Abstract
Melanoma is a highly metastatic and deadly disease. An agent simultaneously targeting COX-2, PI3K/Akt and MAPK signaling pathways that are deregulated in up to 70% of sporadic melanoma might be an effective treatment but no agent of this type exists. To develop a single drug inhibiting COX-2 and PI3K/Akt signaling (and increasing MAPK pathway activity to inhibitory levels as a result of Akt inhibition), a selenium-containing glutathione (GSH) analog of celecoxib, called selenocoxib-1-GSH was synthesized. It killed melanoma cells with an average IC50 of 7.66 µmol/L compared to control celecoxib at 55.6 µmol/L. The IC50 range for normal cells was 36.3–41.2 µmol/L compared to 7.66 µmol/L for cancer cells. Selenocoxib-1-GSH reduced xenografted tumor development by ~70% with negligible toxicity by targeting COX-2, like celecoxib, and having new inhibitory properties acting as a PI3K/Akt inhibitor (and MAPK pathway activator to inhibitory levels due to Akt inhibition). The consequence of this inhibitory activity was an ~80% decrease in cultured cell proliferation and a ~200% increase in apoptosis following 24 hours treatment with 15.5 µmol/L of drug. Thus, this study details development of selenocoxib-1-GSH, which is a non-toxic agent that targets the COX-2 and PI3K/Akt signaling pathways in melanomas to inhibit tumor development.
Keywords: Celecoxib, Selenium, Glutathione, MAPK, Apoptosis
INTRODUCTION
Melanoma remains one of the most invasive and drug resistant cancers, making the development of clinically effective therapies a major obstacle (1). Recent FDA approval of Vemurafenib (PLX-4032) illustrates the drug resistance hurdle faced by melanoma drugs inhibiting single targets. Vemurafenib targets mutant V600EB-Raf present in 50–60% of sporadic melanomas, and has response rates of up to 80% (2, 3). However, nearly all initially responding patients developed recurrent resistant disease within a year (4, 5). Therefore, drugs are needed that can be added to the current arsenal of compounds for use alone or in combination with agents such as Vemurafenib (6). One approach is to improve the therapeutic efficacy of existing agents through chemical modification, enabling them to target multiple key pathways regulating the cancer development (7, 8).
Celecoxib is a drug that inhibits cyclooxygenase-2 (COX-2) activity (9). COX-2 is a ubiquitously expressed inducible enzyme that plays an important role in the production of prostaglandin E2 (PGE2) (10, 11). Celecoxib inhibits COX-2 thereby reducing the production of PGE2 (9). PGE2 affects cellular proliferation, motility, invasiveness, angiogenesis and promotes survival by inhibiting apoptosis (10, 11). Furthermore, PGE2 is a tumor-inducing eicosanoid that promotes tumor development and progression to more invasive disease (12). COX-2 is overexpressed in carcinomas of the colon, breast, lung, prostate, cervix, stomach and melanocyte suggesting it could be an important therapeutic target (13, 14). Initial studies in this report confirm that COX-2 expression is elevated in melanoma cell lines and in tumor biopsies compared to normal human melanocytes and that targeting COX-2 but not COX-1 in melanoma cell lines using siRNA inhibited xenografted melanoma tumor development.
Concentrations of celecoxib required to induce apoptosis of cultured cells are high and ranged from 25–100 µmol/L and clinical use is associated with cardiovascular side effects at doses of 200 mg per day (15, 16). Therefore, scientists are developing a variety of celecoxib analogs that are effective at lower concentrations or have altered properties (17, 18). Interestingly, some analogs maintain COX-2 inhibitory potency, while others do not, but all seem to decrease the viability of cancer cells in culture to varying degrees depending on the new properties of the agents (19–21). Several reports document that selenium incorporation into the structural backbone of certain compounds can enhance the therapeutic potential of the drug by providing it with new inhibitory properties, which involve inhibition of the Akt signaling pathway (22, 23). The Akt pathway is important in melanoma development and it is activated in up to 70% of sporadic melanomas (24). Therefore, selenium incorporation into celecoxib would have potential to enhance its therapeutic efficacy by providing additional inhibitory properties unrelated to its initial COX-2 targeting efficacy. An analog of celecoxib has been developed that contains selenium, called Selenocoxib-1, but the drug is toxic to animals, limiting its use as a therapeutic agent (25). Hence, additional modifications of selenocoxib-1 were needed to maintain cancer cell killing efficacy but decrease toxicity on normal calls.
The novel discovery detailed in this report is that a form of selenocoxib-1 called selenocoxib-1-GSH was developed, which does not exhibit the same toxicity on normal cells as selenocoxib-1, making it a potentially useful therapeutic agent. Selenocoxib-1-GSH more effectively killed melanoma cell lines than celecoxib. The new agent retained COX-2 targeting activity, and as predicted had new Akt signaling inhibitory properties. Mechanistically, selenocoxib-1-GSH inhibited melanoma cell survival by targeting the COX-2 and PI3K/Akt pathways (and increased pErk1/2 to inhibitory levels due to targeting of the Akt pathway), which decreased cellular proliferation and triggered apoptosis mediated through a G0-G1 block, resulting in fewer cells in S and G2-M phases of the cell cycle. Intra-peritoneal administration of selenocoxib-1-GSH retarded the growth of xenografted melanoma tumors up to 70% without affecting animal body weight or major organ functions. Thus, a more effective agent has been developed from a toxic one that can decrease melanoma development by targeting key signaling pathways without causing major-organ related toxicity.
MATERIALS AND METHODS
Cell lines and culture conditions
Human primary melanocytes containing wild-type B-Raf FOM103 and NHEM 558; and human melanoma cell lines harboring mutant V600EB-Raf - WM35, WM115, WM278.1, A375M and 1205 Lu (provided by Dr. Herlyn; Wistar Institute, Philadelphia, PA) were cultured as described (26). Human fibroblast FF2441 cells (provided by Dr. Craig Myers, Penn State College of Medicine, Hershey, PA), metastatic melanoma cell lines UACC 903 (V600EB-Raf; provided by Dr. Mark Nelson University of Arizona, Tucson, AZ). Wild type B-Raf containing C8161.Cl9 (provided by Dr. Danny Welch, University of Kansas, Kansas City, KS) and MelJuSo (provided by Dr. Judith Johnson, Institute for Immunology, Germany) cell lines were maintained in DMEM supplemented with 10% FBS. Cell lines were authenticated and maintained in a 37°C humidified 5% CO2 atmosphere incubator and periodically monitored for genotypic characteristics, tumorigenic potential to confirm cell line identity and phenotypic behavior.
Analysis of human melanoma patient tumors
Tumors were pulverized using a mortar and pestle chilled in liquid nitrogen and protein lysates extracted as reported previously (27). Western blotting was used to measure levels of COX-2 protein, normalized to alpha-enolase using ImageJ software.
SiRNA efficacy and knockdown studies
To determine efficacy of siRNA-mediated knockdown, 200 pmoles of siCOX-2 #1or siCOX-2 #2, was compared to scrambled siRNA or reconstitution buffer following nucleofection into 1×106of 1205 Lu or A375M cells using an Amaxa nucleofector with solution R/program K-17 (1205 Lu) or solution R/program A-23 (A375M). Transfection efficiency of viable cells was >90%. Following siRNA transfection, cells were reseeded and left to recover for 2 days followed by replating in 96-well plates to measure cell viability using the MTS assay (Promega, Madison, WI). To show siRNA-mediated protein knockdown in vitro, 1×106of 1205 Lu, UACC 903 and A375M cells were similarly nucleofected with 200 pmoles of siCOX-2 #1, siCOX-2 #2, and 100 pmoles of V600EB-RAF, MEK1, MEK2, ERK1, and ERK2, scrambled siRNA, reconstitution buffer, and protein lysates were harvested at day 4 or 6, and analyzed by Western blot analysis. Duplexed Stealth siRNA (Invitrogen) was used for these studies. The following siRNA sequences were used: COX-2 #1: UCC AGA CAA GCA GGC UAA UAC UGA U; COX-2 #2: GAG UUA UGU CUU GAC AUC CAG AUC A. SiRNA sequences for scrambled, V600EB-RAF, MEK1, MEK2, ERK1, and ERK2 as previously reported (28).
