Abstract
Alopecia areata (AA) is an autoimmune disease that attacks anagen hair follicles. Gene array in graft-induced C3H/HeJ mice revealed that genes involved in retinoic acid (RA) synthesis were increased, while RA degradation genes were decreased in AA compared to sham controls. This was confirmed by immunohistochemistry in biopsies from patients with AA and both mouse and rat AA models. RA levels were also increased in C3H/HeJ mice with AA. C3H/HeJ mice were fed a purified diet containing one of four levels of dietary vitamin A or an unpurified diet two weeks before grafting and disease progression followed. High vitamin A accelerated AA, while mice fed no vitamin A had more severe disease by the end of the study. More hair follicles were in anagen in mice fed high vitamin A. Both the number and localization of granzyme B positive cells were altered by vitamin A. IFNG was also lowest and IL13 highest in mice fed high vitamin A. Other cytokines were reduced and chemokines increased as the disease progressed, but no additional effects of vitamin A were seen. Combined, these results suggest that vitamin A regulates both the hair cycle and immune response to alter the progression of AA.
Introduction
Alopecia areata (AA) is an autoimmune, non-scarring, hair loss disease affecting up to 1.7 % of humans (Safavi et al., 1995). Current treatments are often ineffective in inducing prolonged remission (Harries et al., 2010; Tosti and Duque-Estrada, 2009). AA is characterized by a loss of hair follicle immune privilege, increased interferon gamma (IFNG) and other T helper 1 (Th1) cytokines, and an increase in CD8+ T cells (Gilhar et al., 2005; King et al., 2008; McElwee et al., 1996). NK or NKT cells and T regulatory (Tregs) cells may also be involved (Ito et al., 2008; McElwee et al., 2005; Petukhova et al., 2010). Studies suggest AA is a complex polygenetic disease (Petukhova et al., 2010; Sundberg et al., 2004). Little is known about environmental factors, such as diet, that impact the course of AA.
Several interactions between retinoids and immunity exist (Duriancik et al., 2010). Vitamin A deficiency impairs the development of cell-mediated immunity (Smith et al., 1987), promotes Th1 responses, while delaying Th2 development (Cantorna et al., 1994). Recently, additional T cell subtypes were appreciated as important in autoimmunity, including Th17 and T regulatory (Tregs) cells, which are both regulated by vitamin A to maintain gut immune tolerance (Sojka et al., 2008; Stockinger et al., 2007). Retinoic acid (RA) synthesis occurs in dendritic cells (DCs) in the gut (Iwata et al., 2004), which increases FOXP3 positive Tregs (Coombes et al., 2007), and inhibits Th17 cell development (Mucida et al., 2007) via RA receptor alpha (RARA) in vitro (Schambach et al., 2007). Inducing in vivo endogenous RA synthesis through activation of toll like receptor 2 (TLR2, Manicassamy et al., 2009) or PPARG agonist (Housley et al., 2009) inhibited Th17 cells and increased FOXP3. Exogenous RA inhibited Th17 cells but had no effect on FOXP3 (Xiao et al., 2008), suggesting RA needs to be induced in a precise location endogenously to maintain gut immune tolerance. Similar to the gut, skin has a major barrier function. DCs in the dermis have similar characteristics to those in the gut, including langerin expression (Bursch et al., 2007). Mouse ear dermal DCs had aldehyde dehydrogenase activity and induced FOXP3 positive Tregs in a RAR dependent manner although it was not confirmed these cells also expressed langerin or retinal dehydrogenase 2 (ALDH1A2, (Guilliams et al., 2010). Collectively, the results from these studies suggest RA synthesis within the gut and skin DCs regulates immune tolerance.
Excess RA leads to alopecia (Ries and Hess, 1999; Ruzicka et al., 1992; Shih et al., 2009), which may result from many factors including dysregulated immune function. Vitamin A deficiency leads to follicular hyperkeratosis and rupture in humans and rodents (Girard et al., 2006; Wolbach and Howe, 1925). In rodents, vitamin A deficiency also leads to a thin hair coat that is frequently seen, but rarely reported (Anzano et al., 1979) (unpublished observation; Everts and Berdanier, 2002). The results from these studies suggest precise RA levels are needed for optimal hair follicle function.
RA synthesis occurs locally in or near the cells where it will ultimately be used. Precise spatial and temporal levels of RA in the skin are achieved by regulating several key steps in cellular vitamin A metabolism: storage as retinyl esters, RA synthesis, and RA degradation (Everts, 2012). Briefly, vitamin A circulates as retinol bound to retinol binding protein (RBP4). Retinol is transported into the cell via stimulated by RA 6 (STRA6) and binds cellular retinol binding protein 1 (RBP1, aka CRBP). This bound retinol can either be esterified by lecithin:retinol acyltransferase (LRAT) for storage, or reversibly oxidized to retinal via retinol dehydrogenases, such as dehydrogenase reductase SDR family member 9 (DHRS9) or RDH10. Retinal is further oxidized to RA by retinal dehydrogenases 1–3 (ALDH1A1-3). RA is then sent to the nucleus with the assistance of cellular RA binding protein 2 (CRABP2) to bind its RA receptors alpha, beta, and gamma (RARA, B, C) and activate the transcription of 500+ genes (Balmer and Blomhoff, 2002). When RBP1/CRBP is saturated or absent, retinol can be esterified by acyl CoA: diacylglycerol acyltransferase 1 (DGAT1) or cleared by conversion to retinal by alcohol dehydrogenases 1–4 (ADH1-4). Retinal is then oxidized to RA via ALDH1A1 and further metabolized by cytochrome P450 26 family members (CYP26A1, B1, C1) with the assistance of cellular RA binding protein 1 (CRABP1).
To better understand AA, transcriptome analysis of C3H/HeJ mice with AA was performed. Since retinoid metabolism was not part of the network analysis software, the expression of retinoid metabolism genes was examined with the hypothesis that retinoic acid metabolism was not altered in AA. Transcripts coding for proteins metabolizing retinoids were altered, which was confirmed in biopsies from AA patients and rodent AA models. High dietary vitamin A accelerated disease progression and numbers of hair follicles in anagen. Lack of vitamin A resulted in a more severe disease. A few immune factors were also altered by diet, suggesting that retinoids alter AA by regulating both the hair cycle and immune response.
