Abstract
Purpose
Using real-time electromagnetic (EM) transponder tracking data recorded by the Calypso® 4D Localization System, we report inter- and intrafractional target motion of the prostate bed, describe a strategy to evaluate treatment adequacy in post-prostatectomy patients receiving intensity modulated radiation therapy (IMRT), and propose an adaptive workflow.
Methods and Materials
Tracking data recorded by Calypso EM transponders was analyzed for post-prostatectomy patients that underwent step-and-shoot IMRT. Rigid target motion parameters during beam delivery were calculated from recorded transponder positions in 16 patients with rigid transponder geometry. The delivered doses to the clinical target volume (CTV) were estimated from the planned dose matrix and the target motion for the first 3, 5, 10, and all fractions. Treatment adequacy was determined by comparing the delivered minimum dose (Dmin) with the planned Dmin to the CTV. Treatments were considered adequate if the delivered CTV Dmin is at least 95% of the planned CTV Dmin.
Results
Translational target motion was minimal for all 16 patients (mean: 0.02 cm; range: − 0.12 cm to 0.07 cm). Rotational motion was patient-specific, and maximum pitch, yaw, and roll were 12.2, 4.1, and 10.5 degrees, respectively. We observed inadequate treatments in 5 patients. In these treatments, we observed greater target rotations along with large distances between the CTV centroid and transponder centroid. The treatment adequacy from the initial 10 fractions successfully predicted the overall adequacy in 4 of 5 inadequate treatments and 10 of 11 adequate treatments.
Conclusion
Target rotational motion could cause under-dosage to partial volume of the post-prostatectomy targets. Our adaptive treatment strategy is applicable to post-prostatectomy patients receiving IMRT to evaluate and improve radiation therapy delivery.
Keywords: Adaptive, Radiotherapy, Prostatectomy, Tracking, Intrafraction
INTRODUCTION
Prostate cancer is the most frequently diagnosed cancer and second-leading cause of cancer death for males in the United States (1). Radiation therapy (RT) is an integral therapeutic modality in definitive, adjuvant and salvage treatment of prostate cancer (2). Accurate target localization is essential to ensure adequate radiation dose delivery to the target volume and minimizing toxicity. Common target localization protocols in RT involve patient alignment and target verification with various imaging modalities immediately before radiation dose delivery. However, it is impractical to track the actual target positions at high temporal frequency using X-ray imaging techniques due to added radiation exposure and intrinsic equipment limitations.
Recently, real-time target motion tracking during treatment delivery became available with the Calypso 4D® Localization System (Varian Medical Systems, Palo Alto, CA) (3). The fiducial-based electromagnetic (EM) tracking method has been shown to provide accurate and reliable real-time localization of the prostate (3). In addition, using real-time tracking information, we have developed an algorithm named SWIFTER (4) to determine the translational and rotational target displacement at each tracking instance and to calculate the actual delivered dose to the targets. Using this algorithm, we have recently reported a practical workflow of adaptive radiotherapy (ART) that allows for the evaluation of radiation dose delivery to intact prostate targets (5). SWIFTER was recently implemented within the research version of the Pinnacle3 (Philips Medical Systems, Madison, WI) treatment planning system (TPS) and named Delivered Dose Investigation Tool (DiDIT) (6). DiDIT allows efficient delivered dose estimation within a commercial TPS environment and enables convenient evaluations of treatment adequacy based on the dosimetric effects of actual target motion.
While fiducial-based tracking has been widely implemented for radiation therapy delivery to intact prostates, only a few studies have reported fiducial use in the prostate bed (7–8). The purpose of this study is to characterize the inter- and intra-fraction motion of the transponders implanted in the prostate bed, estimate the delivered dose to the target utilizing the real-time tracked motion, evaluate the correlation between treatment adequacy and target motion, and evaluate the feasibility of an ART workflow for post-prostatectomy radiotherapy. To our knowledge, this is the first investigation on the dosimetric effects of the inter- and intra-fractional motion of post-prostatectomy targets.