Cyclooxygenase inhibition studies
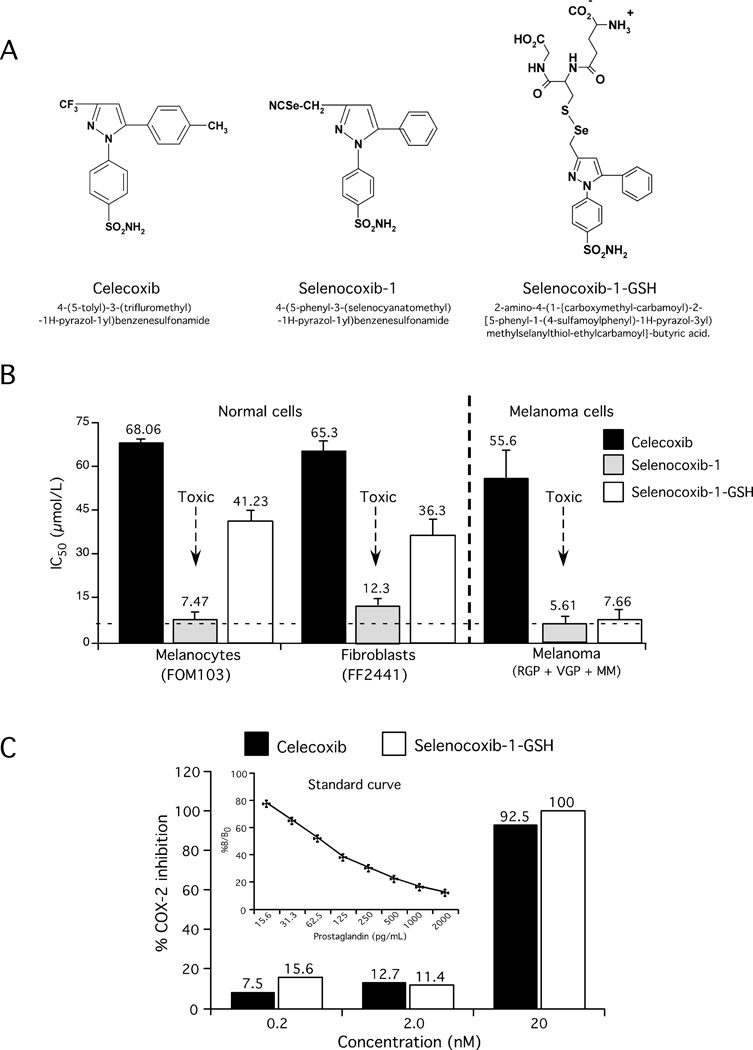
Human recombinant COX-2 activity was assayed using a commercial COX-inhibitor screening assay kit (Cayman Chemical) according to the manufacturer’s protocol. The concentrations of celecoxib and selenocoxib-1-GSH tested were 0.2, 2.0 and 20 nM. SC-560 and DuP-697, standard inhibitors for COX-1 and COX-2 respectively, were used as positive controls. DMSO served as a negative control for 100% activity. The assay was performed in duplicate and repeated twice.
Synthesis of celecoxib, selenocoxib-1 and selenocoxib-1-GSH
Celecoxib was synthesized as synthesized previously (29). Selenocoxib-1 was prepared as reported (25). Selenocoxib-1-GSH conjugate was prepared by reacting molar equivalent selenocoxib-1 with glutathione in THF: H2O (2:1) mixture. pH was adjusted to slightly basic conditions to generate selenocoxib-1-GSH conjugate in a quantitative yield as a yellow powder. MP:196–198°C; 1H NMR (DMSO-d6, 500 MHz) d 1.72–1.83 (m, 3H), 1.88–1.98 (m, 1H), 2.23–2.36 (m, 2H), 2.91 and 2.94 (dd, 1H, J=10 Hz), 3.16 and 3.18 (dd, 1H, J = 4.5 Hz), 3.57–3.72 (m, 3H), 4.18 and 4.23 (dd, 2H, J=12.5 Hz and 22 Hz), 4.51 (td,1H, J = 4.0 Hz), 6.62 (s, 1H, CH), 7.25 (d, 1H, aromatic, J=2.5 Hz), 7.26 (d, 1H, aromatic, J=3.5 Hz), 7.35–7.39 (m, 3H, aromatic), 7.41 (dt, 2H, aromatic, J=8.5 Hz and 2.0 Hz), 7.79 (dt, 2H,aromatic, J = 8.5 Hz and 2.0 Hz), 8.44 (d, 1H, J = 7Hz), 8.75 (ds, 1H); MS (M/Z, Intensity): 681 (M+, 100). Identity of compound was confirmed by NMR as well as MS, and purity >99% was validated by HPLC.
Western blot analysis
Cell lysates were harvested and processed as described previously (28). 1.5×106 melanoma cells were plated in 100 mm culture dishes and 48 h later treated with celecoxib, selenocoxib-1-GSH (5–20 µmol/L), PLX-4032 (0.2–20 µmol/L) or U0126 (2.5–50 µmol/L) for 6–72 h. Protein lysates were collected for Western blotting. Blots were probed with total and pAkt (Ser473), pPRAS40 (Thr246), pErk1/2 (Thr202/Tyr204), total and pMek1/2 (Ser217) and cleaved PARP from Cell Signaling Technology. Total PRAS40 was obtained from Invitrogen. Erk2, cyclin D1, p27, Alpha-enolase and secondary antibodies conjugated with horseradish peroxidase was purchased from Santa Cruz Biotechnology. COX-1 and COX-2 antibodies were obtained from Cayman Chemical Company. Immunoblots were developed using the enhanced chemiluminescence detection system from Amersham Pharmacia Biotech.
Cell viability, proliferation, apoptosis and cell cycle analysis
Viability and IC50 (µmol/L) of normal human melanocytes, fibroblast and melanoma cells following treatment with inhibitors were measured using the MTS assay (22, 23). In brief, 5×103 cells per well in 100 µL of media were plated and grown in a 96-well plate for 36–72 h for melanoma (WM35, WM115, 1205 Lu and UACC 903) and normal cell lines (FOM103 and FF2441). Cells were treated with 0.312–100 µmol/L of celecoxib, selenocoxib-1 and selenocoxib-1-GSH for 24, 48 or 72 h with DMSO as vehicle control. IC50 values for each inhibitor in µmol/L for respective cell lines were measured from three independent experiments using GraphPad Prism version 4.01 from GraphPad Software.
Cellular proliferation and apoptosis rates were measured by seeding 5×103cells in 96-well plates, followed by treatment for 72 h with celecoxib or selenocoxib-1-GSH. Percentage of proliferating or apoptotic cells was quantified using a colorimetric assay using a cell proliferation ELISA BrdU kit from Roche Applied Sciences or Apo-ONE Homogenous caspase-3/7 assay kit from Promega (22), respectively.
Cells in each population of the cell cycle were examined by growing 1205 Lu or UACC 903 melanoma cells in 100-mm culture dishes followed by treatment with 12.5 and 25 µmol/L of celecoxib and selenocoxib-1-GSH for 72 h. The samples were processed as described previously (22, 23). Stained cells were analyzed using the FACScan analyzer from Becton Dickinson and data processed utilizing ModFit LT software from Verity Software House (22, 23). Experiments were replicated twice.