Results
The capacity for RA synthesis was increased in AA
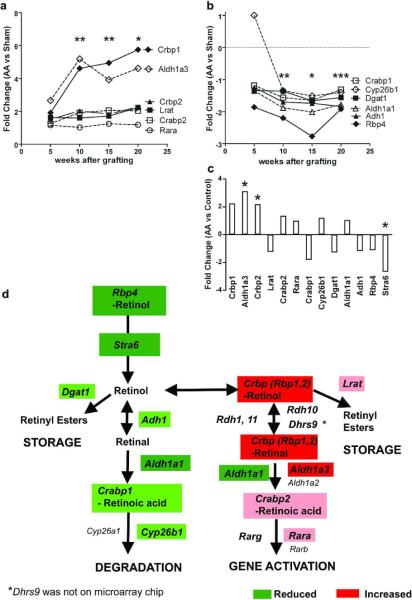
Analysis of graft-induced AA transcripts revealed expression of most genes involved in RA synthesis were significantly increased (Fig.1a), while expression of Rbp4 and RA degradation genes were significantly decreased (Fig.1b) in AA compared to sham controls at 10, 15, and sometimes 20 weeks after grafting. Only Rbp1 (Crbp1), Crabp2, and Aldh1a3 transcripts were significantly increased and Stra6 significantly decreased in mice with spontaneous AA compared to wild type C3H/HeJ mice (Fig.1c,d).
Figure 1. Retinoid metabolism is altered in alopecia areata.
Fold changes (FC) in RA synthesis (a) and retinol degradation (b) proteins determined by microarray analysis between C3H/HeJ mice grafted with alopecia areata skin compared to sham controls 5, 10, 15, and 20 weeks after surgery, or between mice with spontaneous disease compared to unaffected mice (c). *p < 0.05, q < 0.05 all genes, ** p < 0.05, q < 0.05 all genes except Lrat, Rara, and Cyp26b1, *** p < 0.05, q < 0.05 Only Rbp4, Adh1, and Dgat1. d) Diagram of retinoid metabolism with genes significantly altered highlighted. Red, FC > + 3; Pink, FC 0 to +3; Dark green, FC < − 2; Light green, FC 0 to −2.
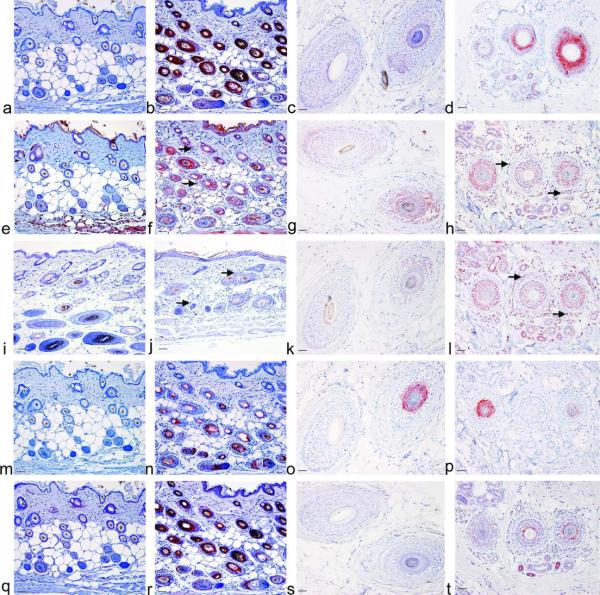
Because differences in the hair cycle between the AA mice and controls were found (Fig.S2) and RA synthesis components changed during the hair cycle (Everts et al., 2007) these gene array results were confirmed using immunohistochemistry on human, DEBR rat, and C3H/HeJ mouse skin, with or without AA, using antibodies against specific RA synthesis and degradation proteins to better control for hair cycle changes. RBP1/CRBP had the greatest increase in immunoreactivity (IR) in biopsies of patients with AA and both rodent models (Fig.2a–d, S3a,b). RBP1/CRBP was also high in CBA/CaHN-Btkxnl/J, SWR/J, and A/J mice with AA, but not C3H/HeOuJ mice with AA (Fig.S4). DHRS9 and CRABP2 were also increased in mice but only slightly increased in AA patients and DEBR rats (Fig.2e–h,q–t, and Fig.S3c,d,g,h). ALDH1A1 and ALDH1A2 were increased in biopsies from humans, but not different in rodent models (Fig.2i–l, and Fig.S6a–d). ALDH1A3 was greatly increased in mice, absent from the premedulla in DEBR rats and not different in biopsies from humans, although no premedulla was present in human samples (Fig.2m–p and Fig.S3e,f arrow). IR of CYP26B1 was not different between C3H/HeJ mice with AA or controls, but localization changed during the hair cycle (data not shown). DHRS9, ALDH1A1, and ALDH1A2 also localized to infiltrating immune cells in biopsies from human patients with AA and C3H/HeJ mice (Fig.2f,h,j,l, and Fig.S6a–d arrow). In DEBR rats, immune cells expressed DHRS9 but not ALDH1A1 or ALDH1A2 (Fig.S3d arrow, data not shown). ALDH1A2 co-localized with dendritic cell (DC) markers langerin and natural killer group 2D (NKG2D; Fig.S6e,f arrow).
Figure 2. Retinoic acid synthesis enzymes and binding proteins are increased in alopecia areata.
Immunohistochemistry (IHC) was performed on C3H/HeJ mouse skin collected 10 weeks after grafting with sham controls (a, e, i, m, q), Alopecia Areata (b, f, j, n, r), biopsies from human patients with tinea capitis controls (c, g, k, o, s) or alopecia areata (d, h, l, p, t) with antibodies against CRBP/RBP1 (a–d), DHRS9 (e–h), ALDH1A1 (i–l), ALDH1A3 (m–p), and CRABP2 (q–t). Bar = 50 μm. Arrow, positive immune cells.
Retinoid levels were measured by LC/MS/MS and HPLC to confirm this expression pattern. RA levels were significantly greater (p<0.05), while retinol levels were lower (p=0.061) in AA mice verses controls (Fig.S7a, b). There was no difference in retinyl ester levels (Fig.S7c).
