Abstract
Hypothesis
We propose that the adrenal gland of an older higher primate female animal model will respond to a human chorionic gonadotropic (hCG) hormone challenge by secreting additional dehydroepiandrosterone sulfate (DHEAS). Such a response in surgically and chemically-castrated animals will provide proof-of-concept and a validated animal model for future studies to explore the rise of DHEAS during the menopausal transition of women.
Methods
Twenty four 18–26 y/o female cynomolgus monkeys were screened for ovarian function then either ovariectomized (n=4) or treated with a gonadotropic releasing hormone agonist (GnRHa) (n=20) to block ovarian steroid production. Following a recovery period from surgery or down-regulation, a single dose challenge (1,000 IU; IM) of human chorionic gonadotropin (hCG) was then administered in order to determine if LH/CG could accelerate circulating DHEAS production. Serum DHEAS, bioactive LH and urinary metabolites of ovarian sex steroids were monitored before, during and following these treatments.
Results
Circulating LH bioactivity and immunoreactive DHEAS concentrations were suppressed in all animals 14 days post administration of GnRHa. Urinary metabolites of estradiol and progesterone remained low following surgery or the flare reaction to GnRHa. Circulating DHEAS levels were increased following hCG administration and the increase in individual animals was proportional to the pre-treatment DHEAS baseline. Circulating DHEAS concentrations were positively correlated to endogenous LH bioactive concentrations prior to, and were increased by hCG challenge while no concomitant change was observed in ovarian steroid hormone excretion.
Conclusion
These data demonstrate a positive adrenal androgen response to LH/CG in older female higher primates and suggests a mechanism for the rise in adrenal androgen production during the menopausal transition in women. These results also illustrate that the nonhuman primate animal model can be effectively used to investigate this phenomenon.
Keywords: DHEAS, menopause, adrenal androgens, LH/hCG
INTRODUCTION
Humans, along with other higher primates, are unique among animal species in having adrenals that secrete large amounts of dehydroepiandrosterone (DHEA) and especially DHEA sulfate (DHEAS). While it is generally accepted that the DHEAS secretion rate decreases with age in humans, and can be used as an index for somatic aging in men, there are recent observations that suggest that this is not true for women. Data indicate that circulating levels of DHEAS, DHEA, androstenediol (Adiol), androstendione (Adione) and testosterone (T) increase during the menopausal transition in most women with differences in absolute levels observed in different ethnic groups (1–5). Similarly, there is an increase in the variability of DHEAS levels (5) as well as a small increase of DHEAS levels as female laboratory macaques progress through the menopausal transition (6). These important observations are relevant to ovarian senescence, decreased estrogen production and the production of androgens as the adrenal becomes the primary source of sex steroids in women approaching and transitioning through menopause (7). This life-stage specific increase in DHEAS concentrations may alter the balance of androgens to estrogens and may, therefore, explain some of the health outcomes that are not explained by the analysis of estradiol and testosterone concentrations.
The relationship between the decline in ovarian cycles and the observed rise in adrenal androgens remains to be explained. It is likely mediated through the hypothalamus/pituitary axis. However, whether pituitary luteinizing hormone (LH) and/or adrenocorticostimulating hormone (ACTH) are involved is not known and several scenarios are possible. The first and simplest scenario is that the rise in adrenal androgens is a response to increasing LH due to the direct withdrawal of the negative feedback on the pituitary that is exerted by the primary ovarian sex steroids. A second scenario is that the classic adrenotropic drive results in a compensatory increase in ACTH as a result of the shunting of pregnenolone from the delta-4 to the delta-5 pathway. However, such a dissociation between the production of cortisol and the adrenal androgens (8) requires a mechanism for the initial delta-4 to delta-5 pathway shunting. Testing either scenario in humans would be challenging because of the requirement for recruiting appropriate study cohorts. Still, there is a strong rationale for conducting such experiments because there is indirect evidence that GnRH pulse amplitude is increased at this time in women (9–13) and the non-human primate animal model (14). This increased GnRH-mediated drive would likely lead to elevations of circulating LH (15) that could be the stimulus for adrenal androgens.
Elevated LH increases DHEAS/DHEA production in postmenopausal women (16) and more recent data indicates that source of the rise of weak androgen during the menopausal transition (MT) is the adrenal gland (3). Ample evidence also exists to support the concept that the primate adrenal gland can respond to LH. Thirty-five years ago it was shown that human chorionic gonadotropin (hCG), an LH-like molecule, was able to stimulate DHEAS secretion from the human adrenal fetal zone in perfusion experiments (17, 18), first suggesting that the human adrenal may have functional LH/hCG or LH/CG-like receptors. It was later confirmed that the human adrenal gland expresses LH/hCG receptors in the reticularis and fasciculata zones (19), and this was further corroborated in a human adrenal cell line (20). However, the functionality of the LH/CG receptor in the human adrenal remains a matter of debate as no major role in the regulation of adrenal androgen production has been identified in healthy women (21).
Taken together, the putative presence of LH receptors in the adrenal gland plus the decline of the negative feedback on LH production during the MT provides the basis to hypothesize a mechanism for the increased adrenal androgen production in mid-aged women. The primary purpose of the current studies was to first test a primary hypothesis that increased LH drive contributes to DHEAS production in a higher primate animal model. A second objective was to validate a non-invasive animal model for future studies to investigate adrenal steroid production in mid-aged women. Two experiments directed to these objectives were carried out using the cynomolgus macaque.