Reactive oxygen species assay (ROS)
The intracellular ROS was monitored according to a published protocol (30). 1.5×106 melanoma cells were plated in 100 mm culture dishes and 48 h later treated with 5–20 µmol/L concentration of celecoxib, selenocoxib-1 or selenocoxib-1-GSH. After 24 h treatment, total cells (floating and adherent) were collected in ice-cold PBS and 5×103 cells / well placed in 100 µL of culture media in a 96-well plate containing 10 µmol/L 2’,7’-dichlorfluorescein-diacetate from Sigma and incubated at 37°C for 30 m. Amount of fluorescent 2’,7’-dichlorfluorescein was measured using a SpectraMax-M2 plate reader. Amount of ROS present compared to DMSO vehicle treated cells was represented in arbitrary units. The assay was performed twice with four replicates each time.
Tumorigenicity assessments following targeting of COX-2 using siRNA
Tumor kinetic studies were undertaken in athymic-Foxn1nu nude mice (Harlan Sprague Dawley, IN). 200 pmoles of siRNA COX-2 #1 or COX-2 #2 were nucleofected into 2×106 1205 Lu cells and after 48 h of recovery, 1×106 cells were collected in 0.2 mL of 10% FBS-DMEM and injected subcutaneously above both the left and right rib cages of 4–6 week old female mice (5 mice/ group; experiments were replicated twice). Dimensions of developing tumors were measured on alternate days up to day 21.5, using calipers by LxWxD (mm3) (22).
Animals studies using selenocoxib-1-GSH for tumorigenicity assessments
Six days after subcutaneous injection of 1×106 1205 Lu or UACC 903 cells in 0.2 mL of DMEM supplemented with 10% FBS in to 4–6 weeks old nude mice, when a fully vascularized tumor (50–75 mm3) had formed (5 mice/group; 2 tumors/mouse). Mice were treated intraperitoneally with selenocoxib-1-GSH (0.127 µmoles, equivalent to 10 ppm selenium) or celecoxib (0.127 µmoles) in DMSO on alternate days for 4 weeks. Body weight (grams) and dimensions of the developing tumors (mm3) were measured at the time of drug treatment (22, 23).
Toxicity assessments
Four to six week old athymic-Foxn1nu nude mice were treated with either vehicle control or selenocoxib-1-GSH (n = 5) as described in tumor kinetics studies. At the end of treatment, blood was collected from each sacrificed animal in a plasma separator tube with lithium heparin (BD Microtainer) following cardiac puncture and analyzed for ALKP (alkaline phosphatase), ALT (alanine aminotransferase), AST (aspartate aminotransferase), ALB (total albumin), TBIL (total bilirubin), CREA (creatinine), BUN (blood urea nitrogen), CHOL (total cholesterol), TRIG (total triglyceride) and GLU (glucose) levels to ascertain possible liver, heart, kidney, and pancreas related toxicity. A portion of vital organs; liver, heart, kidney, pancreas, and spleen-from each animal was formalin-fixed and paraffin-embedded to assess toxicity-associated changes in cell morphology and tissue organization following H&E staining. In addition, the effect of celecoxib, selenocoxib-1 and selenocoxib-1-GSH on the survival of mice was determined by intraperitoneally injecting celecoxib (0.127 µmoles), selenocoxib-1 (0.032–0.064 µmoles) or selenocoxib-1-GSH (0.127–0.254 µmoles) daily for seven days (n = 3). Number of surviving animals or changes in body weight was recorded.
Statistical Analysis
Statistical analysis was performed using Prism 4.01 GraphPad Software. One-way or Two-way Analysis Of Variance (ANOVA) was used for group wise comparisons, followed by the Tukey’s or Bonferroni’s post hoc tests. For comparison between two groups, the t test was used. Results represent at least two to three independent experiments and are shown as averages ± S.E.M. Results with a P value less than 0.05 (95% CI) were considered significant.
RESULTS
COX-2 expression is elevated in advanced-stage melanoma patient tumors and melanoma cell lines
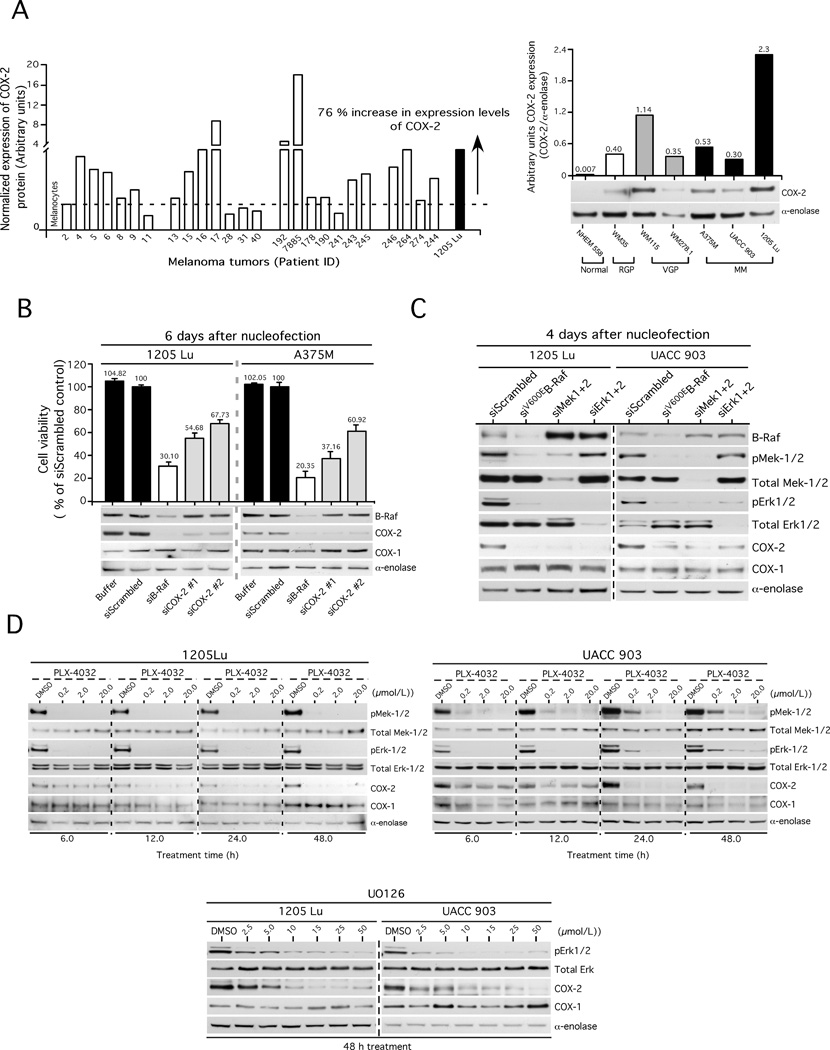
Elevated expression and activity of COX-2 has been reported in cancers of the prostate, breast, colon, kidney, liver and skin (13, 31). To confirm the initial report in melanomas (32), the expression of COX-2 was measured by Western blotting in a panel of melanoma patient tumors and cell lines representing radial (WM35), vertical (WM115, WM278.1) and metastatic (A375M, UACC 903, 1205 Lu) stages of development (Fig. 1A). 76% (19/25) of melanoma patient tumors had elevated COX-2 expression when compared to normal human melanocyte (NHEM) control cells (Fig. 1A, left panel). Similarly, all melanoma cell lines examined contained COX-2 protein higher than that observed in melanocytes, albeit, in varying amounts (Fig. 1A, right panel). Expression of COX-2 in UACC 903, A375M and 1205 Lu cell lines was 43, 76 and 329-fold higher than that observed in melanocytes, respectively.