METHODS AND MATERIALS
Patient characteristics and RT treatments
Under an institutional review board approved protocol, the data of sixteen consecutive patients treated with adjuvant and salvage intensity modulated radiation therapy (IMRT) following prostatectomy using the Calypso 4D® Localization System between May 2010 and July 2011 were used in this study. Patients with non-rigid fiducial geometry and treatments of the whole pelvis were not included. The clinical characteristics of the 16 patients are listed in Table 1. Most patients had high-risk disease (56%), followed by low-risk disease (31%) and intermediate-risk disease (13%).
Table 1.
Patient characteristics (n=16)
| Age: | |
| Range | 46–72 |
| Median | 61 |
| Tumor stage: | |
| cT1c | 1 (6%) |
| cT2a | 1 (6%) |
| cT2c | 6 (38%) |
| cT3a | 5 (31%) |
| cT3b | 3 (19%) |
| Gleason score | |
| 6 | 6 (38%) |
| 7 | 9 (56%) |
| 8 | 1 (6%) |
| PSA, median (range) ng/ml | |
| Pre-op | 5.58 (3.26 – 10.80) |
| Pre-XRT | 0.15 (0.01 – 0.90) |
| RT intent | |
| Adjuvant | 5 (31%) |
| Salvage | 11 (69%) |
Calypso transponder implantation, simulation and treatment planning
All patients had magnetic resonance imaging (MRI) scans prior to transponder implantation. For each patient, three Calypso transponders were implanted under ultrasound guidance into the prostate bed. The transponder positions grossly corresponded to the apex, left base and right base of an intact prostate. Patients then underwent computed tomography (CT) simulation according to in-house protocols (9). Following simulation, the images were transferred to the Pinnacle3 (v9.0) TPS for image fusion, contour delineation, and treatment plan optimization. All 16 patients were prescribed with 6,480 cGy (36 fractions) or 6,660 cGy (37 fractions) to the prostate bed, which was contoured as CTV on CT/MRI fused images according to the Radiation Therapy Oncology Group (RTOG0534) consensus guidelines (10). The planning target volume (PTV) was generated with a 5 mm isotropic expansion from the CTV.
Treatment delivery
Patients were treated with IMRT using a step-and-shoot technique. Five to nine evenly distributed co-planar 18 MV photon beams were delivered by a Varian 2100EX (Varian Medical Systems, Palo Alto, CA) linear accelerator equipped with a Millenium120 multi-leaf collimator (Varian Medical Systems, Palo Alto, CA). Real-time EM tracking was used for all patients; details have been described in our previous article (9). Briefly, the patients were initially setup by skin markers and fine adjusted until the transponder centroid was close to the isocenter (<3 mm in any direction). If a patient had more than 5 consecutive fractions with a rotation >15° in any axis during localization, he was re-simulated and his treatment plan was re-evaluated and adapted if needed. During beam delivery, the transponder centroid position was continuously monitored and if the displacement in any direction was greater than 3 mm relative to the setup position, the beam was turned off until the target moved back within limits or the required readjustment was completed.
Evaluation of rigidity
Our method for evaluation of rigidity is described in detail in another article (11). Briefly, inter-transponder distances were calculated from the tracked transponder positions; and the transponder geometry was considered rigid if the inter-transponder distance variations were less than the cutoff value (the greater of 0.5 cm and 20% of the planned distance) for all three transponder pairs (11). All 16 patients selected for this study had rigid transponder geometry during therapy.
Eccentricity
Due to the arbitrary nature of the implanted transponder positions relative to the prostate bed, the eccentricity, defined as the distance from the CTV centroid to the transponder centroid, was used to describe the geometric relationship between the CTV volume and the implanted transponders.
SWIFTER algorithm
In an earlier work, we had developed a MATLAB-based program called SWIFTER (4) to estimate the delivered dose to CTV. Based on the real-time motion profile, a probability density function (PDF) of the target positions throughout the treatment beam delivery periods was calculated. The delivered dose to each volume element (voxel) within the target was determined by convolving the target position PDFs and the planned dose distribution.
Workflow of DiDIT
Implementation of SWIFTER in the research Pinnacle3 TPS as DiDIT and the general workflow have been reported in another article (6). Here we describe the workflow of the dose estimation using DiDIT for the 16 post-prostatectomy patients investigated in this work. A schematic representation of the workflow is shown in figure 1. The details of the steps are:
Individual transponder data is exported from the Calypso 4D Localization System using a research tool; and converted into 9 columns of data (.txt file) corresponding to the x, y, and z coordinates of transponder number 1, 2, and 3, respectively, at each tracking instance.
