Abstract
BACKGROUND
Prior studies have separately examined the effects of dronabinol (oral THC) on cannabis withdrawal, cognitive performance, and the acute effects of smoked cannabis. A single study examining these clinically relevant domains would benefit the continued evaluation of dronabinol as a potential medication for the treatment of cannabis use disorders.
METHODS
Thirteen daily cannabis smokers completed a within-subject crossover study and received 0, 30, 60 and 120 mg dronabinol per day for 5 consecutive days. Vital signs and subjective ratings of cannabis withdrawal, craving and sleep were obtained daily; outcomes under active dose conditions were compared to those obtained under placebo dosing. On the 5th day of medication maintenance, participants completed a comprehensive cognitive performance battery and then smoked 5 puffs of cannabis for subjective effects evaluation. Each dronabinol maintenance period occurred in a counterbalanced order and was separated by 9 days of ad-libitum cannabis use.
RESULTS
Dronabinol dose-dependently attenuated cannabis withdrawal and resulted in few adverse side effects or decrements in cognitive performance. Surprisingly, dronabinol did not alter the subjective effects of smoked cannabis, but cannabis-induced increases in heart rate were attenuated by the 60 and 120 mg doses.
CONCLUSIONS
Dronabinol’s ability to dose-dependently suppress cannabis withdrawal may be therapeutically beneficial to individuals trying to stop cannabis use. The absence of gross cognitive impairment or side effects in this study supports safety of doses up to 120mg per day. Continued evaluation of dronabinol in targeted clinical studies of cannabis treatment, using an expanded range of doses, is warranted.
Keywords: Cannabis, Marijuana, THC, Dronabinol, Withdrawal, Pharmacotherapy
1. INTRODUCTION
Cannabis (marijuana, hashish) is the most widely used illicit drug in the world (UNODC, 2007). Cannabis dependence develops in a subset of users, typically daily or near daily users. Prevalence rates of cannabis dependence vary by region, but generally exceed the rate for dependence on any other illicit drug (AIHW, 2008; EMCDDA, 2008; SAMHSA, 2008; UNODC, 2007). Clinical trials have demonstrated efficacy for several psychosocial interventions, but, to date, there are no medications known to improve clinical outcomes for those seeking treatment for cannabis use disorders (Benyamina et al., 2008; Nordstrom and Levin, 2007; Vandrey and Haney, 2009).
Considering other drug use disorders, one could argue that the most successful pharmacological treatment interventions involve compounds that target the same neurobiological systems as the drug of abuse. Agonist or partial agonist medications can be used to effectively attenuate drug withdrawal symptoms and reduce the rewarding effects of drugs binding to the same receptor systems. Examples include the use of methadone (mu-opioid agonist) or buprenorphine (mu-opioid partial agonist) for the treatment of heroin or prescription opioid use disorders, and nicotine or varenicline (partial agonist of the A4B2 nicotinic acetylcholine receptor) for the treatment of tobacco use disorders (Raupach and van Schayck, 2011; Stotts et al., 2009). Delta-9-tetrahydrocannabinol (THC) is the primary psychoactive constituent of cannabis responsible for the subjective “high” experienced by users (ElSohly, 2005). Dronabinol is an oral formulation of synthetic THC, commercially available to treat symptoms associated with cancer treatments and advanced HIV/AIDS, that has been investigated as a potential pharmacotherapy for treating cannabis use disorders.
Several laboratory studies have been conducted to examine the effects of dronabinol on 1) cannabis withdrawal expression, 2) the acute effects of smoked cannabis, and 3) “relapse” to cannabis use following a period of abstinence. Dronabinol (30 – 90 mg/day) was shown to reliably and dose-dependently suppress cannabis withdrawal symptoms (Budney et al., 2007a; Haney et al., 2008, 2004). Indeed, in one study, 90 mg/day (30 mg tid) reduced subjective ratings of withdrawal to baseline (ad-libitum cannabis use) levels (Budney et al., 2007a). In another study, 80 mg/day, but not 40 mg/day dronabinol attenuated the subjective effects of smoked cannabis (roughly 50% reduction in ratings of “good drug effect”; Hart et al., 2002a). Dronabinol (60 mg/day) combined with adrenergic agonist lofexedine (2.4 mg/day), but not dronabinol alone (40 – 80 mg/day), has reduced cannabis self-administration in laboratory studies (Haney et al., 2008; Hart et al., 2002a). Thus, in controlled laboratory studies, dronabinol has exhibited dose-related effects on withdrawal and acute cannabis use consistent with other medications conferring clinical benefit in the treatment of drug use disorders. That said, it is important to note that each of these laboratory studies was conducted with daily cannabis users who were not seeking treatment or otherwise trying to reduce their cannabis use.
There are currently two published papers describing the use of dronabinol in the context of treating cannabis use disorders. Levin and Kleber (2008) first presented 2 case reports of treatment resistant cannabis users who successfully sustained cannabis abstinence with the assistance of open-label dronabinol. In one case, dronabinol (40 mg/day) was administered for a finite period of time, followed by a successful dose taper. In the other case, a long-term dronabinol maintenance approach was utilized (40–50 mg/day initially with taper to 15–20 mg/day maintenance). More recently, a controlled clinical trial of dronabinol-assisted treatment was completed (Levin et al., 2011). In this study, dronabinol (up to 40 mg/day) reduced subjective ratings of withdrawal and improved treatment retention, but no differences in cannabis use outcomes were observed when compared with placebo.