Figure 1. COX-2 expression increased in melanomas, regulated through the MAP kinase pathway.
1A (left panel). Elevated levels of COX-2 expression in melanoma patient tumors and cell lines. 1A (right panel). COX-2 expression increased in a cell line based melanoma tumor progression model. 1B. Targeting COX-2 using siRNAs decreased melanoma cell viability. 1C. SiRNA-mediated inhibition of the MAP kinase pathway decreased COX-2 expression in melanomas. 1D (upper panel). PLX-4032 targeting of V600EB-Raf decreased COX-2 expression. 1D (bottom panel). U0126 targeting of Mek1/2 decreased COX-2 expression.
Reduction of COX-2 protein levels using siRNA targeting V600EB-Raf or COX-2 decreased melanoma cell viability
To determine whether targeting COX-2 would reduce viability of melanomas, metastatic 1205 Lu and A375M cells that express relatively high levels of protein were transfected with 2 different siRNAs targeting different regions of the mRNA and cell viability compared to controls nucleofected with a scrambled siRNA, buffer control or siRNA targeting V600EB-Raf (Fig. 1B). In both cell lines, targeting COX-2 reduced melanoma viability by 32–63%. Targeting mutant V600EB-Raf using siRNA reduced COX-2 expression; suggesting protein expression was regulated through this pathway (Fig. 1B).
SiRNA and pharmacological agents targeting the MAP kinase pathway confirm that COX-2 expression is regulated through V600EB-Raf signaling in melanomas
To examine whether siRNA-mediated targeting of Mek1/2 or Erk1/2 downstream of V600EB-Raf would decrease COX-2 expression, siRNA or pharmacological agents were used to decrease protein expression or activity. 1205 Lu and UACC 903 cells were nucleofected with siRNAs inhibiting mutant V600EB-Raf, Mek1/2 or Erk1/2. A significant decrease in COX-2, but not COX-1, was observed when each member of the V600EB-Raf signaling pathway was targeted (Fig. 1C).
Next, Vemurafenib (PLX-4032), a V600EB-Raf inhibitor, was used to inhibit activity of this pathway. Cells treated with PLX-4032 showed decreased COX-2 protein expression beginning after 12 h of treatment for 1205 Lu cells. In the case of UACC 903, a significant decrease was seen from 24 h of treatment (Fig. 1D, upper panels). Similar to siRNA studies (Fig. 1C), no changes were observed in COX-1 expression in either cell line following treatment. A decrease in phosphorylation of Mek1/2 and Erk1/2 proteins showed the inhibitory activity of PLX-4032 on the V600EB-Raf pathway. Like PLX-4032, Mek1/2 inhibitor U0126 also reduced levels of COX-2 protein without affecting COX-1 in 1205 Lu and UACC 903 cell lines (Fig. 1D, lower panel). Therefore, targeting V600EB-Raf or downstream proteins in the signaling cascade reduced expression of COX-2 in melanomas to decrease the proliferative potential of the cells. Thus, COX-2 lies downstream of V600EB-Raf, Mek-1/2 and Erk-1/2 in this important signaling pathway.
Development of selenocoxib-1-GSH retaining COX-2 inhibitory efficacy
Concentrations of celecoxib required to trigger apoptosis in cultured cells range from 25–100 µmol/L and clinical use is associated with cardiovascular side-effects at doses of 200 mg per day (33). To circumvent these concerns, an analog of celecoxib has been created containing selenium that is called selenocoxib-1 (Fig. 2A). While selenocoxib-1 inhibited melanoma cells viability, it was toxic and reduced normal cell growth with similar IC50s to that observed for melanoma cells (Table 1 & Fig. 2B). To lessen the toxicity of selenocoxib-1 on normal but not melanoma cells, the compound was further modified incorporating GSH, generating selenocoxib-1-GSH (Fig. 2A), which significantly decreased the toxicity on normal cells but maintained its melanoma cell-killing efficacy (Fig. 2B). Furthermore, selenocoxib-1-GSH inhibited the growth of melanoma cell lines irrespective of B-Raf mutation status (data not shown). Toxicity limiting the potential clinical utility of selenocoxib-1 but not selenocoxib-1-GSH was corroborated in animal studies (Supplementary Table 1). Celecoxib at 0.127 µmoles led to death of all animals following 7 days of treatment, while selenocoxib-1 at concentrations of 0.032–0.064 µmoles, led to weight losses of 14% or 100% animal mortality after seven days of treatment. In contrast, animals receiving 0.127–0.254 µmoles (equivalent to 5 to 10 ppm of selenium) of selenocoxib-1-GSH exhibited negligible weight loss of ~2% and no mortality (Supplementary Table 1). Due to the toxicity associated with selenocoxib-1, subsequent studies focused on selenocoxib-1-GSH. Selenocoxib-1-GSH was not predicted to have altered COX-2 inhibitory activity by incorporating selenium in the place of sulfur but rather to have Akt inhibitory properties and as predicted, it retained COX-2 inhibitory activity similar to that of celecoxib (Fig. 2C).
Figure 2. Development of selenocoxib-1-GSH.
2A. Structure of celecoxib, selenocoxib-1, and selenocoxib-1-GSH. 2B. Selenocoxib-1-GSH kills cancer more effectively that normal cells. 2C. Selenocoxib-1-GSH retained COX-2 inhibitory activity.
Table 1. Selenocoxib-1-GSH kills melanoma cells more effectively than normal cells.
Normal and melanoma cells were seeded in to a 96-well plate and after 36 to 72 h, treated with increasing concentrations of celecoxib, selenocoxib-1 or selenocoxib-1-GSH for the indicated time period. Number of viable cells was measured using MTS and percentage decrease in viability calculated. IC50 values for each inhibitor in µmol/L for respective cell lines were measured from three independent experiments using GraphPad Prism version 4.01 (GraphPad Software, La Jolla, CA).