The .txt file is copied to the Research Pinnacle3 workstation.
The patient’s treatment plan is transferred to the Research Pinnacle3 using the file archived in the clinical Pinnacle3 TPS, and the dose is recomputed in the research Pinnacle3 using the same beams and machine commissioning data as in the clinical Pinnacle3 system.
The DiDIT script reads the re-computed original planned dose and uses it as a time-invariant dose cloud for the delivered dose calculation.
DiDIT calculates the target position PDFs based on data in the .txt file, including rotation, and convolves the PDFs with the original planned dose matrix, and generates a new Pinnacle3 “trial” with updated dose in the target volume.
In addition, DiDIT also saves the estimated translational and rotational motion of the target at each tracking instance as a .txt file for further analyses if needed.
Figure 1.
The schematic workflow of the Delivered Dose Investigation Tool (DiDIT) implemented in the commercial Pinnacle3 treatment planning system. The processes inside the dashed rectangle are performed in the Pinnacle3 workstation.
The blocks inside the dashed rectangle represent processes built into and executed within the Research Pinnacle3 workstation. DiDIT is the implementation of the SWIFTER algorithm in a commercial TPS, there is no difference in the method of delivered dose calculation.
Motion analysis and evaluation of treatment adequacy
The magnitude of the target translational and rotational motion at each tracking instance was readily available after the DiDIT tool calculated the delivered dose. The ratio of delivered minimal dose to the CTV (CTV Dmin) over planned CTV Dmin was used to determine treatment adequacy. For post-prostatectomy radiotherapy, the treatment is considered adequate when the delivered Dmin is at least 95% of the planned Dmin for the CTV. In addition, the minimal dose received by 95% of CTV (CTV D95) is also evaluated in order to confirm that only a very small volume of the target is under-dosed in inadequate treatments.
Correlation of treatment adequacy to target motion
Student’s t-test was performed to determine whether the treatment adequacy is correlated to the target translational motion, mean pitch, yaw, and roll, the sum of mean pitch, yaw, roll, the maximum of mean pitch, yaw, and roll, the eccentricity, and the CTV volume.
RESULTS
Rigidity
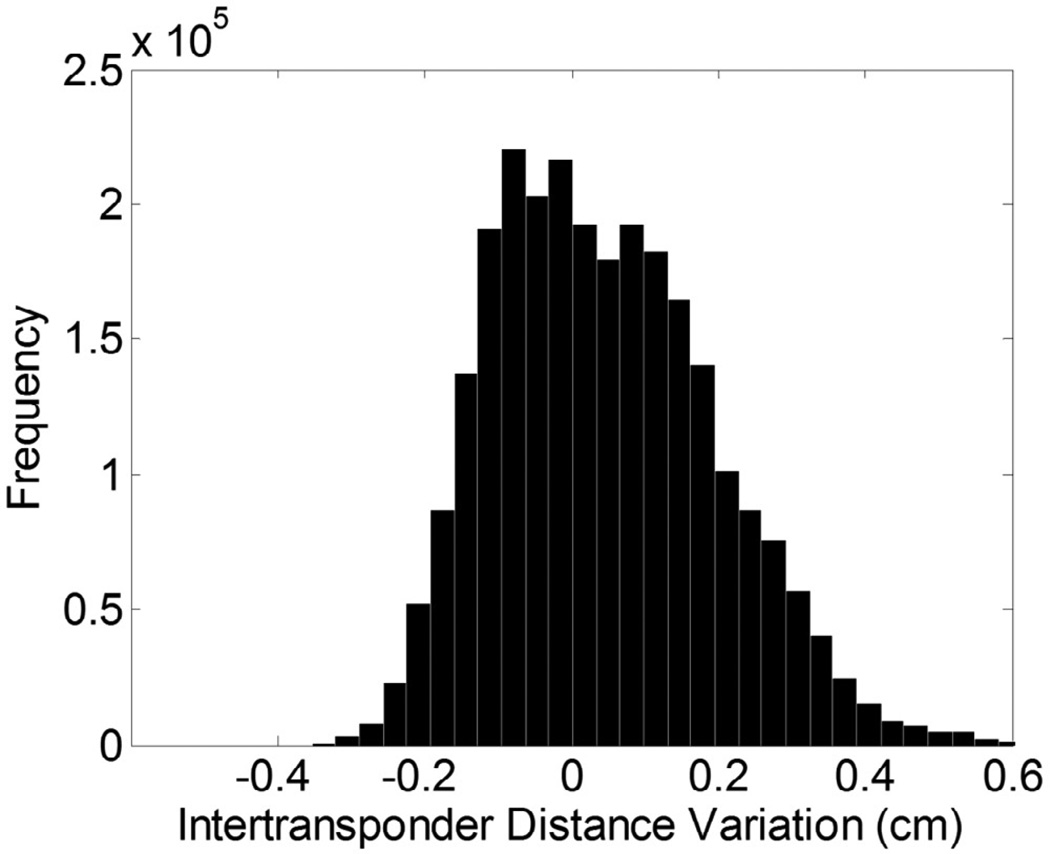
The histogram of inter-transponder distance variation throughout all treatment fractions for all 16 patients is plotted in figure 2. The overall inter-transponder-distance variation was small (0.05 ± 0.15 cm).