Dietary vitamin A altered the progression of AA
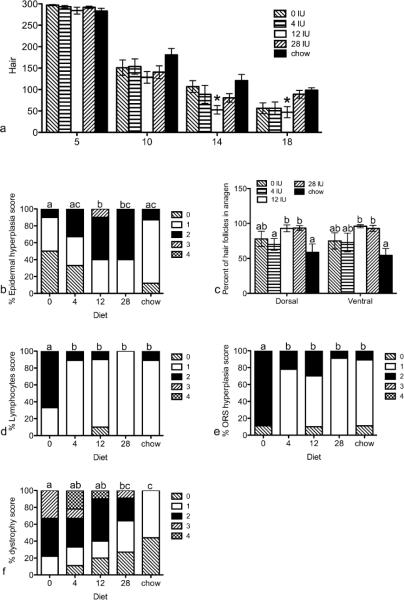
To determine if dietary vitamin A altered AA, C3H/HeJ mice were fed one of five diets, starting 2 weeks before receiving a graft from a mouse with AA, and the progression of the disease was analyzed. Ventral hair loss was significantly increased at 13, 14, and 18 weeks post grafting in mice fed high vitamin A (12 IU, VAH) compared to mice fed unpurified chow (control, Fig.3a). There was also a trend at 14 weeks with mice fed VAH diet having more hair loss than mice fed the vitamin A deficient diet (VAD, p = 0.055). Fifteen weeks after grafting, mice fed VAH diet had significantly more epidermal hyperplasia in ventral skin compared to mice fed VAD or adequate vitamin A (4 IU, VAA), or control diets (Fig.3b). Mice fed excess vitamin A (28 IU, VAE) also had significantly more epidermal hyperplasia in ventral skin than mice fed VAD diet. The percent of hair follicles in mid-anagen through mid-catagen, when active disease is seen, was also greater in mice fed VAH and VAE diets compared to the control diet in both ventral and dorsal skin (Fig.3c). In the dorsal skin these findings were also significantly greater than mice fed VAA diet. By contrast, at 20 weeks post grafting, mice fed VAD diet had significantly more lymphocytes and outer root sheath hyperplasia in ventral skin than all other diets (Fig.3d,e). Follicular dystrophy was significantly greater in mice fed VAD, VAA, and VAH diets compared to mice fed control diet (Fig.3f). Follicular dystrophy was also significantly greater in mice fed VAD compared to mice fed VAE diets. Similar results were seen in dorsal skin with mice fed VAD diet having significantly more follicular dystrophy than mice fed VAH and VAE diets (p < 0.05, data not shown). Mice fed VAA diet also had more follicular dystrophy than mice fed VAH diet (p < 0.05, data not shown).
Figure 3. Progression of AA was altered by dietary vitamin A.
Mice were fed purified diets containing 0, 4, 12, or 28 IU dietary vitamin A/g diet, or a control chow diet starting two weeks prior to grafting and sacrificed 5, 10, 15 (b, c), or 20 (d, e, f) weeks post grafting. Ventral hair loss (a) was measured weekly with 300 representing a full coat of hair. Data are shown as mean ±SEM. H&E slides were scored by JP Sundberg on a scale of 0–4. Data are shown as percent of each score for epidermal hyperplasia (b), anagen (c), lymphocytes (d), outer root sheath hyperplasia (e), and follicular dystrophy (f). n=8–11. *=significantly different from chow fed mice, p<0.05, different letters are significantly different, p<0.05.
There were no differences in body weight between any of the groups (Fig.S8a). Mice fed the control diet ate more food than any of the other diets (p < 0.001), while mice fed VAH diet ate significantly less than mice fed VAD, VAE, and control diets (p < 0.05; Fig.S8b). Both food intake and body weight increased with time as expected, yet neither was significantly different between the mice receiving the AA verse sham grafts (Fig.S8a,b).
Dietary vitamin A altered the number and localization of granzyme B (GZMB) positive cells
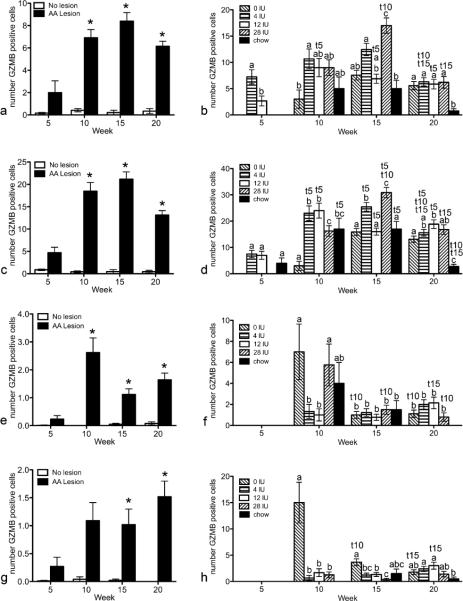
To determine how dietary vitamin A altered activated immune cells in AA, IHC of GZMB was performed and positive cells counted in the bulb, superbular, isthmus, and infundibulum of staged hair follicles. GZMB was significantly greater in AA mice versus shams (Fig.4a,c,e,g). Further statistical analysis was performed with only AA mice with hair follicles in mid-anagen thru mid-catagen, when disease is seen. GZMB was greatest in mice fed VAE diet 15 weeks post grafting in and around the isthmus (Fig.4d). GZMB peaked at 10 weeks in and around the isthmus for mice fed VAH diet, yet was high at both 10 and 15 weeks for mice fed VAA and control diets. In contrast, mice fed VAD diet had a different localization pattern with significantly more positive cells in and around the bulb at 10 and 15 weeks than mice fed the other diets (Fig.4h). At 10 weeks most of the positive cells were in and around the bulb and superbulbar, but by week 15 these mice had higher numbers of cells in and around the isthmus (Fig.4d,f,h).
Figure 4. Granzyme B (GZMB) is significantly increased in mice with AA and altered by vitamin A.
IHC was performed and GZMB positive cells enumerated in the infundibulum (a, b), isthmus (c, d), suprabulbar (e, f), and bulb (g, h). Mann Whitney tests were performed to compare mice with AA lesions to mice without lesions (a, c, e, g; n=40–50). Generalized linear model with the Poisson response function was then performed on mice that received the AA graft with hair follicles in mid-anagen thru midcatagen (b, d, f, h; n=1–10). Data are shown as mean ± SE. * p<0.05 vs no lesion; different letters within a time point are significantly different p<0.05; t5, t10, t15 significantly different from 5, 10, and 15 weeks respectively p<0.05.
High vitamin A increased IL13 and reduced IFNG
Cytokines are differentially regulated in AA (Carroll et al., 2002; Deeths et al., 2006; Freyschmidt-Paul et al., 2006; McPhee et al., 2012). ELISAs were performed to determine if Th1, Th2, or Th17 cytokine protein levels were differentially regulated by diet in mice. There were a few diet differences. Mice fed VAH diet had significantly more IL13 than control chow fed mice, but there were no differences between the AA and sham controls (Fig.5a). Levels of IFNG varied at different times with different diets with low IFNG seen in mice fed VAH diet at 15 weeks and mice fed VAE diet at 20 weeks (Fig.5b).
Figure 5. Alterations in protein levels of cytokines by high vitamin A during AA progression in C3H/HeJ mice.
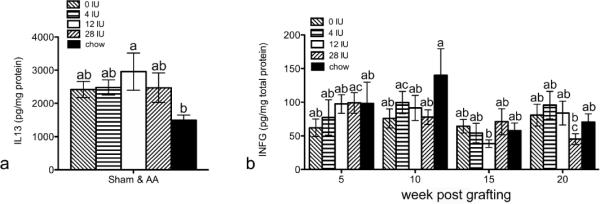
Protein levels of cytokines IL13 (a) and IFNG (b, c) were determined by ELISA from dorsal lumbar skin. Data were natural log (ln) transformed and analysis of variance performed using SPSS, v19. Data are shown as mean ±SE. n=63–65 for a, and n=16–19 for b. different letters are significantly different p<0.05.