MATERIALS AND METHODS
Animals
Two approaches were employed to meet all the goals of the studies described here. First, a small group of animals (n=4) were used in an invasive pilot study to established proof-of-concept. These animals were surgically castrated to rule out ovarian steroid contribution. A more conservative non-invasive approach was used in a larger group of animals (n=20) that were chemically castrated to prevent ovarian activity. Cynomolgus macaques (Macaca fascicularis) ranging in age from 18 to 26 years old and weighing between 2.7 and 5.9 kg were selected for this study. Animals were housed indoors in individual cages at the California National Primate Research Center (CNPRC). Animal rooms were maintained on a 12L:12D cycle (lights on at 06:00), at a constant temperature of approximately 22° C and 60% relative humidity. Animals had ad libitum access to tap water and were fed standard Purina Monkey Chow (Ralston- Purina Co., St. Louis, MO) twice daily, supplemented with fresh seasonal fruit. The CNPRC is fully accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). The University of California Davis Animal Use and Care Committee, the Campus Veterinarian, and the CNPRC approved the research protocol and procedures used for the study described in this report.
Urine Samples and Hormone Analyses
Daily urine samples were collected from each animal for 30 days starting at least 10 days prior to the initiation of experimental treatments and continuing until at least 6 days post hCG challenge. Urine samples were stored at −20° C until assayed for ovarian steroid hormone metabolites, estrone conjugates (E1C) and pregnanediol-glucuronide (PdG), by immunoassay using the automated ACS-180 chemiluminescent immunoassay analyzer (Bayer Diagnostics Corp., Norwood, MA) as previously described (22). Briefly, urine was pre-diluted in distilled water and incubated with the following specific reagent pairs: R6590 rabbit-anti-E1C polyclonal antiserum and E1C conjugated to horseradish peroxidase and dimethyl acridinium ester (E1C-HRP-DMAE) or R13904 rabbit-anti-PdG polyclonal antiserum and PdG-HRP-DMAE (22). In both cases, the ACS-180 autoanalyzer performed all sample/reagent addition, incubation, and chemiluminescent signal-reading steps. Creatinine (CR) concentration was measured in all urine samples following a previously described method (23). The E1C and PdG results are expressed as ng/mg CR for each sample. The ovarian cycle phase was determined by the urinary steroid metabolites (E1C and PdG) profile in retrospect. The CNPRC maintains a menses calendar database that was used to determine if the animals were menstruating as an indicator of ovarian cyclic activity for one year prior to the study.
GnRH agonist (GnRHa) Treatment
GnRHa is commonly used to disrupt the hypothalamus-pituitary-ovary (HPO) axis and stop ovarian activity by decreasing gonadotropin (24). Leuprolide has been used in the past to both stimulate (25) and down-regulate pituitary response to GnRH (26, 27). Based on that literature, a single dose (0.25 mg/kg BW) of leuprolide acetate (TAP Pharmaceutical Products Inc., Lake Forest, Illinois 60045) was administered intramuscularly (IM). Blood samples (3 mL) were collected by venipuncture of the femoral vein before, 2 days and 14 days after the GnRHa injection.
Surgery
Animals were sedated with 10 mg/kg ketamine IM, given 0.04 mg/kg atropine s.c., then intubated, placed on isoflurane anesthesia to effect, and positioned in dorsal recumbancy. A ventral caudal midline abdominal incision was made to visualize the body of the uterus and both ovaries. Ovarian vessels and the Fallopian tubes were isolated, ligated, and severed. The abdominal wall was then closed in three layers with two layers of 2/0 absorbable suture and the final subcuticular layer with 3/0 absorbable suture. The animals were then allowed to recover in the CNPRC surgical recovery unit for three days of immediate post-operative analgesia (1.5 mg/kg oxymorphone IM) three times daily followed by a two week complete recovery period.
CG/LH challenge
Two weeks after surgery or the GnRHa treatment, an LH challenge experiment was performed to study the direct effect of LH/hCG on DHEAS production from the adrenals. A commercial hCG preparation (NOVAREL, Ferring Pharmaceuticals, Suffern, NY 10901) was administered intramuscularly (1000 IU/animal) to supplement the loss of the endogenous LH after the GnRHa treatment.
Serum Samples and Hormone Analyses
A mid-morning blood sample (3 mL) was taken on restrained, non-anesthetized animals immediately before and then 3, 6, 24 and 48 hours after the hCG treatment. Sera were obtained by centrifugation of the blood samples and stored at −20°C until analysis. DHEAS was measured by radioimmunoassay using a commercially available kit (Coat-A-Count, Diagnostic Products Corporation, Los Angeles, CA). Circulating bioactive LH/CG was measured using a stably transfected cell line as previously described (28).
Data Analysis
Descriptive statistics as well as individual hormone profiles were used to identify the individual differences at baseline and after treatment. Grouped data are expressed as mean ± SE in all the figures. For nonparametric values, significant statistic differences among the groups were analyzed by Friedman repeated measures ANOVA on ranks, followed by an all-pairwise multiple comparison procedure using the Student-Newman-Keuls method. Mann-Whitney rank sum test was used to determine differences in hormone levels at baseline, 2 days and 14 days after GnRHa treatment between the monkeys with or without menstrual cycle activity. Pearson Product Moment Correlation was used to analyze the relationship among baseline hormone levels and different post-treatment time points. All statistical analyses were performed using Sigma Stat software (Systat, San Jose, CA). Significance was set at P<0.05
RESULTS
Animals
Twenty-four (24) animals were recruited for both studies and ten (10) of these were experiencing cyclic ovarian activity as indicated by urinary metabolite excretion during the immediate ten days prior to the initiation of the study. Another ten animals had normal menstrual flow during the previous year as verified by routine check of the colony for vaginal bleeding. Only four animals were considered noncyclic or post-menopausal.
Pilot Study
Three (3) of the four (4) surgically castrated animals exhibited a rise in DHEAS levels in response to hCG challenge that followed a fall in mean DHEAS levels from 12.6 ± 3.8 ug/dL pre-surgery to 8.5 ± 3.0 ug/dL immediately prior to hCG challenge two weeks later. Mean DHEAS levels then rose acutely in response to the hCG to 13.4 ±4.9 ug/dL (p.>0.05) six hours post injection. Daily E1C and PdG levels were consistently low in all animals following recovery from surgery (data not shown). The average E1C value was below 25 ng/mg Cr while the mean PdG value was below 28 ng/mg CR with no difference for either E1C or PdG levels before or after hCG treatment. These steroid hormone metabolite profiles were compared to corresponding data from previous studies in which cyclic, non-cyclic and castrate animals were characterized using the same methods.