Figure 2.
Histogram of the inter-transponder distance variation compared with planned distances for all treatment session of all 16 patients. The overall variation was 0.05 ± 0.15 cm.
Translational and rotational motion
Figure 3(a) and (b) show the amount of translational displacements and rotational motion of the targets during all the treatment fractions. All patients had target mean translational motion less than 1.2 mm with standard deviations less than 1.0 mm in the lateral, anterior-posterior, and superior-inferior directions. Rotational motion, on the other hand, varied greatly among patients. The maximum mean rotations were 12.2, 4.1, and 10.5 degrees in the pitch, yaw, and roll directions, respectively; and for the majority of the patients, the pitch was greater than yaw and roll.
Figure 3.
(a) Mean translational displacement and standard deviation in left-right (LR), anterior-posterior (AP), and superior-inferior (SI) directions. (b) Mean magnitude of rotation and the standard deviation in pitch, yaw, and roll directions. (c) Eccentricity of all 16 patients. (d) The delivered to planned Dmin ratio of after all treatment sessions. Rectangles indicate patients with inadequate treatment to the CTV in terms of Dmin.
Eccentricity
Figure 3(c) shows the values of the eccentricity measured from images acquired during simulations. The eccentricity was less than 1 cm for 5 patients, between 1 and 2 cm for 6 patients, and larger than 2 cm for the remainder 5 patients. The largest value was 3.4 cm.
Treatment adequacy: Dmin and D95 of CTV
The delivered Dmin and D95 of CTV, based on motion of initial 3, 5, and 10 and all treatment fractions, were compared with the planned values, and the ratios are tabulated in Table 2. Although 5 patients’ treatments were inadequate when gauged by the Dmin to CTV, the under-dosed volume was small as evaluated by the D95 to CTV. Figure 3 (d) shows Dmin ratio based on all treatment fractions for the 16 patients; points above the dashed line indicate the patients’ treatment were adequate in terms of Dmin. The 5 red rectangles indicate the patients with inadequate treatments.
Table 2.
Treatment adequacy by comparing the delivered and planned minimum dose (Dmin) to the clinic target volume (CTV) and the minimal dose received by the highest 95% of CTV (D95 of CTV) based on the target motions tracked during the first 3, 5, 10, and all treatment fractions. The treatment is considered adequate when the delivered Dmin is at least 95% of the planned Dmin of the CTV. Bold indicates inadequate treatment.