Cytokine production and PDL1 was reduced while CXCL9, and CCL5 increased in AA
Protein levels of IL4, IL10, IL22, and IFNG were elevated in AA mice verses sham mice at 5 weeks, but this was only significant for IL4 (Fig.S9a–c, f). All cytokines progressively decreased in the AA mice and rose in the sham mice with time after grafting. By 20 weeks post grafting IL10, IFNG, IL17, IL21, and IL22 were significantly decreased in mice who received the AA graft compared to sham controls (Fig.S9a–f). Two factors essential for tolerance, CTLA4 and PDL1 (CD274), were also tested in the skin by ELISA and IHC, repectively. CTLA4 was significantly lower by 20 weeks, but not significantly different between AA and sham mice (Fig.S9g). In contrast, PDL1 (CD274) was significantly lower in mice who received the AA graft vs sham graft 15 and 20 weeks post grafting (Fig.S9h).
Quantitative real-time PCR (QPCR) analysis revealed that Ifng mRNA levels were significantly increased in AA mice 15 weeks post grafting compared to sham controls, although no diet effect was seen (Fig.S10).
To better select additional immune factors to analyze, an ELISArray (Qiagen, Frederick, MD) was performed based on results from a gene microarray and QPCR arrays (McPhee et al., 2012), ELISArray data not shown). CCL5 (RANTES) and CXCL9 (MIG) were significantly increased in the skin of mice receiving AA grafts compared to sham controls at 10, 15, and 20 weeks post grafting (Fig.S11a, b). Neither chemokine was altered by dietary vitamin A. Data for the control fed mice were previously published (McPhee et al., 2012).
Discussion
This report shows that expression of retinoid synthesis enzymes and binding proteins are increased in human patients with AA and in two rodent models. DCs and NKG2D positive cells also contained RA synthesis enzymes. RA levels were also increased in C3H/HeJ mice with AA. Feeding high levels of dietary vitamin A combined with increased RA synthesis accelerated the onset of AA. Yet feeding no vitamin A resulted in more severe disease by the end of the study. This duality of responses suggests precise vitamin A levels are needed. Analysis of immune factors showed dietary vitamin A altered GZMB, IL13, and IFNG, and provided additional findings on AA pathogenesis. Regulation of the hair cycle may have also contributed to the accelerated disease.
This report shows increased retinoic acid synthesis in AA. To our knowledge any studies of RA alterations in AA are previously unreported. RA levels were also increased in the skin specific (Krt14-cre recombinase) Scd1tm2Ntam null mice with cicatricial alopecia (CA, (Flowers et al., 2011). Expression of RA synthesis components were also increased in patients and C57BL/6J mice with CA (Everts et al in press). Thus RA synthesis maybe secondary to these diseases. Regardless of how changes occurred, alteration in retinoid metabolism may make patients with AA more sensitive to exogenous retinoids.
Vitamin A toxicity leads to alopecia (Ries and Hess, 1999; Ruzicka et al., 1992; Shih et al., 2009). In mice, excess retinol and all transRA (Dgat1tm2Far null mice) within the basal epidermis and outer root sheath (Krt14 cre) led to progressive cyclical alopecia with accelerated telogen to anagen transition (Shih et al, 2009). This increased anagen induction and alopecia could be reduced in Dgat1tm2Far null mice by severely reducing dietary vitamin A intake. In this study, a moderate 3 fold increase in dietary vitamin A accelerated AA onset, possibly by inducing anagen, the target of AA. This dietary vitamin A level is well within the amount consumed by Americans who take supplements (Park et al, 2008). Thus, one mechanism for the increased AA with high vitamin A may be to regulate the hair cycle.
Vitamin A may also directly regulate the immune response, as RA regulates numerous immune cells (Duriancik et al., 2010). Vitamin A increases Th2 and reduces Th1 cytokines (Hoag et al., 2002; Iwata et al., 2003). The current study found high vitamin A reduced protein levels of the Th1 cytokine IFNG and increased Th2 cytokine IL13 over the control diet. Note that many things are different between these diets besides the vitamin A content. The reduction in IFNG may be important as it was shown to play a key role in the etiology of AA (Freyschmidt-Paul et al., 2005; Freyschmidt-Paul et al., 2006; Gilhar et al, 2005; Nakamura et al, 2008). Several studies reported increased skin Ifng mRNA levels, similar to the current finding (Carroll et al., 2002; McPhee et al, 2012). IFNG is regulated posttranscriptionally (Khabar and Young, 2007), yet few studies have measured IFNG protein levels in AA. Serum IFNG protein levels were increased in patients with AA (Barahmani 2010, Arca 2004). Within the skin of patients with long standing AA IFNG levels were reduced (Deeths et al., 2006). This is consistant with the drop in skin IFNG found in the current study. Collectively, the results from these studies suggest that while IFNG is essential for AA onset, skin IFNG levels may drop as AA progresses. High vitamin A may accelerate AA towards a chronic stage and associated reduced IFNG. Future studies should analyze IFNG protein levels in serum, spleen, and/or lymph nodes.
Vitamin A plays a large role in maintaining gut immune privilege (Duriancik et al., 2010). RA synthesized in DCs increased FOXP3+ Tregs and reduced Th17 cells to maintain mucosal immune tolerance. Increased Th17 cytokine levels are hallmarks of autoimmune disease (Langrish et al., 2005; Nakae et al., 2003; Pelidou et al., 2000). The current report shows that ALDH1A2 localized to dermal DCs, but Th17 cells likely do not contribute to AA in C3H/HeJ mice as cytokine levels were reduced, not elevated. Th17 cytokines levels may have been too reduced for vitamin A to have any further effect. IL10, produced by numerous cell types (reviewed in O'Garra et al., 2008), is an key immunosuppressive cytokine in Treg differentiation (Asseman et al., 1999; Asseman and Powrie, 1998). IL10 can also activate CD8+ T cells (Groux et al., 1998), and IL10 therapy produced variable clinical outcomes (O'Garra et al., 2008). AA frequency is reduced in IL10 deficient mice (Freyschmidt-Paul et al., 2002), suggesting IL10 may activate CD8+ T cells and exacerbate the disease. Vitamin A may regulate IL10, but results are conflicting (Cantorna et al., 1994; Maynard et al., 2009; Stephensen et al., 2004). In the current report, IL10 was highest at 10 weeks, then became significantly reduced as AA progressed but vitamin A had no effects. Since IL10 plays many roles, it may be immunosuppressive in sham mice but proinflammatory in AA mice. The reduction in IL10 at 20 weeks in AA may point to a loss in function or a reduction in the number or Tregs in AA. Other studies (McElwee et al., 2005; Petukhova et al., 2010; Zoller et al., 2002) provide indirect evidence that Tregs are important in AA, as interleukin 2 (IL2) and its receptor IL2A (CD25) were reduced in AA. IL2 is essential in the maintenance and survival of Treg cells (Fontenot et al., 2005). Overall the results of this study suggest that while Th17 cells may not be involved in AA, Tregs possibly are. There was no effect of dietary vitamin A on the immune factors tested but RA synthesis enzymes are localized to DCs. Future studies on AA should examine factors such as FOXP3 and Treg function to further analyze role(s) of vitamin A in Treg responses in AA.