Principal Study
Baseline DHEAS concentrations ranged from non-detectable (analytical limit of detection [LOD] = 1.1 ug/dL) to a maximum of 12.7 ug/dL in the twenty animals. Five (5) of 20 animals in the principal study had no detectable levels of DHEAS and were not responsive to any manipulations during the entire study period and thus were not included in further analysis. For all the data collected from the remaining fifteen (15) animals, there were eight time points that DHEAS concentrations were below the LOD (8 of 105 data points) and were replaced with LOD divided by √2, a standard replacement technique (29). The basal DHEAS level was 4.8 ± 0.98 ug/dL. The GnRHa treatment decreased DHEAS mean concentrations at 14 days post-treatment to 3.4 ± 0.59 ug/dL (median: 2.8 vs. 2.2 ug/dL, respectively, P < 0.05, Friedman repeated measures ANOVA on ranks, Figure 1). Daily E1C levels significantly increased two days after GnRHa treatment (pre-treatment: 72.9 ± 6.17 vs. 101.5 ± 12.3 ng/mg CR, respectively, P < 0.05, one-way ANOVA with repeated measures. Figure 2), reaching the peak value on post-GnRHa treatment day four (119.4 ±17.8 ng/mg CR) and then decreasing steadily to post-treatment day 14 (37.5 ± 4.7 ng/mg CR, P < 0.05, one-way ANOVA with repeated measures). A similar pattern was observed for PdG levels, which reached the peak value on post GnRHa treatment day 4 followed by a significant decrease on post-GnRHa treatment day 14 (pre-treatment: 77.3 ± 7.2 ng/mg CR; post-GnRHa treatment day 4: 110.6 ± 14.4 ng/mg CR; post-GnRHa treatment day 14: 41.3 ± 4.8 ng/mg CR, P < 0.05, one way ANOVA with repeated measures, Figure 3). The average E1C value was below 27 ng/mg Cr while the mean PdG value was below 31 ng/mg CR prior to hCG challenge and remained consistently low in all animals following hCG challenge (Figures 4 and 5). Following the hCG treatment, mean DHEAS significantly increased to 5.9 ± 1.2 ug/dL at 3 hours and remain elevated for at least 48 hours after treatment (means: (6 hours) 10.9 ± 1.9 ug/dL; (24 hours) 8.04 ± 1.4 ug/dL; (48 hours) 7.9 ± 1.4 ug/dL; medians: (6 hours) 8.7 ug/dL; (24 hours) 6.7 ug/dL; (48 hours) 7.2 ug/dL, Figure 6). There was a positive correlation between the concentration of circulating DHEAS levels before and 6 hours post-hCG challenge (4.81 ± 0.98 ug/dL vs. 10.9 ± 1.9 ug/dL, Pearson’s r = 0.63, P < 0.05, Figure 2). Four animals showed no cyclic activity and among them, only one animal had consistent non-detectable DHEAS regardless of any manipulations. There were no differences observed for DHEAS levels when animals with and without apparent menstrual activity (based on colony records) were compared at any of the time points analyzed before or after the treatments (P > 0.05).
FIG. 1.
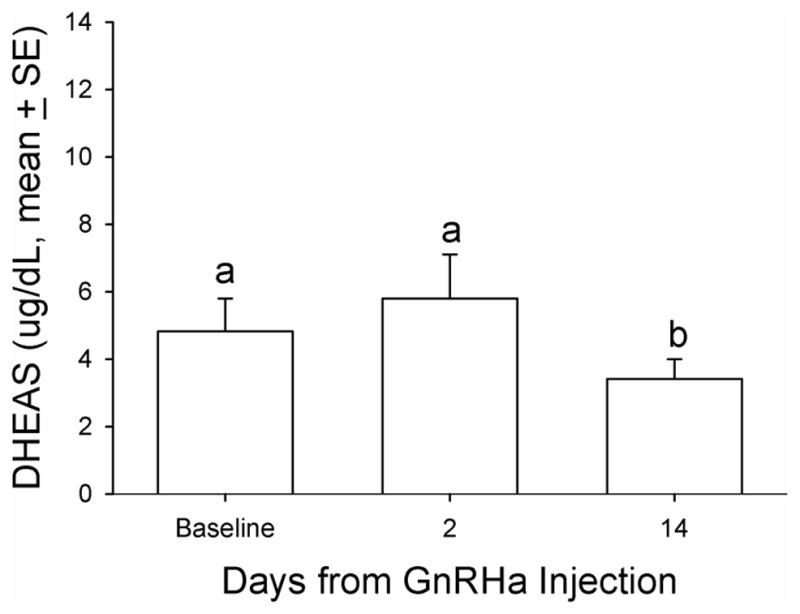
Mean serum DHEAS concentration relative to GnRHa treatment in aging female macaques. A single dose (0.25 mg/kg of body weight) of GnRHa (leuprolide acetate) was administered intramuscularly. DHEAS was measured in serum obtained before (baseline), 2 days after, and 14 days after the GnRHa injection. Friedman repeated-measures analysis of variance on ranks was conducted for comparison. Levels not linked by the same letter indicate a statistically significant difference (P<0.05). DHEAS, dehydroepiandrosterone sulfate; GnRHa, gonadotropin-releasing hormone agonist.
FIG. 2.