| FOM103 | FF2441 | WM35 | WM115 | UACC903 | 1205 Lu | ||
|---|---|---|---|---|---|---|---|
| Celecoxib | >100 | >100 | 51.3 ± 2.3 | 54.4 ± 3.6 | >100 | >100 | |
| Selenocoxib-1-GSH | 66.3 ± 3.0 | >100 | 52.6 ± 3.8 | 30.1 ± 3.3 | 30.9 ± 2.8 | 24.6 ± 2.2 | |
| Celecoxib | >100 | >100 | 42.3 ± 1.8 | 45.3 ± 2.7 | >100 | 83.6 ± 3.3 | |
| Selenocoxib-1-GSH | 53.4 ± 4.3 | 75.5 ± 5.6 | 4.1 ± 0.8 | 5.8± 0.9 | 20.6 ± 1.7 | 17.2 ± 1.6 | |
| Celecoxib | 68.0 ± 1.2 | 65.3 ± 3.3 | 37.9 ± 3.3 | 41.8 ± 2.9 | 76.6 ± 4.4 | 66.1 ± 3.2 | |
| Selenocoxib-1-GSH | 41.2 ± 3.2 | 36.3 ± 5.6 | 2.7 ± 0.4 | 3.1 ± 0.4 | 14.2 ± 1.2 | 10.6± 2.6 | |
| Normal | Radial | Vertical | Metastatic | ||||
Selenocoxib-1-GSH inhibited melanoma cellular proliferation and increased apoptosis by arresting cells in the G0-G1 phase of the cell cycle
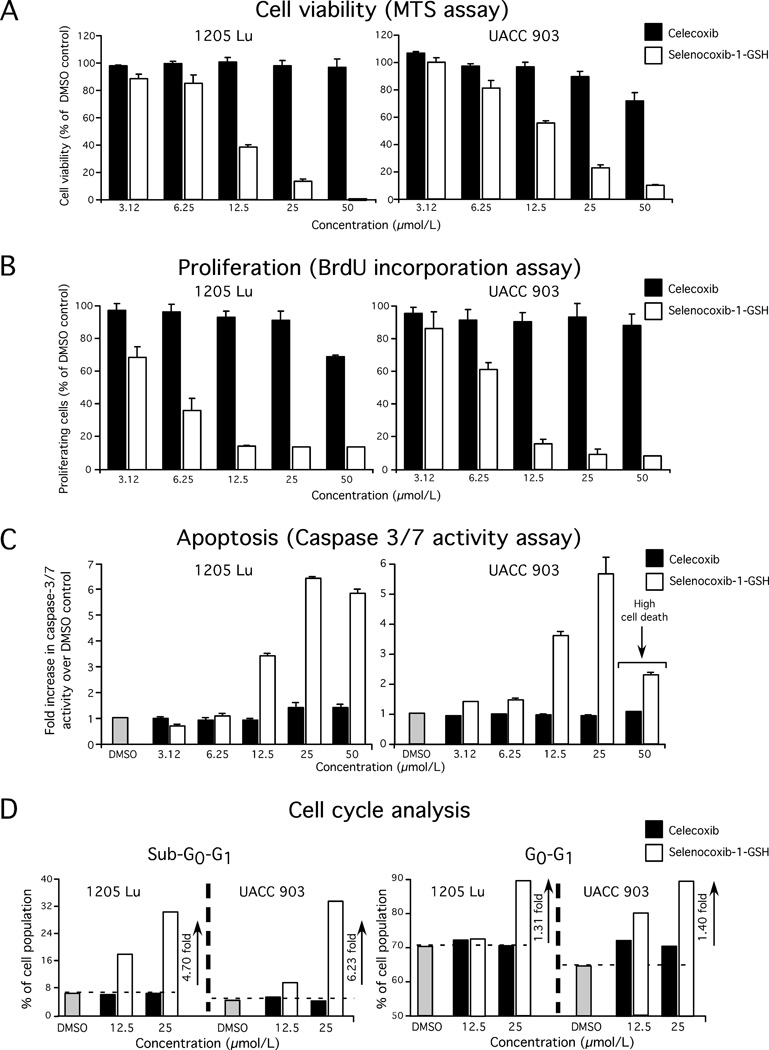
Selenocoxib-1-GSH but not celecoxib inhibited melanoma cell viability in a dose responsive manner (Fig. 3A). At 12.5 µmol/L, selenocoxib-1-GSH led to a 40–60% decrease in cell viability compared to control DMSO vehicle treated cells. Next, mechanisms leading to cell growth inhibition after treatment with selenocoxib-1-GSH were examined by measuring the level of cellular proliferation, apoptosis, and the percentage of cells in the various phases of the cell cycle. Selenocoxib-1-GSH reduced proliferation of 1205 Lu and UACC 903 melanoma cells (Fig. 3B) and increased caspase-3/7 activity, which is an indicator of apoptosis (Fig. 3C). A significant decrease in caspase-3/7 activity was observed when UACC 903 cells were treated with 50 µmol/L selenocoxib-1-GSH, which can be attributed to massive cell death (Fig. 3C). The effect of selenocoxib-1-GSH on cell cycle distribution was measured by analyzing propidium iodide stained 1205 Lu and UACC 903 cells using a BD FACScan. Selenocoxib-1-GSH treatment increased the sub-G0-G1 cell population, which is indicative of cellular apoptosis. The sub-G0-G1 cell population increased by 6.2 and 4.7-fold, respectively, when 1205 Lu and UACC 903 cells were treated with 25 µmol/L selenocoxib-1-GSH (Fig. 3D). In addition, an increase in the G0-G1 cell population was also observed at 12.5 and/or 25 µmol/L selenocoxib-1-GSH (Fig. 3D). Thus, selenocoxib-1-GSH inhibited cellular proliferation and triggered apoptosis mediated through a G0-G1 block, resulting in fewer cells in the S and G2-M phases of the cell cycle.
Figure 3. Selenocoxib-1-GSH inhibited melanoma cell growth by reducing cellular proliferation, triggering apoptosis, and arresting melanoma cells in the G0-G1 phase of the cell cycle.
3A, 3B & 3C. Selenocoxib-1-GSH, but not celecoxib, inhibited melanoma cell proliferation and induced apoptosis. 3D. Selenocoxib-1-GSH arrested melanoma cells in the G0-G1 phase of the cell cycle.
Selenocoxib-1-GSH inhibited Akt signaling, which activates MAP kinase activity to reduce melanoma cellular proliferation and promote apoptosis
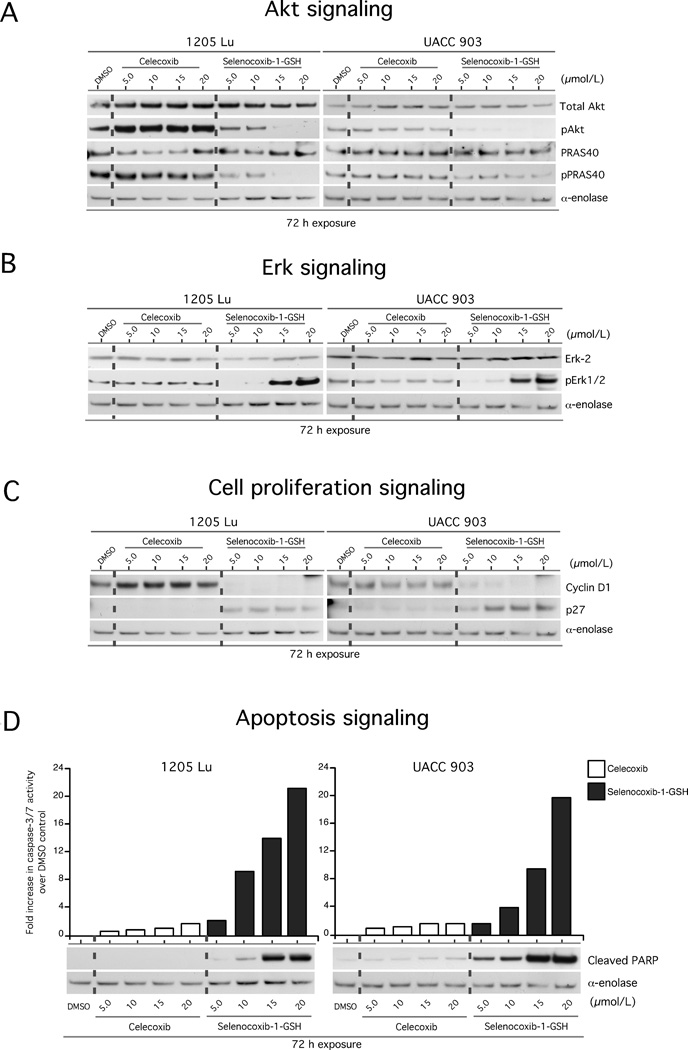
Selenium incorporation into the structural backbone of certain agents can enhance the therapeutic potential of the agent by providing the compound with new inhibitory properties (19, 22, 23, 34). Selenocoxib-1-GSH retained COX-2 inhibitory activity as predicted (Fig. 2C). To determine whether selenium incorporation into selenocoxib-1-GSH provided the compound with new Akt pathway inhibitory properties, pAkt levels were examined in melanoma cells following treatment. Compared to celecoxib, selenocoxib-1-GSH treatment inhibited Akt phosphorylation in a dose dependent manner (Fig. 4A). Furthermore, phosphorylation of the downstream Akt3 substrate PRAS40 was significantly inhibited.
Figure 4. Selenocoxib-1-GSH inhibited Akt signaling to reduce the proliferative potential and promote apoptotic signaling in melanoma cells.