Both CTLA4 and PD1 (CD279) and its ligand PDL1 (CD274) are essential for suppressing T cell responses directly and indirectly via Treg activation and preventing autoimmune diseases (Fife and Bluestone, 2008). PDL1 is expressed in many immune privileged sites. To our knowledge a reduction in PDL1 in AA affected mouse hair follicles is previously unreported. PDL1 reduction later in disease is consistent with compromised immune tolerance (privilege) in AA. In contrast, while CTLA4 decreased with time post grafting in AA affected mouse skin, there were no significant differences between AA and sham mice. These results conflict with gene association studies in humans (John et al., 2011; Petukhova et al., 2010) and functional studies in C3H/HeJ mice (Carroll et al., 2002; McElwee et al., 2002; Sundberg et al., 2011) linking CTLA4 to the pathogenesis of AA. One potential reason for this difference may be the site tested. Skin was examined in this study, but CTLA4 alterations may occur in the spleen and/or lymph nodes and should be examined in future studies in these sites. The PD1 pathway, a second co-stimulatory pathway, should be examined for its role(s) in the pathogenesis of AA.
Increased GZMB expression occurs in numerous autoimmune and skin diseases (Afonina et al., 2010; Boivin et al., 2009). GZMB was localized to the dermis, subcutis, and hair follicle connective tissue sheath in some but not all studies on AA patients (Sato-Kawamura et al., 2003). Small numbers of GZMB positive cells were detected in the dermis of C3H/HeJ mice and were increased by tachykinin 1 (substance P, (Siebenhaar et al., 2007). None of these studies reported the GZMB positive cells localization within the hair follicle. In the current study, GZMB positive cells were detected around, within the hair follicle, and in the isthmus, the site of hair follicle stem cells (Cotsarelis et al., 1990) and immune privilege collapse in AA (Meyer et al., 2008). Most studies only mention GZMB's role in inducing apoptosis and some argue against GZMB having a major role in killing follicular cells in AA. More recent studies reveal additional roles for GZMB in cleaving extracellular matrix (ECM) proteins, autoantigens, and receptors NOTCH1 and FGFR1 (Boivin et al., 2009). These effects could negatively impact the hair follicle in AA by altering both the stucture of the connective tissue sheath and signaling within the hair follicle stem cells and dermal papilla. Breakdown of the ECM can cause cell death due to loss of cell-matrix interactions, allow more immune cells access to follicles and contribute to collapse of hair follicle immune privilege. Thus, altered immunolocalization of GZMB by vitamin A may cause different types of cellular damage at different follicular sites.
The chemokines tested in this study increased consistent with recruitment of immune cells (Flier et al., 2001; Hayashi, 1982; Rathanaswami et al., 1993; Shimokawa et al., 1983). The increase in CCL5 and CXCL9 in C3H/HeJ mice is similar to reported increases in gene and protein expression (Kuwano et al., 2007; Subramanya et al., 2010) in patients with AA. The co-localization of RA synthesis enzymes with NKG2D cells suggests AA may be altered by RA effects on these cells. RA upregulates several NKG2D ligands (Cerwenka et al., 2000; Jinushi et al., 2003; Poggi et al., 2009), which activate NK, NKT, and CD8+ T cells (Mistry and O'Callaghan, 2007), but if and how vitamin A regulates NKG2D and its ligands is unknown.
Initial attempts to treat AA with retinoids gave promising results. Topical tretinoin (0.05%) alone (Much, 1976), or combined with intralesional triamcinolone acetonide (Kubeyinje and Cmathur, 1997) were effective in some patients. The RXR agonist, bexarotene, also induced hair regrowth in some patients (Talpur et al., 2009). None of these studies had more than 42 patients nor were they controlled for spontaneous hair regrowth. In the current study, high vitamin A accelerated the disease arguing against pharmacologic retinoids as an effective treatment for AA. It is also possible the patients who responded to retinoid treatment were marginally vitamin A deficient and treatment corrected this deficency. AA waxes and wanes, therefore the stage of disease at time of treatment may also alter retinoids effectiveness. In addition, studies in the gut suggest exogenous retinoids do not alter Tregs (Xiao et al., 2008), while upregulating RA within DCs did (Housley et al., 2009; Manicassamy et al., 2009) suggests regulating DC RA production may be an effective treatment. This study also highlights the need for precise regulation of RA in AA patients with adequate dietary vitamin A, as excess vitamin A from physicians and commercial sources such as skin and hair care products (Arechalde and Saurat, 2000) and dietary supplements (Park et al., 2008) may be detrimental.
Materials and Methods
All procedures were done with approval by The Jackson Laboratory IACUC for the microarray study, The Ohio State University IACUC for the diet study, and both IACUC boards for the mice used for retinoid analysis. The microarray study was previously reported (McPhee et al., 2012). Tissue arrays were created from archived tissues (McElwee et al., 1999; McElwee et al., 1998b; Sundberg et al., 1994; Sundberg et al., 1995). Biopsies from human patients (n=2) were obtained from archives at Vanderbilt University (Nashville, TN) and Baylor Hair Research and Treatment Center (Dallas, TX, Table S1). The Vanderbilt University Institutional Review Board approved all human work. Rat paraffin blocks of skin with or without AA (n = 3) were provided by Kevin McElwee at the University of British Columbia. IHC and IFA was performed as previously described (Duncan et al., 2009; Everts et al., 2007). Primary antibodies against Langerin (eBiosciences, San Diego, CA), NKG2D, GZMB and PDL1 (CD274; R&D, Minneapolis, MN) were purchased, while the rest were made and validated in Dr. Ong's laboratory (Everts et al., 2007).
In the second study recipient mice (n=10) were fed purified AIN93M diets containing either 0 (VAD), 4 (VAA), 12 (VAH), or 28 (VAE) IU retinyl acetate/g diet (Research Diets Inc., New Brunswick, NJ), or control unpurified diet (chow, Harlen Teklad 7912, Indianapolis, IN) starting two weeks before grafting according to McElwee et al., (1998a). Body weight, food intake, and hair loss were measured (Tang et al., 2004) weekly and samples collected 5, 10, 15, and 20 weeks post grafting. ELISA kits were purchased from R&D Systems (Minneapolis, MN), while the ELISA array was purchased from SABiosciences (Frederick, MD), and analyses performed according to kit instructions. RNA was isolated using the RNAeasy Fibrous tissue kit (Qiagen, Valencia, CA), cDNA made (High Capacity cDNA Archive Kit, Applied Biosystems, Carlsbad, CA), and QPCR performed using a Taqman kit (Applied Biosystems, Carlsbad, CA).