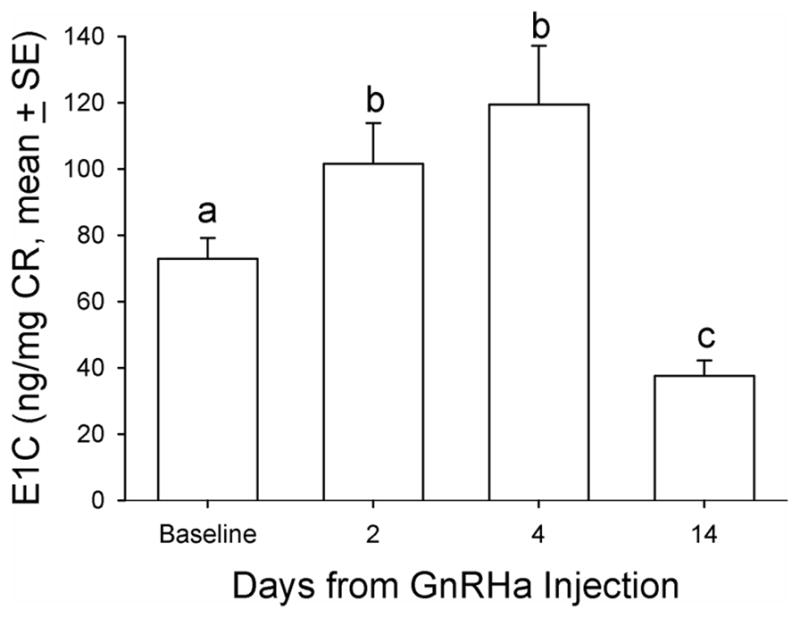
Mean urinary estrogen metabolite (estrone conjugate [E1C]) concentration indexed by CR concentration before and after GnRHa treatment in aging female macaques. A single dose (0.25 mg/kg of body weight) of GnRHa (leuprolide acetate) was administered intramuscularly. Friedman repeated-measures analysis of variance on ranks was conducted for comparison. Levels not linked by the same letter indicate a statistically significant difference (P<0.05). CR, creatinine; GnRHa, gonadotropin-releasing hormone agonist.
FIG. 3.
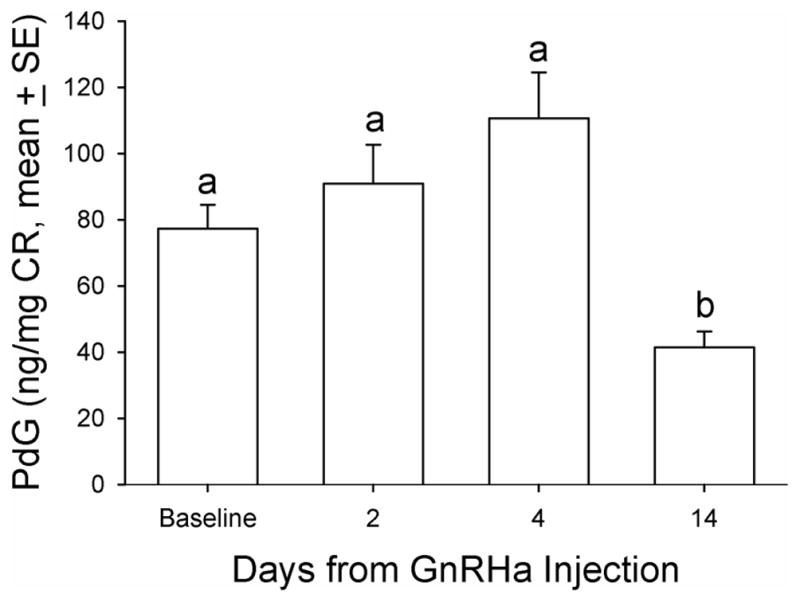
Mean urinary progesterone metabolite (pregnanediol glucuronide [PdG]) concentration before and after GnRHa treatment in aging female macaques. A single dose (0.25 mg/kg of body weight) of GnRHa (leuprolide acetate) was administered intramuscularly. Friedman repeated-measures analysis of variance on ranks was conducted for comparison. Levels not linked by the same letter indicate a statistically significant difference (P<0.05). CR, creatinine; GnRHa, gonadotropin-releasing hormone agonist.
FIG. 4.
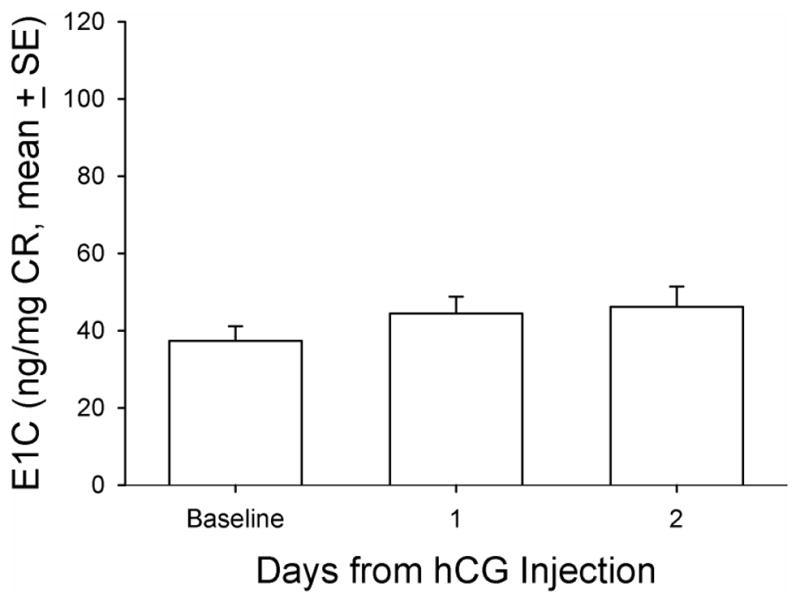
Mean urinary estrogen metabolite (estrone conjugate [E1C]) concentration indexed by CR concentration before, 1 day after, and 2 days after hCG treatment in aging female macaques. A single dose (1,000 IU/animal) of hCG (NOVAREL) was administered intramuscularly 14 days after GnRHa treatment. CR, creatinine; GnRHa, gonadotropin-releasing hormone agonist; hCG, human chorionic gonadotropin.