| Patient Number |
Dmin ratio (Dmin, deliver/Dmin, plan) | D95 ratio (D95deliver/D95plan) | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 fx | 5 fx | 10 fx | All fx | 3 fx | 5 fx | 10 fx | All fx | |
| 1 | 1.00 | 1.00 | 1.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.00 | 1.00 | 1.00 | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 |
| 3 | 0.98 | 0.96 | 0.94 | 0.97 | 1.00 | 1.00 | 1.00 | 1.00 |
| 4 | 0.95 | 0.96 | 0.96 | 0.98 | 1.00 | 1.00 | 1.00 | 1.00 |
| 5 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6 | 0.98 | 0.99 | 0.97 | 0.93 | 1.00 | 1.00 | 1.00 | 1.00 |
| 7 | 0.89 | 0.91 | 0.98 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 8 | 0.94 | 0.96 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 9 | 0.88 | 0.92 | 0.94 | 0.93 | 1.00 | 1.00 | 1.00 | 1.00 |
| 10 | 0.90 | 0.90 | 0.90 | 0.84 | 1.00 | 1.00 | 1.00 | 0.99 |
| 11 | 0.75 | 0.70 | 0.73 | 0.79 | 0.99 | 0.99 | 0.99 | 1.00 |
| 12 | 1.00 | 1.00 | 1.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13 | 0.93 | 0.95 | 0.99 | 0.96 | 1.00 | 1.00 | 1.01 | 1.01 |
| 14 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 15 | 1.00 | 0.96 | 0.98 | 0.98 | 1.01 | 1.01 | 1.01 | 1.01 |
| 16 | 0.90 | 0.86 | 0.87 | 0.86 | 1.00 | 1.00 | 1.00 | 1.00 |
Dose effects of target motion
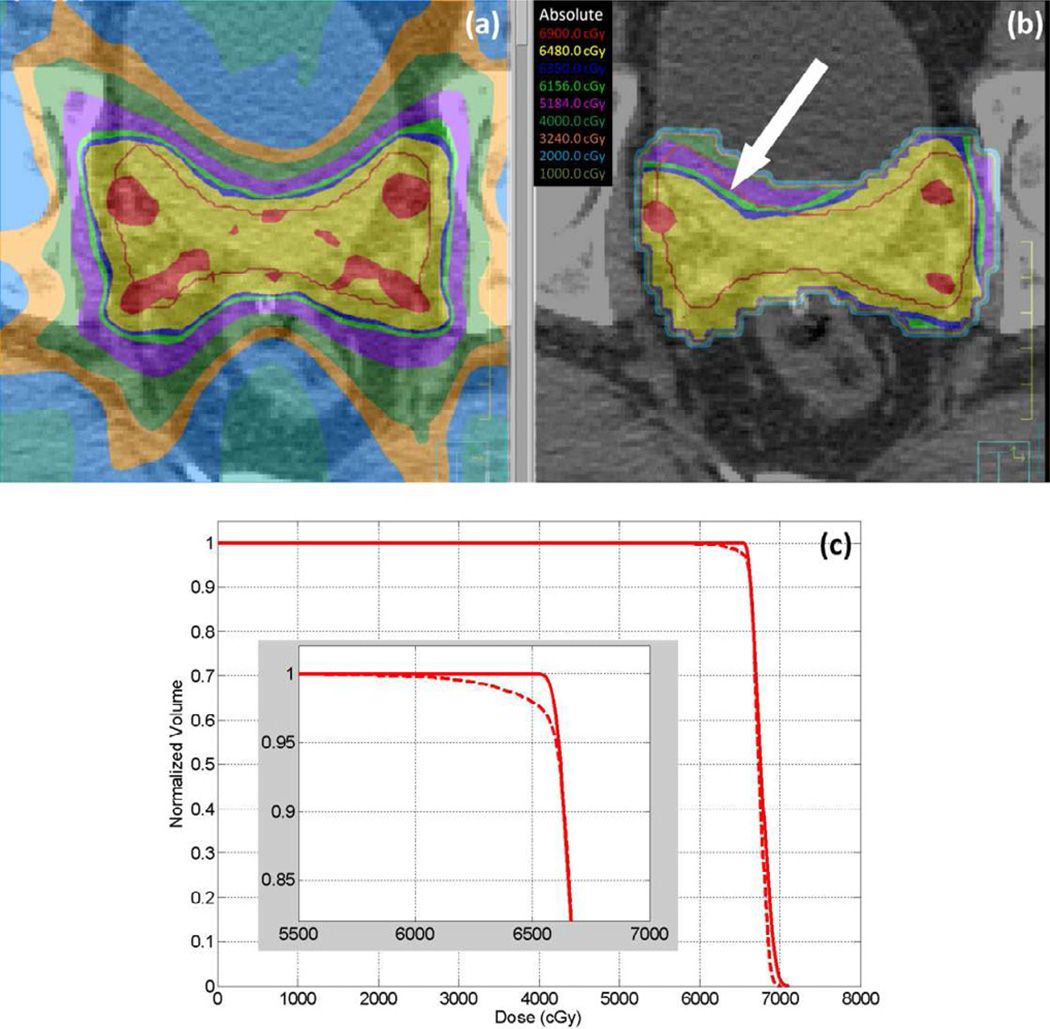
Delivered doses were calculated for all patients using the motion data tracked during the initial 3, 5, and 10 and all treatment fractions. An example of the planned and delivered dose comparison being evaluated in the TPS is shown in figure 4(a) and (b). The red line represents the contour of the CTV in the selected slice, and a cold spot in the estimated delivered dose is pointed out by a white arrow. The dose-volume histogram is displayed in figure 4(c) for the same patient.