Additional AA and sham mice (n=6) were obtained (JPS) and retinoids analyzed as previously described (Kane et al., 2008a; Kane et al., 2008b).
Data were analyzed using SPSS, v19 (IBM; Armonk, New York) after consultation with the OSU statistical consulting services. Histological scores were analysed using a generalized linear model with the multinomial logistic response function at each time point. See the figure legends for additional analyses.
See Supplemental Data online for more details.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Alopecia Areata Foundation (to HBE, LEK, and JPS) and the National Institutes of Health (AR052009 to HBE, AR041943 to LEK, and AR056635 to JPS). The authors thank Jeni Squiric and Steven Naber from the OSU statistical department for their guidance.
Abbreviations
- RA
retinoic acid
- STRA6
Stimulated by retinoic acid 6
- RBP1 (formerly CRBP)
Cellular retinol binding protein 1
- DHRS9
Dehydrogenase reductase (SDR family) member 9
- ALDH1A1, 2, 3
retinal dehydrogenase1, 2, 3
- CRABP1
Cellular retinoic acid binding protein I
- CRABP2
Cellular retinoic acid binding protein II
- RARA, B, G
retinoic acid receptor alpha, beta, gamma
- Roldhs
retinol dehydrogenases
- Raldhs
retinal dehydrogenases
- IR
immunoreactivity
- VAD
vitamin A deficient
- VAA
vitamin A adequate
- VAH
vitamin A high
- VAE
vitamin A excess. Genes and RNA message expression is italicized, while proteins are not. Proteins from mouse, rat, and human are all capital letters.
References
- Afonina IS, Cullen SP, Martin SJ. Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunological Reviews. 2010;235:105–116. doi: 10.1111/j.0105-2896.2010.00908.x. [DOI] [PubMed] [Google Scholar]
- Anzano MA, Lamb AJ, Olson JA. Growth, appetite, sequence of pathological signs and survival following the induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr. 1979;109:1419–1431. doi: 10.1093/jn/109.8.1419. [DOI] [PubMed] [Google Scholar]
- Arechalde A, Saurat JH. Retinoids: Unapproved uses or indications. Clinics in Dermatology. 2000;18:63–76. doi: 10.1016/s0738-081x(99)00095-4. [DOI] [PubMed] [Google Scholar]
- Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseman C, Powrie F. Interleukin 10 is a growth factor for a population of regulatory T cells. Gut. 1998;42:157–158. doi: 10.1136/gut.42.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- Boivin WA, Cooper DM, Hiebert PR, Granville DJ. Intracellular versus extracellular granzyme B in immunity and disease: challenging the dogma. Laboratory Investigation. 2009;89:1195–1220. doi: 10.1038/labinvest.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch LS, Wang L, Igyarto B, Kissenpfennig A, Malissen B, Kaplan DH, et al. Identification of a novel population of Langerin(+) dendritic cells. J Exp Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, Nashold FE, Hayes CE. In vitamin A deficiency multiple mechanisms establish a regulatory T-helper cell imbalance with excess Th1 and insufficent Th2 function. J Immunol. 1994;152:1515–1522. [PubMed] [Google Scholar]
- Carroll JM, McElwee KJ, King LEJ, Byrne MC, Sundberg JP. Gene array profiling and immunomodulation studies define a cell-mediated immune response underlying the pathogenesis of alopecia areata in a mouse model and humans. J Invest Dermatol. 2002;119:392–402. doi: 10.1046/j.1523-1747.2002.01811.x. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Bakker ABH, McClanahan T, Wagner J, Wu J, Phillips JH, et al. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KRR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103(+) DCs induces Foxp3(+) regulatory T cells via a TGF-beta- and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Deeths MJ, Endrizzi BT, Irvin ML, Steiner LP, Ericson ME, Hordinsky MK. Phenotypic analysis of T-cells in extensive alopecia areata scalp suggests partial tolerance. J Invest Dermatol. 2006;126:366–373. doi: 10.1038/sj.jid.5700054. [DOI] [PubMed] [Google Scholar]
- Duncan FJ, Martin JR, Wulff BC, Stoner GD, Tober KL, Oberyszyn TM, et al. Topical Treatment with Black Raspberry Extract Reduces Cutaneous UVB-Induced Carcinogenesis and Inflammation. Cancer Prev Res. 2009;2:665–672. doi: 10.1158/1940-6207.CAPR-08-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duriancik DM, Lackey DE, Hoag KA. Vitamin A as a Regulator of Antigen Presenting Cells. J Nutr. 2010;140:1395–1399. doi: 10.3945/jn.110.124461. [DOI] [PubMed] [Google Scholar]
- Everts HB. Endogenous retinoids in the hair follicle and sebaceous gland. Biochim Biophys Acta. 2012;1821:222–229. doi: 10.1016/j.bbalip.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts HB, Berdanier CD. Nutrient-gene interactions in mitochondrial function: vitamin A needs are increased in BHE/Cdb rats. IUBMB Life. 2002;53:289–294. doi: 10.1080/15216540213464. [DOI] [PubMed] [Google Scholar]
- Everts HB, Sundberg JP, King LE, Jr., Ong DE. Immunolocalization of enzymes, binding proteins, and receptors sufficient for retinoic acid synthesis and signaling during the hair cycle. J Invest Dermatol. 2007;127:1593–1604. doi: 10.1038/sj.jid.5700753. [DOI] [PubMed] [Google Scholar]
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunological Reviews. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Flowers MT, Paton CM, O'Byrne SM, Schiesser K, Dawson JA, Blaner WS, et al. Metabolic Changes in Skin Caused by Scd1 Deficiency: A Focus on Retinol Metabolism. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nature Immunology. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, McElwee KJ, Happle R, Kissling S, Wenzel E, Sundberg JP, et al. Interleukin-10-deficient mice are less susceptible to the induction of alopecia areata. J Invest Dermatol. 2002;119:980–982. doi: 10.1046/j.1523-1747.2002.00230.x. [DOI] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, Sundberg JP, Kissling S, Hummel S, et al. Reduced expression of interleukin-2 decreases the frequency of alopecia areata onset in C3H/HeJ mice. J Invest Dermatol. 2005;125:945–951. doi: 10.1111/j.0022-202X.2005.23888.x. [DOI] [PubMed] [Google Scholar]
- Freyschmidt-Paul P, McElwee KJ, Hoffmann R, Sundberg JP, Vitacolonna M, Kissling S, et al. Interferon-gamma-deficient mice are resistant to the development of alopecia areata. Br J Dermatol. 2006;155:515–521. doi: 10.1111/j.1365-2133.2006.07377.x. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Kam Y, Assy B, Kalish RS. Alopecia areata induced in C3H/HeJ mice by interferon-gamma: evidence for loss of immune privilege. J Invest Dermatol. 2005;124:288–289. doi: 10.1111/j.0022-202X.2004.23580.x. [DOI] [PubMed] [Google Scholar]