FIG. 5.
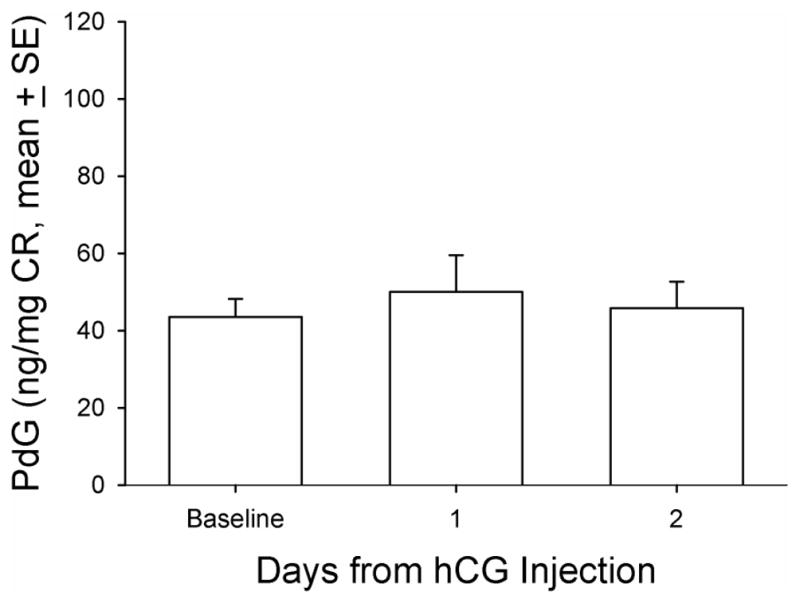
Mean urinary progesterone metabolite (pregnanediol glucuronide [PdG]) concentration before, 1 day after, and 2 days after hCG treatment in aging female macaques. A single dose (1,000 IU/animal) of hCG (NOVAREL) was administered intramuscularly 14 days after GnRHa treatment. CR, creatinine; GnRHa, gonadotropin-releasing hormone agonist; hCG, human chorionic gonadotropin.
FIG. 6.
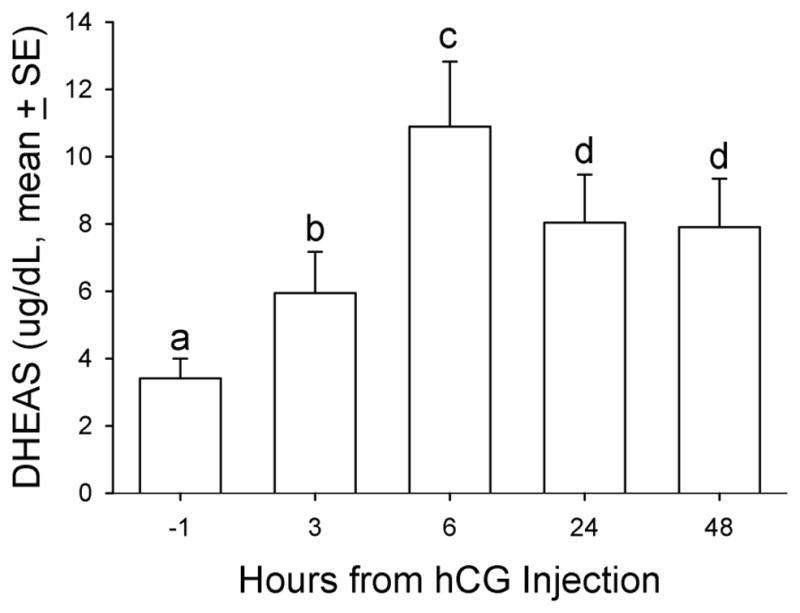
Mean serum DHEAS concentration relative to hCG treatment in aging female macaques. A single dose (1,000 IU/animal) of hCG (NOVAREL) was administered intramuscularly 14 days after GnRHa treatment. DHEAS was measured in serum samples before and at several time points after hCG treatment. Friedman repeated-measures analysis of variance on ranks was conducted for comparison. Levels not linked by the same letter indicate a statistically significant difference (P<0.05). DHEAS, dehydroepiandrosterone sulfate; GnRHa, gonadotropin-releasing hormone agonist; hCG, human chorionic gonadotropin.
There was no observed correlation between DHEAS and PdG levels at baseline (Pearson’s r = −0.18, P > 0.05) or at 6 hours post-hCG treatment (Pearson’s r = 0.12, P > 0.05). Serum LH/CG bioactivity levels were observed to have a modest increase 2 days post-GnRHa treatment followed by a substantial and significant decrease 14 days post-GnRHa treatment (basal level: 0.47 ± 0.17; post-GnRHa treatment day 2: 0.63 ± 0.15 and post-GnRHa treatment day 14: 0.06 ± 0.01 ng/mL, P < 0.05, one way ANOVA with repeated measures, Figure 7). The animals lacking recent or regular menstrual activity (4 of 20) had circulating LH levels greater than those with normal menstrual calendars at baseline (median: 1.80 vs. 0.09 ng/mL, mean: 1.85 ± 0.19 vs. 0.16 ± 0.05 ng/mL, P < 0.05, Mann-Whitney Rank Sum Test) as well as at two days post-GnRHa treatment (median: 1.73 vs. 0.31 ng/mL, mean: 2.12 ± 0.64 vs. 0.42 ± 0.11 ng/mL, respectively, P<0.05, Mann-Whitney Rank Sum Test). While all animals were suppressed, there was still a significant difference in circulating bioactive LH between cyclic and noncyclic animals at 14 days post GnRHa.
FIG. 7.