Figure 4.
The planned (a) and estimated delivered (b) dose to the target for patient 11 overlaying on the CT images. The white arrow in (b) indicates a cold spot. The DVH (c) of the planned (solid) and delivered (dashed) dose to CTV shows that only a small percentage of the CTV volume is under-dosed.
Correlation of treatment adequacy to target motion
The treatment adequacy is correlated to the mean target pitch (p=0.0097), the maximum of mean pitch, yaw, and roll (p=0.0006), and the sum of mean pitch, yaw, and roll (p=0.0053). There was not statistically significant correlation between treatment adequacy and the translational motion, the eccentricity, or the CTV volume.
DISCUSSION
For post-prostatectomy targets, large set-up errors and inter-fraction motion were observed using 3D ultrasound, weekly CT, electronic portal imaging device, cone beam CT (CBCT), and orthogonal on-board imaging (7, 12–16), however, there is not report on the dosimetric impact of target motion. Although multiple systems are being developed for continuous localization of radiation therapy targets (3, 17–19). widespread clinical implementation has thus far been limited to implanted EM transponders. In this paper, we report the utility of this information to determine target coverage using a novel TPS tool. We have shown that even with careful setup and continuous localization, there may be a potential underdosage of the CTV, which may be a concern clinically depending on the margins physicians used to contour the CTV.
For all patients in this study, target translational displacements were small since clinical practice was to hold the beam when displacement exceeded 3 mm. However, rotational motions can be substantial. Therefore, rotation motion is an important factor to consider when evaluating the treatment adequacy of post-prostatectomy patients. Furthermore, eccentricity can be critical in evaluation of the delivered dose. As pointed out by Olsen, et al., (5) motion magnitude of a specific voxel in target volume is affected by angle of the estimated target rotation and its distance from the point of rotation (which is the transponder centroid). With a large eccentricity, a portion of CTV can move out of the radiation field even for small angles of rotation and can therefore result in a cold spot.
The 5 inadequate treatments are related to a large rotational motion (patient #6, 9, 10 and 16) or a large eccentricity with some rotational motion (patient 11). For the 5 patients with large eccentricity (number 1, 3, 13, 14, and 15) and adequate treatments, the rotational motions were all relatively small. The other 6 patients with adequate treatment (patient 2, 4, 5, 7, 8, and 12) had both small eccentricity (less than 1.2 cm) and rotational motion (within −6.3 to +5.0 degrees). We demonstrated that there is correlation between the treatment adequacy and the magnitude of the target rotational motion, but not the eccentricity. Possible reason is that the lever-arm effect of eccentricity is critical only with sufficient rotation. We were not able to draw a cut-off rotation based on this preliminary data.
In our study, judging from the Dmin criterion of 0.95, overall treatments were adequate for 11 patients and inadequate for the remaining 5 patients. The probability of correctly predicting treatment adequacy from the initial 10 fractions was 87.5% (14 out of 16). For inadequate treatments, 4 out of 5 (80.0%) cases were predicted by the adequacy of the first 10 fractions. For 11 adequate treatments, 10 (90.9%) were consistent with the first 10 fraction predicting adequacy. The high sensitivity (80%) and specificity (90.9 %) indicate that accurate prediction of the final treatment adequacy from the tracking record of the initial fractions is probable though continuing investigation with more patients is required for confirmation. It is worth mentioning that for the two failed predictions (patient 3 and 6), the difference between the Dmin ratio for 10 fractions and overall treatment were small (0.94 vs. 0.97 and 0.97 vs. 0.93).