- Girard C, Dereure O, Blatiere V, Guillot B, Bessis D. Vitamin A deficiency phrynoderma associated with chronic giardiasis. Pediatr Dermatol. 2006;23:346–349. doi: 10.1111/j.1525-1470.2006.00261.x. [DOI] [PubMed] [Google Scholar]
- Groux H, Bigler M, de Vries JE, Roncarolo MG. Inhibitory and stimulatory effects of IL-10 on human CD8(+) T cells. J Immunol. 1998;160:3188–3193. [PubMed] [Google Scholar]
- Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, et al. Skin-draining lymph nodes contain dermis-derived CD103(−) dendritic cells that constitutively produce retinoic acid and induce Foxp3(+) regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- Harries MJ, Sun J, Paus R, King LE. Management of alopecia areata. BMJ. 2010;341:c3671. doi: 10.1136/bmj.c3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H. A review on the natural mediators of inflammatory leucotaxis. Acta Pathol Jpn. 1982;32(Suppl 2):271–284. [PubMed] [Google Scholar]
- Hoag KA, Nashold FE, Goverman J, Hayes CE. Retinoic acid enhances the T helper 2 cell development that is essential for robust antibody responses through its action on antigen-presenting cells. J Nutr. 2002;132:3736–3739. doi: 10.1093/jn/132.12.3736. [DOI] [PubMed] [Google Scholar]
- Housley WJ, O'Conor CA, Nichols F, Puddington L, Lingenheld EG, Zhu L, et al. PPAR gamma regulates retinoic acid-mediated DC induction of Tregs. Journal of Leukocyte Biology. 2009;86:293–301. doi: 10.1189/jlb.1208733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Ito N, Saatoff M, Hashizume H, Fukamizu H, Nickoloff BJ, et al. Maintenance of hair follicle immune privilege is linked to prevention of NK cell attack. J Invest Dermatol. 2008;128:1196–1206. doi: 10.1038/sj.jid.5701183. [DOI] [PubMed] [Google Scholar]
- Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress T(h)1 development and enhance T(h)2 development via retinoic acid receptors. International Immunology. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, et al. Expression and role of MICA and MICB in human hepatocellular carcinomas and their regulation by retinoic acid. Int J Cancer. 2003;104:354–361. doi: 10.1002/ijc.10966. [DOI] [PubMed] [Google Scholar]
- John KK, Brockschmidt FF, Redler S, Herold C, Hanneken S, Eigelshoven S, et al. Genetic Variants in CTLA4 Are Strongly Associated with Alopecia Areata. J Invest Dermatol. 2011 doi: 10.1038/jid.2010.427. ePub 2/25/11. [DOI] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Napoli JL. HPLC/UV quantitation of retinal, retinol, and retinyl esters in serum and tissues. Anal Biochem. 2008a;378:71–79. doi: 10.1016/j.ab.2008.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008b;80:1702–1708. doi: 10.1021/ac702030f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabar KSA, Young HA. Post-transcriptional control of the interferon system. Biochimie. 2007;89:761–769. doi: 10.1016/j.biochi.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LE, McElwee KJ, Sundberg JP. Alopecia Areata. In: Nickoloff BJ, Nestle FO, editors. Dermatologic Immunity, Curr Dir Autoimmun. Karger; Basel: 2008. pp. 280–312. [DOI] [PubMed] [Google Scholar]
- Kubeyinje EP, Cmathur M. Topical tretinoin as an adjunctive therapy with intralesional triamcinolone acetonide for alopecia areata. Clinical experience in northern Saudi Arabia. Int J Dermatol. 1997;36:320–320. doi: 10.1111/j.1365-4362.1997.tb03060.x. [DOI] [PubMed] [Google Scholar]
- Kuwano Y, Fujimoto M, Watanabe R, Ishiura N, Nakashima H, Ohno Y, et al. Serum chemokine profiles in patients with alopecia areata. Br J Dermatol. 2007;157:466–473. doi: 10.1111/j.1365-2133.2007.07943.x. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manicassamy S, Ravindran R, Deng JS, Oluoch H, Denning TL, Kasturi SP, et al. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nature Medicine. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT. Contrasting roles for all-trans retinoic acid in TGF-beta-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. J Exp Med. 2009;206:343–357. doi: 10.1084/jem.20080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwee KJ, Boggess D, King LE, Jr., Sundberg JP. Experimental induction of alopecia areata-like hair loss in C3H/HeJ mice using full-thickness skin grafts. J Invest Dermatol. 1998a;111:797–803. doi: 10.1046/j.1523-1747.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Boggess D, Miller J, King LE, Sundberg JP. Spontaneous alopecia areata-like hair loss in one congenic and seven inbred laboratory mouse strains. J Invest Dermatol Symp Proc. 1999;4:202–206. doi: 10.1038/sj.jidsp.5640211. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Boggess D, Olivry T, Oliver RF, Whiting D, Tobin DJ, et al. Comparison of alopecia areata in human and nonhuman mammalian species. Pathobiology. 1998b;66:90–107. doi: 10.1159/000028002. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Freyschmidt-Paul P, Hoffmann R, Kissling S, Hummel S, Vitacolonna M, et al. Transfer of CD8(+) cells induces localized hair loss whereas CD4(+)/CD25(−) cells promote systemic alopecia areata and CD4(+)/CD25(+) cells blockade disease onset in the C3H/HeJ mouse model. J Invest Dermatol. 2005;124:947–957. doi: 10.1111/j.0022-202X.2005.23692.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Hoffmann R, Freyschmidt-Paul P, Wenzel E, Kissling S, Sundberg JP, et al. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4+ and CD8+ cells. J Invest Dermatol. 2002;119:1426–1433. doi: 10.1046/j.1523-1747.2002.19620.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Spiers EM, Oliver RF. In vivo depletion of CD8+ T cells restores hair growth in the DEBR model for alopecia areata. Br J Dermatol. 1996;135:211–217. [PubMed] [Google Scholar]
- McPhee CG, Duncan FJ, Silva KA, King LE, HogenEsch H, Roopenian DC, et al. Increased Expression of Cxcr3 and Its Ligands, Cxcl9 and Cxcl10, during the Development of Alopecia Areata in the Mouse. J Invest Dermatol. 2012;132:1736–1738. doi: 10.1038/jid.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer KC, Klatte JE, Dinh HV, Harries MJ, Reithmayer K, Meyer W, et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br J Dermatol. 2008;159:1077–1085. doi: 10.1111/j.1365-2133.2008.08818.x. [DOI] [PubMed] [Google Scholar]