4A. Selenocoxib-1-GSH inhibits the PI3K/Akt signaling pathway. 4B. Selenocoxib-1-GSH activates the MAPK signaling pathway. 4C. Selenocoxib-1-GSH decreased cyclin D1 protein levels indicating a reduction in cellular proliferation. 4D. Selenocoxib-1-GSH increased levels of cellular apoptosis.
1205 Lu and UACC 903 melanoma cells have elevated MAPK activities due to the presence of constitutively active V600EB-Raf; however, the levels are moderated into a range that promotes rather than inhibits cellular proliferation (35). Akt3 has been shown to phosphorylate V600EB-Raf to lower MAP kinase pathway activity to promote cellular proliferation (35). Treatment of melanoma cells with selenocoxib-1-GSH, which decreased Akt activity, led to a significant increase in pERK1/2 levels (the indicator of MAP kinase pathway activity) (Fig. 4B), to a point where it no longer promoted proliferation but led to cell senescence. This was due to decreased phosphorylation and regulation of V600EB-Raf by Akt (35). In addition, selenocoxib-1-GSH inhibited expression of cyclin D1 and increased levels of p27 (Fig. 4C). Finally, increased caspase 3/7 and cleaved PARP levels were observed indicating higher levels of apoptosis in selenocoxib-1-GSH compared to celecoxib treated cells (Fig. 4D).
Selenocoxib-1-GSH inhibited melanoma tumor development in mice without significant toxicity
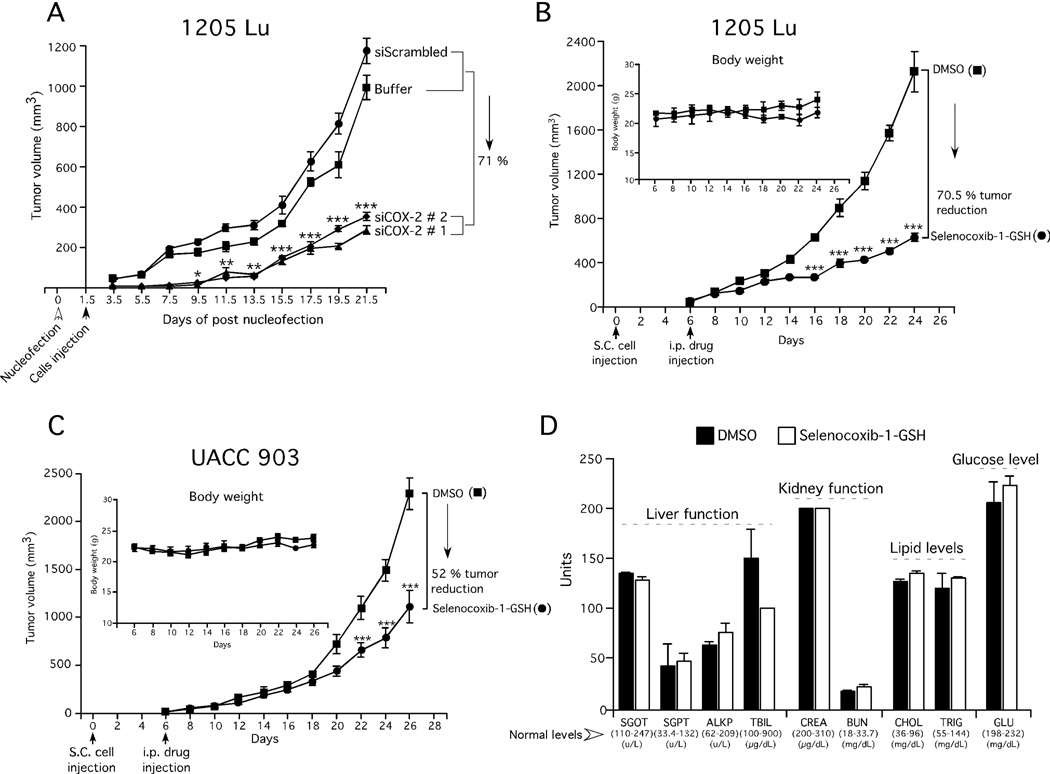
Initially, siRNA-targeting COX-2 was used to reduce protein expression in melanoma cells to measure the effect on melanoma tumor development to serve as a control. Inhibition of COX-2 protein expression using siRNAs, reduced xenografted melanoma tumor development by an average of 71% after 21 days compared to controls, suggesting COX-2 was a good therapeutic target in melanomas (Fig. 5A). Next, the effect of intraperitoneal administration of selenocoxib-1-GSH on xenografted melanoma tumor development was examined (Figs. 5B & 5C). Decreased xenografted tumor development compared to control treated mice was observed from day 16 in 1205 Lu tumors (Fig. 5B). Similarly, a significant decrease was observed in UACC 903 tumors from day 22 (Fig. 5C). For both cell lines at the end of treatment, up to a 70% decrease in tumor volume was observed following selenocoxib-1-GSH treatment compared to controls (Figs. 5B & 5C). No noticeable changes in animal body weight were observed (Figs. 5B & 5C; insets). The levels of blood markers for major organ related toxicity and analysis of H & E stained tissue sections showed negligible differences compared to controls at the concentrations examined (Fig. 5D and Supplementary Fig. 1). These data suggest that selenocoxib-1-GSH can inhibit melanoma tumor development without significant organ related toxicity.
Figure 5. Targeting COX-2 inhibited melanoma tumor development with negligible toxicity.
5A. SiRNA-mediated reduction of COX-2 protein levels decreased melanoma tumor development in mice. 5B & 5C. Selenocoxib-1-GSH treatment decreased xenografted melanoma tumor development. No significant difference was observed in body weight of mice treated with the drug indicating negligible toxicity (Figs. 5B & 5C; inset). 5D. Selenocoxib-1-GSH does not affect blood biomarkers indicative of major organ related toxicity.
DISCUSSION
Incidence and mortality rates of malignant melanoma continue to increase annually (36). Although efforts have been made to design structurally well-defined small molecular inhibitors that interact with protein targets in melanoma cells, these efforts have failed due to development of resistant disease (37). Therefore, the realization now is that multiple important targets driving the development of this disease will need to be simultaneously targeted to most effectively manage melanoma and reduce the probability of resistant disease development. This may be achievable through the use of drug cocktails or a single drug simultaneously inhibiting multiple key signaling pathways implicated in melanoma development (38). In addition, selection of patients expressing proteins targeted by the drug would be a key factor that could lead to better results in the clinic. In this study, COX-2 protein levels are shown to be elevated in 76% of melanoma patient tumors and cell lines. Targeting COX-2 using siRNA, inhibited the growth of metastatic melanoma cells in culture and retarded the development of xenografted melanoma tumors in mice, indicating that COX-2 would be a good therapeutic target.
Since siRNA mediated inhibition of COX-2 expression decreased melanoma tumor growth, pharmacological agents inhibiting COX-2 activity with high selectivity may have therapeutic potential for inhibiting melanomas. However, the COX-2 selective inhibitor celecoxib has an effect on melanoma cell proliferation but only at very high concentrations, necessitating the development of analogs better able to kill these cells at lower concentrations (39, 40). Initially an analog of celecoxib was developed that contained selenium, called selenocoxib-1, but the drug was toxic to normal cells and lethal to animals, which limited its use as a therapeutic agent (18). To manage this concern, a glutathione (GSH) derivative called selenocoxib-1-GSH was developed. It killed cultured cancer cells at doses 5-fold lower than those required to kill normal cells.