Based on the results demonstrated in this work, we propose the following adaptive treatment planning workflow for post-prostatectomy patients undergoing EBRT. After the first 10 fractions of treatment, the transponder geometry should be evaluated using tracked motion data. For patients with rigid transponder geometry, the delivered dose to the target is estimated and treatment adequacy is evaluated. If initial treatments are adequate, then it is possible to reduce the CTV-PTV margin to further spare surrounding tissues provided that the initial treatment is still adequate with reduced margin (5). If initial treatments are inadequate, an expansion of margin might be necessary to ensure complete tumor coverage. The benefit of this adaptive radiation therapy workflow is two-fold. For patients with small target motion, CTV-PTV margin can be shrunk to reduce dose delivered to adjacent normal tissue and minimize toxicity, especially rectal morbidities. For patients with large target motion, margins can be expanded to ensure complete coverage.
Because the DiDIT application is readily incorporated in the Pinnacle3 TPS, our ART workflow requires minimal extra effort. The only input to the DiDIT application is the tracked motion data as a .txt file, which is usually generated within 5–10 minutes. The DiDIT calculation requires about 5–10 minutes depending on the target volume, motion pattern, and the number of fractions. In our opinion, the 10–20 minutes extra effort is worth the potential benefit of improved overall treatment quality with reduced toxicity and higher disease-free survival rate.
There are some shortcomings in our study. First, the EM fiducials are used as surrogates for the prostate bed and the rigid transponder geometry may not necessary correspond to a rigid prostate bed. We did not have correlative imaging, and it is not clear that the prostate bed moves as a rigid structure. Unfortunately, in clinical practice, the delivered dose is always to a rigid structure defined in 3D space. Future studies with inter- and intra-fraction using MRI (20) may determine if the rigidity of the fiducial geometry correlates well with rigidity of the prostate bed.
CONCLUSION
We used EM fiducials as surrogates to monitor real-time motion of prostate bed in 16 post-prostatectomy patients receiving IMRT. Our results indicate that a subset of patients has large eccentricity and significant rotational motion of the clinical target volume, resulting in inadequate treatments with CTV Dmin less than 0.95. To predict the overall treatment adequacy from the initial 10 fractions, the sensitivity and specificity were 80.0% and 90.9%, respectively. Our ART workflow may identify patients at risk of receiving inadequate treatment.
SUMMARY.
Real-time electromagnetic fiducials can be used as surrogate of tumor motion for patients receiving post-prostatectomy radiotherapy. Inter- and intra-fractional prostatic-bed motions of 16 patients were assessed. Translational target motions are small for all patients, while rotational motions can be substantially large. The minimum dose (Dmin) delivered to the clinical-target-volume (CTV) was inadequate for 5 of the 16 patients. The overall CTV Dmin adequacy could be predicted based on the initial ten fractions of treatment.
Acknowledgments
CONFLICT OF INTEREST
Work supported by NCI R01CA134541, Philips Healthcare. Shyam Bharat is an employee of Philips Research North America, and was involved in the implementation of DiDIT workflow in the research Pinnacle system. Parag Parikh receives research funding from NIH/NCI, Philips Healthcare, Varian Medical and Calypso Medical. Parag Parikh, Shyam Bharat and Mingyao Zhu have pending patents related to this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures 2011. 2011 Available at: http://wwwcancerorg/Research/CancerFactsFigures/CancerFactsFigures/cancer-factsfigures-2011;
- 2.Kwok Y, Yovino S. Update on radiation-based therapies for prostate cancer. Current Opinion in Oncology. 2010;22:257–262. doi: 10.1097/CCO.0b013e3283378c84. [DOI] [PubMed] [Google Scholar]