- Mistry AR, O'Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121:439–447. doi: 10.1111/j.1365-2567.2007.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Much T. Treatment of alopecia areata with vitamin A acid. Zeitschrift fur Hautkrankheiten. 1976;51:993–998. [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal T(H)17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Jo J, Tabata Y, Ishikawa O. Controlled delivery of T-box21 small interfering RNA ameliorates autoimmune alopecia (Alopecia Areata) in a C3H/HeJ mouse model. Am J Pathol. 2008;172:650–658. doi: 10.2353/ajpath.2008.061249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A, Barrat FJ, Castro AG, Vicari A, Hawrylowicz C. Strategies for use of IL-10 or its antagonists in human disease. Immunological Reviews. 2008;223:114–131. doi: 10.1111/j.1600-065X.2008.00635.x. [DOI] [PubMed] [Google Scholar]
- Park SY, Murphy SP, Martin CL, Kolonel LN. Nutrient intake from multivitamin/mineral supplements is similar among users from five ethnic groups: The Multiethnic Cohort Study. J Am Diet Assoc. 2008;108:529–533. doi: 10.1016/j.jada.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Pelidou SH, Zou LP, Deretzi G, Oniding C, Mix E, Zhu J. Enhancement of acute phase and inhibition of chronic phase of experimental autoimmune neuritis in Lewis rats by intranasal administration of recombinant mouse interleukin 17: potential immunoregulatory role. Exp Neurol. 2000;163:165–172. doi: 10.1006/exnr.2000.7357. [DOI] [PubMed] [Google Scholar]
- Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggi A, Catellani S, Garuti A, Pierri I, Gobbi M, Zocchi MR. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-trans-retinoic acid or sodium valproate. Leukemia. 2009;23:641–648. doi: 10.1038/leu.2008.354. [DOI] [PubMed] [Google Scholar]
- Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES and interleukin-8 genes by inflammatory cytokines. J Biol Chem. 1993;268:5834–5839. [PubMed] [Google Scholar]
- Ries G, Hess R. Retinol: Safety considerations for its use in cosmetic products. J Toxicol, Cutaneous Ocul Toxicol. 1999;18:169–185. [Google Scholar]
- Ruzicka T, Sommerburg C, Goerz G, Kind P, Mensing H. Treatment of cutaneous lupus-erythematosus with acitretin and hydroxychloroquine. Br J Dermatol. 1992;127:513–518. doi: 10.1111/j.1365-2133.1992.tb14851.x. [DOI] [PubMed] [Google Scholar]
- Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–633. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- Sato-Kawamura M, Aiba S, Tagami H. Strong expression of CD40, CD54 and HLA-DR antigen and lack of evidence for direct cellular cytotoxicity are unique immunohistopathological features in alopecia areata. Arch Dermatol Res. 2003;294:536–543. doi: 10.1007/s00403-002-0354-7. [DOI] [PubMed] [Google Scholar]
- Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- Shih MYS, Kane MA, Zhou P, Yen CLE, Streeper RS, Napoli JL, et al. Retinol Esterification by DGAT1 Is Essential for Retinoid Homeostasis in Murine Skin. J Biol Chem. 2009;284:4292–4299. doi: 10.1074/jbc.M807503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa Y, Miura K, Hifumi M, Hayashi H. Lymphocyte chemotaxis in inflammation. VI. Lyt phenotype analysis of effector cells responsible for producing murine lymphocyte chemotactic factor. Immunology. 1983;49:95–102. [PMC free article] [PubMed] [Google Scholar]
- Siebenhaar F, Sharov AA, Peters EM, Sharova TY, Syska W, Mardaryev AN, et al. Substance P as an immunomodulatory neuropeptide in a mouse model for autoimmune hair loss (alopecia areata) J Invest Dermatol. 2007;127:1489–1497. doi: 10.1038/sj.jid.5700704. [DOI] [PubMed] [Google Scholar]
- Smith SM, Levy NS, Hayes CE. Impaired immunity in vitamin A deficient mice. J Nutr. 1987;117:857–865. doi: 10.1093/jn/117.5.857. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression a diverse arsenal for a moving target. Immunology. 2008;124:13–22. doi: 10.1111/j.1365-2567.2008.02813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephensen CB, Jiang XW, Freytag T. Vitamin A deficiency increases the in vivo development of IL-10-positive Th2 cells and decreases development of Th1 cells in mice. J Nutr. 2004;134:2660–2666. doi: 10.1093/jn/134.10.2660. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M, Martin B. Th17 T cells: Linking innate and adaptive immunity. Seminars in Immunology. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Subramanya RD, Coda AB, Sinha AA. Transcriptional profiling in alopecia areata defines immune and cell cycle control related genes within disease-specific signatures. Genomics. 2010;96:146–153. doi: 10.1016/j.ygeno.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Cordy WR, King LE., Jr. Alopecia areata in aging C3H/HeJ mice. J Invest Dermatol. 1994;102:847–856. doi: 10.1111/1523-1747.ep12382416. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, McElwee KJ, Carroll JM, King LE. Hypothesis Testing: CTLA4 Co- Stimulatory Pathways Critical in the Pathogenesis of Human and Mouse Alopecia Areata. J Invest Dermatol. 2011;131:2323–2324. doi: 10.1038/jid.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg JP, Oliver RF, McElwee KJ, King LE., Jr. Alopecia areata in humans and other mammalian species. J Invest Dermatol. 1995;104:32S–33S. doi: 10.1038/jid.1995.51. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Silva KA, Li RH, Cox GA, King LE. Adult-onset alopecia areata is a complex polygenic trait in the C3H/HeJ mouse model. J Invest Dermatol. 2004;123:294–297. doi: 10.1111/j.0022-202X.2004.23222.x. [DOI] [PubMed] [Google Scholar]
- Talpur R, Vu J, Bassett R, Stevens V, Duvic M. Phase I/II randomized bilateral half-head comparison of topical bexarotene 1% gel for alopecia areata. J Am Acad Dermatol. 2009;61:592–598. doi: 10.1016/j.jaad.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Tang L, Cao L, Sundberg JP, Lui H, Shapiro J. Restoration of hair growth in mice with an alopecia areata-like disease using topical anthralin. Exp Dermatol. 2004;13:5–10. doi: 10.1111/j.0906-6705.2004.00098.x. [DOI] [PubMed] [Google Scholar]
- Tosti A, Duque-Estrada B. Treatment strategies for alopecia. Expert Opinion on Pharmacotherapy. 2009;10:1017–1026. doi: 10.1517/14656560902876368. [DOI] [PubMed] [Google Scholar]
- Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble A vitamin. J Exp Med. 1925;42:753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, et al. Retinoic acid increases Foxp3(+) regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M, McElwee KJ, Engel P, Hoffmann R. Transient CD44 variant isoform expression and reduction in CD4(+)/CD25(+) regulatory T cells in C3H/HeJ mice with alopecia areata. J Invest Dermatol. 2002;118:983–992. doi: 10.1046/j.1523-1747.2002.01745.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.