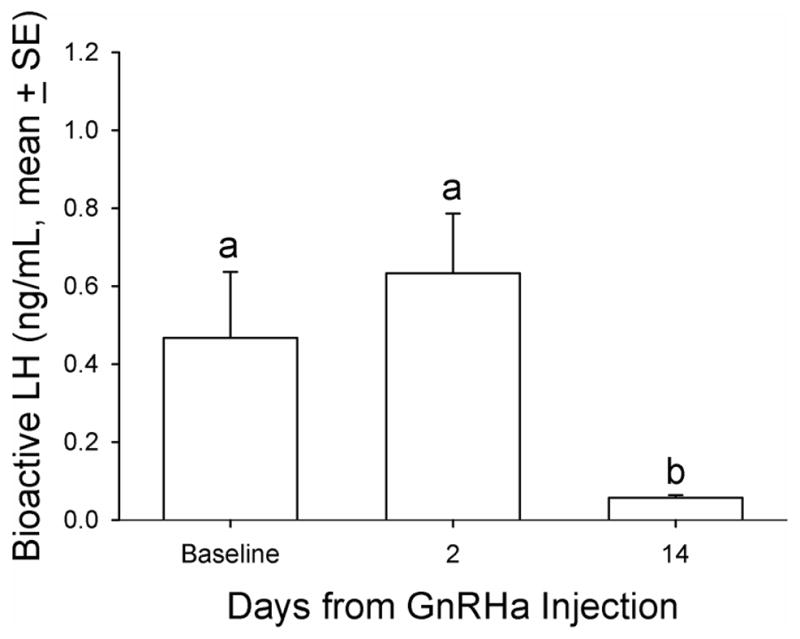
Mean bioactive LH concentration relative to GnRHa treatment in aging female macaques. A single dose (0.25 mg/kg of body weight) of GnRHa (leuprolide acetate) was administered intramuscularly. Bioactive LH in the three serum samples before the hCG challenge was measured for LH. Friedman repeated-measures analysis of variance on ranks was conducted for comparison. Levels not linked by the same letter indicate a statistically significant difference (P<0.05). GnRHa, gonadotropin-releasing hormone agonist; LH, luteinizing hormone.
DISCUSSION
In order to avoid as much stress-induced adrenal steroid production as possible, urinary monitoring was used in this study whenever appropriate and animals were handled as little as possible. In one group of animals, the natural source of gonadotropin support was withdrawn by down-regulating the GnRH receptor by a single injection of GnRHa. This “castration effect” of GnRHa treatment has been observed to be 100% effective in most models, yet it is reversible and is not associated with significant side effects beyond the reduction of gonadotropin secretion. In the other group of animals, complete recovery from ovariectomy was allowed before hCG challenge. Thus, the minimization of physical manipulations and the collection of sequential urine rather than blood samples for endocrine evaluations in our experimental design obviated most concerns regarding a stress-induced increase in adrenal steroid production. The gonadotropin challenge in this study was delivered as a single treatment with exogenous hCG, which is known to have no effect beyond the induction of signal transduction mediated through LH/CG receptors. We hypothesized that DHEAS production could be accelerated using a non-invasive experimental design with a non-human primate animal model. This was based on the premise that DHEAS is primarily an adrenal product (30, 31) and hCG/LH receptors may be present and functional in the adrenal cortex of older female primates. However, as Havelock et al (16) have demonstrated, the post-menopausal ovary and perhaps the pre-menopausal ovary may also have the capacity to respond to LH/hCG and produce delta-5 steroids. This possibility was ruled out in the current design by ovariectomy.
The GnRHa treatment induced an initial modest increase in DHEAS concentration followed by a significant decrease, supporting the concept that circulating LH maintains adrenal DHEAS production since LH concentrations followed the same trajectory as DHEAS after the GnRHa treatment. Furthermore, the hCG challenge triggered a rapid response in circulating DHEAS concentrations which was sustained for at least 48 hours. The GnRHa treatment also induced a transient increase in E1C and PdG followed by a significant decrease in both ovarian steroid metabolites. This supports the concept that GnRHa is effective in decreasing ovarian steroidogenesis following a flare reaction (Figures 2 and 3).
These experimental data indicate that the non-human primate animal model is appropriate for studies relating to the endocrinology of the MT. The explanation that LH receptors are responsible for the rise in adrenal androgen production in later life is based largely on observations (32), partly on speculation, and even less on experimental evidence. In fact, few of the mechanisms for androgen production in older women have been well characterized, and the source of androgens is still a controversial issue (30). This uncertainty makes the broad use of DHEAS therapy controversial, and there is mounting evidence that such supplements may not provide the expected benefit to women who already exhibit increased DHEAS production and healthy aging. Such issues may be best resolved by dissecting the problem empirically using an appropriate animal model as we have attempted to do here.
There are five important observations that arise from this study. The first is that GnRHa treatment decreased DHEAS concentrations consistently in all animals, demonstrating involvement of the pituitary-adrenal axis signal pathway in the production of androgens of adrenal and/or ovarian origins. The second observation is that the suppressed DHEAS secretion, either spontaneous or caused by GnRHa was consistently stimulated following hCG treatment in all animals including 3 animals that had baseline DHEAS levels below the assay sensitivity prior to hCG challenge. This suggests a potential up-regulation of adrenal sensitivity in response to gonadotropin challenge following reduced LH support. The third observation is that DHEAS was detected immediately in most animals at 6 hours post-hCG challenge (Figure 6), suggesting that the adrenal response to gonadotropic stimulation persists during pituitary down-regulation, and that all the animals responded in a consistent temporal fashion to the gonadotropin challenge even though they had different, and sometimes undetectable, basal circulating DHEAS concentrations. The fourth observation is the conspicuous absence of an acute increase in levels of ovarian steroids metabolites (E1C and PdG) immediately following the hCG challenge. This suggests that the observed DHEAS elevation was of adrenal origin with little or modest involvement of the ovary in the chemically castrated group. The fifth and final observation is the positive correlation between the DHEAS levels before and at different time points after hCG treatment, together with the proportionality of the response to hCG treatment with baseline DHEAS levels. These data suggest different sensitivities to the endogenous LH and exogenous hCG stimuli among the animals producing different levels at baseline and after the hCG challenge, respectively. The pulsatile nature of LH secretion precludes any expectation of a stronger correlation among basal levels of LH and the corresponding DHEAS levels. The significance of these observations, however, is that they suggest that DHEAS secretion in these animals was mediated by the GnRH/LH pathway and was stimulated through LH/hCG receptors in the adrenal that mediated a consistent response. Thus, the individual differences observed in circulating DHEAS levels were quantitatively but not qualitatively different.
