colonic manometry as predictor of cecostomy success in children with defecation disorders. J Pediatr Surg. 2006;41(4):730–6. doi: 10.1016/j.jpedsurg.2005.12.018. discussion 730–6. [DOI] [PubMed] [Google Scholar]
- 12.Youssef NN, et al. Is there a role for surgery beyond colonic aganglionosis and anorectal malformations in children with intractable constipation? J Pediatr Surg. 2004;39(1):73–7. doi: 10.1016/j.jpedsurg.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Villarreal J, et al. Colonic diversion for intractable constipation in children: colonic manometry helps guide clinical decisions. J Pediatr Gastroenterol Nutr. 2001;33(5):588–91. doi: 10.1097/00005176-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Aspirot A, et al. Antegrade enemas for defecation disorders: do they improve the colonic motility? J Pediatr Surg. 2009;44(8):1575–80. doi: 10.1016/j.jpedsurg.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Vetuschi A, et al. Smad3-null mice lack interstitial cells of Cajal in the colonic wall. Eur J Clin Invest. 2006;36(1):41–8. doi: 10.1111/j.1365-2362.2006.01593.x. [DOI] [PubMed] [Google Scholar]