Addition of GSH to a compound can be used to increase bioavailability and reduce cytotoxicity as has been seen with the GSH conjugate of benzyl selenocyanate for inhibiting of colonic preneoplastic lesions and aberrant crypt foci development (41). One mechanism by which GSH conjugates inhibit cancer, involves reduction of GSH reductase activity, which depletes intracellular reduced glutathione thereby enhancing reactive oxygen species (ROS) mediated cell death (42, 43). Others have reported that GSH depleting agents can selectively sensitize cancer cells to high ROS levels (44). Another mechanism by which GSH conjugated anticancer agents inhibit cancer cells growth involves reduction of elevated ROS levels. High ROS levels mediate cancer development and reduction reverses this process (30). Our data suggests that selenocoxib-1-GSH reduces ROS levels in melanoma cells more effectively than celecoxib or selenocoxib-1 to kill these cells (Supplementary Fig. 2).
Enhanced growth inhibitory properties of selenocoxib-1-GSH could also be attributed to the incorporation of selenium into the structure of celecoxib. Selenium is an antioxidant nutrient reported to inhibit oncogenic Akt and NFkB pathways as well as inducing the expression of tumor suppressors PTEN, p53 and KLF-4 to mediate apoptotic cell death (25, 45). Incorporation of selenium into the structure of drugs has been shown to increase the agent’s potency by inhibiting Akt signaling (22, 23, 46). The selenium-containing analogs of the PBIT and PBITC called PBISe and ISC-4, respectively, inhibited melanoma cell growth and suppressed tumor development in animals more effectively than the sulfur containing parental compound (22, 23). In addition, since very low levels of selenium occur in the majority of melanoma patients, incorporating selenium may not only provide this micronutrient but also increase tumor cell killing efficacy (34). While several reports document loss of COX-2 inhibitory activity when celecoxib was derivatized or analogs were synthesized, selenocoxib-1-GSH retained COX-2 inhibitory activity and had new Akt targeting capabilities. In contrast, 2, 5-dimethyl-celecoxib, lacked the ability to inhibit COX-2, but still had anti-tumor activity (47).
The PI3K/Akt and MAP kinase signaling pathways are constitutively activated in melanoma and play a prominent role in the development of recurrent resistant disease (22, 23, 48). Selenocoxib-1-GSH while retaining COX-2 inhibitory activity at levels seen with celecoxib also blocked Akt signaling. Decreasing levels of active pAkt3 increased V600EB-Raf activity and downstream MAP kinase-signaling activity to levels that are inhibitory, inducing cellular senescence (35, 49). This phenomenon occurs following selenocoxib-1-GSH treatment of melanoma cells. Selenium containing PBISe treatment acts in a similar manner to decrease Akt activity, consequently increasing MAP kinase pathways activity to inhibitory levels (23). Other studies and this one found that increased pErk-1/2 in turn up-regulated COX-2 protein expression, consistent with COX-2 lying downstream in the MAP kinase pathway (50). High MAP kinase pathway activity mediated by selenocoxib-1-GSH or PBISe, induced cell senescence arresting cells in G0-G1 phase of the cell cycle. Combined targeting of these pathways inhibited cell proliferation by lowering cyclin D1 and increasing p27 levels, which enhanced rates of cellular apoptosis.
In conclusion, selenium-containing selenocoxib-1-GSH retains COX-2 inhibitory activity and has new PI3K/Akt inhibitory activity to decrease melanoma cell growth by arresting cells in the G0-G1 phase of the cell cycle, to promote melanoma cell apoptosis and inhibit cellular proliferation. Thus, a potentially clinically viable drug has been developed from a toxic one that can decrease melanoma development by targeting the COX-2 and PI3K/Akt signaling pathways without causing major-organ related toxicity.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Arati Sharma, Omer Kuzu, Virginia Robertson and Anton Mulder for technical assistance.
GRANT SUPPORT
NIH CA-127892-01A (GP Robertson), The Foreman Foundation for Melanoma Research (GP Robertson).
Footnotes
Conflict of Interest: None
REFERENCES
- 1.Oliveria SA, Hay JL, Geller AC, Heneghan MK, McCabe MS, Halpern AC. Melanoma survivorship: research opportunities. J Cancer Surviv. 2007;1:87–97. doi: 10.1007/s11764-007-0009-y. [DOI] [PubMed] [Google Scholar]
- 2.Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 4.Smalley KS, Sondak VK. Melanoma--an unlikely poster child for personalized cancer therapy. N Engl J Med. 363:876–878. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- 5.Fedorenko IV, Paraiso KH, Smalley KS. Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol. 2011;82:201–209. doi: 10.1016/j.bcp.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villanueva J, Vultur A, Herlyn M. Resistance to BRAF inhibitors: unraveling mechanisms and future treatment options. Cancer Res. 2011;71:7137–7140. doi: 10.1158/0008-5472.CAN-11-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen N, Sharma A, Sharma AK, Desai D, Huh SJ, Amin S, et al. Melanoma chemoprevention in skin reconstructs and mouse xenografts using isoselenocyanate-4. Cancer Prev Res (Phila) 4:248–258. doi: 10.1158/1940-6207.CAPR-10-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolaou VA, Stratigos AJ, Flaherty KT, Tsao H. Melanoma: new insights and new therapies. J Invest Dermatol. 132:854–863. doi: 10.1038/jid.2011.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hawk ET, Viner JL, Dannenberg A, DuBois RN. COX-2 in cancer--a player that's defining the rules. J Natl Cancer Inst. 2002;94:545–546. doi: 10.1093/jnci/94.8.545. [DOI] [PubMed] [Google Scholar]
- 10.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol. 2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meric JB, Rottey S, Olaussen K, Soria JC, Khayat D, Rixe O, et al. Cyclooxygenase-2 as a t arget for anticancer drug development. Crit Rev Oncol Hematol. 2006;59:51–64. doi: 10.1016/j.critrevonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Biberstine KJ, Darr DS, Rosenthal RS. Tolerance to appetite suppression induced by peptidoglycan. Infect Immun. 1996;64:3641–3645. doi: 10.1128/iai.64.9.3641-3645.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker MR, Siegelin MD, Rompel R, Enk AH, Gaiser T. COX-2 expression in malignant melanoma: a novel prognostic marker? Melanoma Res. 2009;19:8–16. doi: 10.1097/CMR.0b013e32831d7f52. [DOI] [PubMed] [Google Scholar]
- 14.Denkert C, Kobel M, Berger S, Siegert A, Leclere A, Trefzer U, et al. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 2001;61:303–308. [PubMed] [Google Scholar]
- 15.Waskewich C, Blumenthal RD, Li H, Stein R, Goldenberg DM, Burton J. Celecoxib exhibits the greatest potency amongst cyclooxygenase (COX) inhibitors for growth inhibition of COX-2-negative hematopoietic and epithelial cell lines. Cancer Res. 2002;62:2029–2033. [PubMed] [Google Scholar]
- 16.Drazen JM. COX-2 inhibitors--a lesson in unexpected problems. N Engl J Med. 2005;352:1131–1132. doi: 10.1056/NEJMe058038. [DOI] [PubMed] [Google Scholar]
- 17.Jawabrah Al-Hourani B, Sharma SK, Suresh M, Wuest F. Cyclooxygenase-2 inhibitors: a literature and patent review (2009 – 2010) Expert Opin Ther Pat. 2011;21:1339–1432. doi: 10.1517/13543776.2011.593510. [DOI] [PubMed] [Google Scholar]