To our knowledge, there has been no previous evidence that hCG can stimulate adrenal steroid production in vivo in either mid-aged women or laboratory macaques, however, the current data suggest that a change in ovarian status (through ending the negative feedback on gonadotropins) may contribute indirectly to an increased adrenal sensitivity to an LH-like stimulation. It is possible that the increased levels of DHEAS observed in this study were due not only to direct LH/CG stimulation, but also to a decreased metabolic clearance. However, the fact that DHEAS decreased similarly after GnRHa treatment in all animals argues strongly against this possibility.
Recently, SWAN (Study of Women Across the Nation) investigators have shown that neither cardiovascular disease nor cardiovascular risk factors are positively related to anovulation or longer menstrual cycles in mid-aged women (33). However, a significant negative correlation was found between these two health factors and increased urinary excretion of estrogen and progesterone metabolites when daily hormone patterns were examined. This observation suggests that the maintenance of normal ovarian steroid production may prevent the MT rise in LH and the attendant increase in adrenal androgen production. In this study, the mean LH/CG bioactivity and DHEAS profiles followed similar patterns (Figures 1 and 7) and the decrease in DHEAS levels following GnRHa treatment (Figure 1) suggests that the pituitary drive is responsible for the increase in DHEAS secretion. One possible explanation for not showing a direct positive relationship between circulating LH/hCG and DHEAS concentrations may be that differences in DHEAS production primarily reflect differences in the adrenal sensitivity to modestly increased LH concentrations. This seems unlikely because most of the animals responded to the hCG challenge in a qualitatively similar fashion (Figure 6). A more attractive possibility, and one that is most consistent with all observations, is that a tropic compound that is LH or structurally similar to LH is produced by the pituitary and under the control of GnRH, is capable of stimulating adrenal or ovarian DHEAS production. The cognate receptor in the adrenal for this tropic compound would be or resemble the LH receptor.
Conclusion
These results are consistent with the recent findings that the adrenal glands of mid-aged women have the capacity to increase the production of the delta-4 adrenal steroids. This increase in adrenal delta-5 steroid appears to be under the control of LH. In addition, the results reported here indicate that this increase in adrenal delta-5 steroids during the menopausal transition is independent of increased ovarian steroidogenesis.
Acknowledgments
The authors gratefully acknowledge the extraordinary support of the Research Services Unit at CNPRC and financial support from: NIEHS Superfund Basic Research Program (P42 ES04699); NIEHS (P01 ES06198 and P30 ES005707); NIH (P51 RR000169) and NIA AG02224 and AG17164
Footnotes
Disclosure information:
The authors declare they have no competing financial interests.
Disclaimers: None
References
- 1.Lasley BL, Santoro N, Randolph J, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87:3760–3767. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 2.Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley BL. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94(8):2945–51. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasley BL, Crawford SL, Laughlin GA, Santoro N, McConnell DS, Crandall C, Greendale GA, Polotsky AJ, Vuga M. Circulating dehydroepiandrosterone sulfate levels in women who underwent bilateral salpingo-oophorectomy during the menopausal transition. Menopause. 2011;18(5):494–498. doi: 10.1097/gme.0b013e3181fb53fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasley BL, Stanczyk FX, Gee NA, Chen J, Khoudary El, Crawford S, McConnell DS. Androstenediol complements estradiol during the menopausal transition. Menopause. 2012;19(6):650–657. doi: 10.1097/gme.0b013e31823df577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemnitz JW, Roecker EB, Haffa AL, Pinheiro J, Kurzman I, Ramsey JJ, MacEwen EG. Serum dehydroepiandrosterone sulfate concentrations across the life span of laboratory-housed rhesus monkeys. J Med Primatol. 2000;29:330–7. doi: 10.1034/j.1600-0684.2000.290504.x. [DOI] [PubMed] [Google Scholar]
- 6.Shideler S, Gee N, Chen J, Lasley B. Estrogen and progesterone metabolites, and follicle stimulating hormone in the aged macaque female. Biol Reprod. 2001;65:1718–1725. doi: 10.1095/biolreprod65.6.1718. [DOI] [PubMed] [Google Scholar]
- 7.Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: Intracrinology. Frontiers in Neuroendocrinology. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- 8.McKenna TJ, Fearon U, Clarke D, Cunningham SK. A critical review of the origin and control of adrenal androgens. Baillieres Clin Obstet Gynaecol. 1997;11:229–48. doi: 10.1016/s0950-3552(97)80035-1. [DOI] [PubMed] [Google Scholar]
- 9.Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal Women. J Clin Endocrinol Metab. 2000;85:1794–1800. doi: 10.1210/jcem.85.5.6612. [DOI] [PubMed] [Google Scholar]