- 3.Willoughby TR, Kupelian PA, Pouliot J, et al. Target localization and real-time tracking using the Calypso 4D localization system in patients with localized prostate cancer. Int J Radiation Oncology Biol Phys. 2006;65:528–534. doi: 10.1016/j.ijrobp.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 4.Noel CE, Santanam L, Olsen JR, et al. An automated method for adaptive radiation therapy for prostate cancer patients using continuous fiducial-based tracking. Phys Med Biol. 2010;55:65. doi: 10.1088/0031-9155/55/1/005. [DOI] [PubMed] [Google Scholar]
- 5.Olsen JR, Noel CE, Baker K, et al. Practical Method of Adaptive Radiotherapy for Prostate Cancer Using Real-Time Electromagnetic Tracking. Int J Radiation Oncology Biol Phys. 2012;82:1903–1911. doi: 10.1016/j.ijrobp.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharat S, Parikh P, Noel C, et al. Motion-compensated estimation of delivered dose during external beam radiation therapy; Implementation in Philips' Pinnacle3 treatment planning system. Med Phys. 2012;39:437–443. doi: 10.1118/1.3670374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffner DC, Gottschalk AR, Lometti M, et al. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiation Oncology Biol Phys. 2007;67:610–619. doi: 10.1016/j.ijrobp.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 8.Noel CE, Higham-Kessler J, Olsen J, et al. Feasibility of Fiducial-based Electromagnetic Tracking for Postoperative Radiotherapy to the Prostate Bed. Int J Radiation Oncology Biol Phys. 2010;78:S668–S669. [Google Scholar]
- 9.Olsen JR, Parikh PJ. Image Guided Radiation Therapy. 1st ed. New York: McGraw-Hill; 2011. Calypso real-time localization and tracking for treatment of prostate cancer with external beam radiotherapy; pp. 407–410. [Google Scholar]
- 10.Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG Consensus Guidelines for the Definition of the Clinical Target Volume for Postoperative Conformal Radiation Therapy for Prostate Cancer. Int J Radiation Oncology Biol Phys. 2010;76:361–368. doi: 10.1016/j.ijrobp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu M, Hou W-H, Gay HA, et al. Electromagnetic Tracking Shows Deformation of the Prostate Bed (ROB-D-12-00824) Int J Radiation Oncology Biol Phys. submitted; [Google Scholar]
- 12.Chinnaiyan P, Tome W, Patel R, et al. 3D-Ultrasound Guided Radiation Therapy in the Post-Prostatectomy Setting. Technology in Cancer Research & Treatment. 2003;2:455–458. doi: 10.1177/153303460300200511. [DOI] [PubMed] [Google Scholar]
- 13.Paskalev K, Feigenberg S, Jacob R, et al. Target localization for post-prostatectomy patients using CT and ultrasound image guidance. J Appl Clinc Med Phys. 2005;6:40–49. doi: 10.1120/jacmp.v6i4.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorino C, Foppiano F, Franzone P, et al. Rectal and bladder motion during conformal radiotherapy after radical prostatectomy. Radiotherapy and Oncology. 2005;74:187–195. doi: 10.1016/j.radonc.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu A, Sethi R, Rice R, et al. Prostate bed localization with image-guided approach using on-board imaging: Reporting acute toxicity and implications for radiation therapy planning following prostatectomy. Radiotherapy and Oncology. 2008;88:20–25. doi: 10.1016/j.radonc.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Ost P, De Meerleer G, De Gersem W, et al. Analysis of Prostate Bed Motion Using Daily Cone-Beam Computed Tomography During Postprostatectomy Radiotherapy. Int J Radiation Oncology Biol Phys. 2011;79:188–194. doi: 10.1016/j.ijrobp.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 17.Shirato H, Shimizu S, Shimizu T, et al. Real-time tumour-tracking radiotherapy. The Lancet. 1999;353:1331–1332. doi: 10.1016/S0140-6736(99)00700-X. [DOI] [PubMed] [Google Scholar]
- 18.Kindblom J, Ekelund-Olvenmark A-M, Syren H, et al. High precision transponder localization using a novel electromagnetic positioning system in patients with localized prostate cancer. Radiotherapy and Oncology. 2009;90:307–311. doi: 10.1016/j.radonc.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Shchory T, Schifter D, Lichtman R, et al. Tracking Accuracy of a Real-Time Fiducial Tracking System for Patient Positioning and Monitoring in Radiation Therapy. Int J Radiation Oncology Biol Phys. 2010;78:1227–1234. doi: 10.1016/j.ijrobp.2010.01.067. [DOI] [PubMed] [Google Scholar]
- 20.Lagendijk JJW, Raaymakers BW, Raaijmakers AJE, et al. MRI/linac integration. Radiotherapy and Oncology. 2008;86:25–29. doi: 10.1016/j.radonc.2007.10.034. [DOI] [PubMed] [Google Scholar]