- 18.Desai D, Sinha I, Null K, Wolter W, Suckow MA, King T, et al. Synthesis and antitumor properties of selenocoxib-1 against rat prostate adenocarcinoma cells. Int J Cancer. 2010;127:230–238. doi: 10.1002/ijc.25033. [DOI] [PubMed] [Google Scholar]
- 19.Desai D, Kaushal N, Gandhi UH, Arner RJ, D'Souza C, Chen G, et al. Synthesis and evaluation of the anti-inflammatory properties of selenium-derivatives of celecoxib. Chem Biol Interact. 2010;188:446–456. doi: 10.1016/j.cbi.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang HC, Kardosh A, Gaffney KJ, Petasis NA, Schonthal AH. COX-2 inhibition is neither necessary nor sufficient for celecoxib to suppress tumor cell proliferation and focus formation in vitro. Mol Cancer. 2008;7:38. doi: 10.1186/1476-4598-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang JT, Kim YM, Surh YJ, Baik HW, Lee SK, Ha J, et al. Selenium regulates cyclooxygenase-2 and extracellular signal-regulated kinase signaling pathways by activating AMP-activated protein kinase in colon cancer cells. Cancer Res. 2006;66:10057–10063. doi: 10.1158/0008-5472.CAN-06-1814. [DOI] [PubMed] [Google Scholar]
- 22.Sharma A, Sharma AK, Madhunapantula SV, Desai D, Huh SJ, Mosca P, et al. Targeting Akt3 signaling in malignant melanoma using isoselenocyanates. Clin Cancer Res. 2009;15:1674–1685. doi: 10.1158/1078-0432.CCR-08-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madhunapantula SV, Desai D, Sharma A, Huh SJ, Amin S, Robertson GP. PBISe, a novel selenium-containing drug for the treatment of malignant melanoma. Mol Cancer Ther. 2008;7:1297–1308. doi: 10.1158/1535-7163.MCT-07-2267. [DOI] [PubMed] [Google Scholar]
- 24.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desai D, Kaushal N, Gandhi UH, Arner RJ, D'Souza C, Chen G, et al. Synthesis and evaluation of the anti-inflammatory properties of selenium-derivatives of celecoxib. Chem Biol Interact. 188:446–456. doi: 10.1016/j.cbi.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, et al. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7(Suppl 2):S35–S42. [PubMed] [Google Scholar]
- 27.Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res. 2007;67:3626–3636. doi: 10.1158/0008-5472.CAN-06-4234. [DOI] [PubMed] [Google Scholar]
- 28.Sharma A, Tran MA, Liang S, Sharma AK, Amin S, Smith CD, et al. Targeting mitogen-activated protein kinase/extracellular signal-regulated kinase kinase in the mutant (V600E) B-Raf signaling cascade effectively inhibits melanoma lung metastases. Cancer Res. 2006;66:8200–8209. doi: 10.1158/0008-5472.CAN-06-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, et al. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib) J Med Chem. 1997;40:1347–1365. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 30.Somasundaram S, Edmund NA, Moore DT, Small GW, Shi YY, Orlowski RZ. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62:3868–3875. [PubMed] [Google Scholar]
- 31.Ghosh N, Chaki R, Mandal V, Mandal SC. COX-2 as a target for cancer chemotherapy. Pharmacol Rep. 62:233–244. doi: 10.1016/s1734-1140(10)70262-0. [DOI] [PubMed] [Google Scholar]
- 32.Flockhart RJ, Armstrong JL, Reynolds NJ, Lovat PE. NFAT signalling is a novel target of oncogenic BRAF in metastatic melanoma. Br J Cancer. 2009;101:1448–1455. doi: 10.1038/sj.bjc.6605277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senior K. COX-2 inhibitors: cancer prevention or cardiovascular risk? Lancet Oncol. 2005;6:68. doi: 10.1016/s1470-2045(05)01720-1. [DOI] [PubMed] [Google Scholar]
- 34.Gowda R, Madhunapantula SV, Desai D, Amin S, Robertson GP. Selenium-containing histone deacetylase inhibitors for melanoma management. Cancer Biol Ther. 2012;13 doi: 10.4161/cbt.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung M, Sharma A, Madhunapantula SV, Robertson GP. Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res. 2008;68:3429–3439. doi: 10.1158/0008-5472.CAN-07-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 37.Kitano H. A robustness-based approach to systems-oriented drug design. Nat Rev Drug Discov. 2007;6:202–210. doi: 10.1038/nrd2195. [DOI] [PubMed] [Google Scholar]
- 38.Lasithiotakis KG, Sinnberg TW, Schittek B, Flaherty KT, Kulms D, Maczey E, et al. Combined inhibition of MAPK and mTOR signaling inhibits growth, induces cell death, and abrogates invasive growth of melanoma cells. J Invest Dermatol. 2008;128:2013–2023. doi: 10.1038/jid.2008.44. [DOI] [PubMed] [Google Scholar]
- 39.Bundscherer A, Hafner C, Maisch T, Becker B, Landthaler M, Vogt T. Antiproliferative and proapoptotic effects of rapamycin and celecoxib in malignant melanoma cell lines. Oncol Rep. 2008;19:547–553. [PubMed] [Google Scholar]
- 40.Wilgus TA, Breza TS, Jr, Tober KL, Oberyszyn TM. Treatment with 5-fluorouracil and celecoxib displays synergistic regression of ultraviolet light B-induced skin tumors. J Invest Dermatol. 2004;122:1488–1494. doi: 10.1111/j.0022-202X.2004.22606.x. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa T, Okuno T, Nakamuro K, Sayato Y. Identification and metabolism of selenocysteine-glutathione selenenyl sulfide (CySeSG) in small intestine of mice orally exposed to selenocystine. Arch Toxicol. 1996;71:39–44. doi: 10.1007/s002040050356. [DOI] [PubMed] [Google Scholar]
- 42.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 43.Burg D, Mulder GJ. Glutathione conjugates and their synthetic derivatives as inhibitors of glutathione-dependent enzymes involved in cancer and drug resistance. Drug Metab Rev. 2002;34:821–863. doi: 10.1081/dmr-120015695. [DOI] [PubMed] [Google Scholar]
- 44.Gouaze V, Mirault ME, Carpentier S, Salvayre R, Levade T, Andrieu-Abadie N. Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol Pharmacol. 2001;60:488–496. [PubMed] [Google Scholar]
- 45.Bureau C, Hanoun N, Torrisani J, Vinel JP, Buscail L, Cordelier P. Expression and Function of Kruppel Like-Factors (KLF) in Carcinogenesis. Curr Genomics. 2009;10:353–360. doi: 10.2174/138920209788921010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Das A, Bortner J, Desai D, Amin S, El-Bayoumy K. The selenium analog of the chemopreventive compound S,S'-(1,4-phenylenebis[1,2-ethanediyl])bisisothiourea is a remarkable inducer of apoptosis and inhibitor of cell growth in human non-small cell lung cancer. Chem Biol Interact. 2009;180:158–164. doi: 10.1016/j.cbi.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schonthal AH, Chen TC, Hofman FM, Louie SG, Petasis NA. Celecoxib analogs that lack COX-2 inhibitory function: preclinical development of novel anticancer drugs. Expert Opin Investig Drugs. 2008;17:197–208. doi: 10.1517/13543784.17.2.197. [DOI] [PubMed] [Google Scholar]
- 48.Tran MA, Gowda R, Sharma A, Park EJ, Adair J, Kester M, et al. Targeting V600EB-Raf and Akt3 using nanoliposomal-small interfering RNA inhibits cutaneous melanocytic lesion development. Cancer Res. 2008;68:7638–7649. doi: 10.1158/0008-5472.CAN-07-6614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madhunapantula SV, Hengst J, Gowda R, Fox TE, Yun JK, Robertson GP. Targeting sphingosine kinase-1 to inhibit melanoma. Pigment Cell Melanoma Res. 2012;25:259–274. doi: 10.1111/j.1755-148X.2012.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elder DJ, Halton DE, Playle LC, Paraskeva C. The MEK/ERK pathway mediates COX-2-selective NSAID-induced apoptosis and induced COX-2 protein expression in colorectal carcinoma cells. Int J Cancer. 2002;99:323–327. doi: 10.1002/ijc.10330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.