- 10.Sharpless JL, Supko JG, Martin KA, Hall JE. Disappearance of endogenous luteinizing hormone is prolonged in postmenopausal women. J Clin Endocrinol Metab. 1999;84:688–694. doi: 10.1210/jcem.84.2.5433. [DOI] [PubMed] [Google Scholar]
- 11.Santoro N, Banwell T, Tortoriello D, Lieman H, Adel T, Skurnick J. Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obset Gynecol. 1998;178:732–741. doi: 10.1016/s0002-9378(98)70483-1. [DOI] [PubMed] [Google Scholar]
- 12.Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- 13.Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2290–2296. doi: 10.1210/jcem.87.5.8508. [DOI] [PubMed] [Google Scholar]
- 14.Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- 15.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 1995;21:103–113. doi: 10.1016/0378-5122(94)00869-9. [DOI] [PubMed] [Google Scholar]
- 16.Havelock JC, Rainey WE, Bradshaw KD, Carr BR. The post-menopausal ovary displays a unique pattern of steroidogenic enzyme expression. Hum Reprod. 2006;21:309–17. doi: 10.1093/humrep/dei373. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann WD, Lauritzen C. HCG + ACTH stimulation of in vitro dehydroepiandrosterone production in human fetal adrenals from precursor cholesterol and delta5-pregnenolone. J Perinat Med. 1975;3:231–236. doi: 10.1515/jpme.1975.3.4.231. [DOI] [PubMed] [Google Scholar]
- 18.Seron-Ferre M, Lawrence C, Jaffe R. Role of hCG in regulation of the fetal zone of the human fetal adrenal gland. J Clin Endocrinol Metab. 1978;46:834–837. doi: 10.1210/jcem-46-5-834. [DOI] [PubMed] [Google Scholar]
- 19.Pabon J, Li X, Lei Z, Sanfilippo J, Yussman M, Rao C. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J Clin Endocrinol Metab. 1996;81:2397–2400. doi: 10.1210/jcem.81.6.8964884. [DOI] [PubMed] [Google Scholar]
- 20.Rao CV, Zhou XL, Lei ZM. Functional luteinizing hormone/chorionic gonadotropin receptors in human adrenal cortical H295R cells. Biol Reprod. 2004;71:579–587. doi: 10.1095/biolreprod.104.027300. [DOI] [PubMed] [Google Scholar]
- 21.Piltonen T, Koivunen R, Morin-Papunen L, Ruokonen A, Huhtaniemi IT, Tapanainen JS. Ovarian and adrenal steroid production: regulatory role of LH/HCG. Hum Reprod. 2002;17:620–4. doi: 10.1093/humrep/17.3.620. [DOI] [PubMed] [Google Scholar]
- 22.Santoro N, Crawford SL, Allsworth JE, Gold EB, Greendale GA, Korenman S, Lasley BL, McConnell D, McGaffigan P, Midgely R, Schocken M, Sowers M, Weiss G. Assessing menstrual cycles with urinary hormone assays. Am J Physiol Endocrinol Metab. 2003;284:E521–530. doi: 10.1152/ajpendo.00381.2002. [DOI] [PubMed] [Google Scholar]
- 23.Taussky HH. A microcolorimetric determination of creatinine in urine by the Jaffé reaction. J Biol Chem. 1954;208:853–861. [PubMed] [Google Scholar]
- 24.Conn PM, Crowley WF. Gonadotropin-releasing hormone and its analogs. Annu Rev Med. 1994;45:391–405. doi: 10.1146/annurev.med.45.1.391. [DOI] [PubMed] [Google Scholar]
- 25.Kuehl TJ, Davis TW, Young C, Nunez P, Robinson MR, Huddleston KP, Wincek TJ, Pliego JF, Dukelow WR. Incorporation of a GnRH agonist, leuprolide acetate, into regimens with exogenous gonadotropins to produce ovarian stimulation and ovulation in the nonpregnant squirrel monkey. Am J Primatol. 1999;49:153–64. doi: 10.1002/(SICI)1098-2345(199910)49:2<153::AID-AJP6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Golub MS, Styne DM, Wheeler MD, Keen CL, Hendrickx AG, Moran F, Gershwin ME. Growth retardation in premenarchial female rhesus monkeys during chronic administration of GnRH agonist (leuprolide acetate) J Med Primatol. 1997;26:248–56. doi: 10.1111/j.1600-0684.1997.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 27.Grow DR, Williams RF, Hsiu JG, Hodgen GD. Antiprogestin and/or gonadotropin-releasing hormone agonist for endometriosis treatment and bone maintenance: a 1-year primate study. J Clin Endocrinol Metab. 1996;81:1933–9. doi: 10.1210/jcem.81.5.8626860. [DOI] [PubMed] [Google Scholar]
- 28.Jia XC, Perlas E, Jyuan-Gwo JS, Morán F, Lasley BL, Ny T, Hsueh AJW. Luminescence luteinizing hormone/choriogonadotropin (LH/CG) bioassay: measurement of serum bioactive LH/CG during early pregnancy in human and macaque. Biol Reprod. 1993;49:1310–1316. doi: 10.1095/biolreprod49.6.1310. [DOI] [PubMed] [Google Scholar]
- 29.Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5(1):46–51. [Google Scholar]
- 30.Couzinet B, Meduri G, Lecce MG, Young J, Brailly S, Loosfelt H, Milgrom E, Schaison G. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab. 2001;86:5060–5066. doi: 10.1210/jcem.86.10.7900. [DOI] [PubMed] [Google Scholar]
- 31.Arlt W, Justl HG, Callies F, Reincke M, Hubler D, Oettel M, Ernst M, Schulte HM, Allolio B. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab. 1998;83:1928–1934. doi: 10.1210/jcem.83.6.4850. [DOI] [PubMed] [Google Scholar]
- 32.Alevizaki M, Saltiki K, Mantzou E, Anastasiou E, Huhtaniemi I. The adrenal gland may be a target of LH action in postmenopausal women. Eur J Endocrinol. 2006;154:875–81. doi: 10.1530/eje.1.02165. [DOI] [PubMed] [Google Scholar]
- 33.Matthews KA, Santoro N, Lasley B, Chang Y, Crawford S, Pasternak RC, Sutton-Tyrrell K, Sowers M. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91:1789–95. doi: 10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]