In summary, there is ample evidence that dronabinol can attenuate cannabis withdrawal severity. Mixed results have been observed regarding the effects of dronabinol on the acute effects of smoked cannabis and cessation during treatment. Thus, while the overall clinical benefit seems somewhat unclear, one consistent finding across studies is that effects are clearly dose dependent. This is important because the maximum dose administered in the lone controlled clinical trial conducted was relatively low compared with doses that had the greatest effect on withdrawal and acute cannabis effects in laboratory studies.
As described by Levin et al. (2011), clinicians are hesitant to administer high doses of dronabinol in a clinical setting due to concerns about intoxication and patient safety and/or acceptability. Though safety-related measures in prior studies suggest little or no cognitive impairment, decreased performance following dronabinol administration has been observed on measures of memory, attention, and psychomotor ability in some studies following doses lower than the 90 mg/day dose that demonstrated the greatest suppression of withdrawal in the outpatient study by Budney and colleagues (Curran et al., 2002; Haney et al., 2004; Hart et al., 2002a; Kamien et al., 1994). Also, 2 of 12 participants experienced significant side effects following 4 daily 30 mg doses of dronabinol in another study (Haney et al., 1999). However, among studies in which dronabinol has been administered chronically, participants were not always heavy/daily cannabis users, and the cognitive performance measures used have not been comprehensively studied with regards to assessments important for daily functioning (psychomotor ability, focused and divided attention, decision-making, problem solving, and multiple domains of memory ability) within the same participants. Thus, additional research is needed to explore the safety, tolerability, and cognitive effects of higher doses of dronabinol among heavy cannabis users to help define an upper limit that balances safety and efficacy for clinical use.
The present study examined the dose effects of dronabinol on cannabis withdrawal, the acute effects of smoked cannabis, cognitive performance, and subjective ratings of side effects among daily cannabis smokers. Based on prior research, we hypothesized that dronabinol would dose-dependently reduce withdrawal severity, the acute effects of smoked cannabis, and cognitive performance on measures associated with memory and divided attention. The study extends prior research by including a larger range of doses than has been studied previously in the same individuals. Also, the combined assessment of outcomes believed to be related to clinical benefit (reduction of withdrawal and smoked cannabis effects) and safety (cognitive performance and side effects) will help inform dosing strategy for clinical programs considering use of dronabinol in the treatment of cannabis use disorders.
2. METHODS
2.1. Participants
Cannabis users were recruited through newspaper advertisements and flyers posted on campus and community bulletin boards. Volunteers were eligible for the study if they: 1) were 18–55 years old; 2) self-reported cannabis use at least 25 days per month for the prior 3 months and provided a urine specimen positive for cannabinoids; 3) reported experiencing withdrawal during periods of cannabis abstinence; 4) had at least an 8th grade level of education and demonstrated literacy; 5) were not taking psychoactive medication; 6) did not meet criteria for Axis I psychiatric disorders (DSM-IV-TR) other than nicotine or cannabis dependence; 7) were not seeking treatment for cannabis-related problems or using cannabis for a medical disorder; 8) had a negative urine toxicology test for drugs other than cannabis (cocaine, opioids, amphetamine, methamphetamine, PCP, benzodiazepines, barbiturates, methadone, and MDMA) and a negative breath alcohol test on the day of study admission; 9) had a negative urine pregnancy test; 10) had no history of seizures, severe hepatic impairment, or conditions associated with clinically significant cognitive impairment.
Study eligibility was determined based on an initial telephone interview followed by screening assessments conducted in the laboratory. Demographic, health and drug history inventories were obtained with locally developed questionnaires. The Time-Line Follow-Back (TLFB) method (Sobell and Sobell, 1992) was used to obtain the amount and frequency of substance use during the previous 3 months. Urine testing for recent drug use and pregnancy was conducted using qualitative rapid tests. The DSM Checklist (Hudziak et al., 1993) was used to diagnose current Axis I psychiatric disorders. An ECG test was conducted to assess cardiovascular health. The Marijuana Quit Questionnaire (Boyd et al., 2005) and Marijuana Withdrawal Checklist (MWC) (Budney et al., 1999) were conducted to obtain a detailed history of cannabis use, quit attempts, and presence of cannabis withdrawal symptoms during prior periods of abstinence.
Written informed consent was obtained prior to study participation. The study was approved by the John Hopkins Medicine IRB and conducted in accordance with the ethical standards of the Helsinki Declaration. Thirteen of 25 enrolled participants completed the study and were included in data analyses. Two were discharged on the day of admission due to ECG abnormalities not detected during the screening ECG test, 3 were discharged for personal conduct violations, 6 volunteers did not complete the study for personal reasons, and 1 study completer was excluded from analysis due to invalid reporting on subjective assessments (e.g., numerous conflicting statements suggesting extremely poor compliance/comprehension of the assessments). Study completers had an average (SD) age of 34 (9) years, 92% were male (12 males/1 female), and 100% were African American. Participants on average (SD) first used cannabis at 14 (2) years of age, had been using cannabis regularly for 18 (9) years, and currently smoked cannabis 4 (2) times per day. Eleven participants met DSM-IV-TR criteria for cannabis dependence. The most common route of cannabis administration was via “blunt,” cannabis rolled in a hollowed out cigar. Use of drugs other than cannabis was infrequent.
2.2. Procedures
A within-subject crossover design was used to allow for a controlled comparison of placebo with 3 doses of dronabinol (30, 60, and 120 mg/day) on cannabis withdrawal expression and severity during cannabis abstinence, side effects and cognitive performance following repeated dronabinol exposure, and subjective and physiological response to the acute effects of smoked cannabis after a period of abstinence. The study lasted 51 days, requiring overnight inpatient housing at the Johns Hopkins Bayview Medical Center (JHBMC). The first 4 days served as a baseline period for acclimation to the laboratory and training on study tasks. This was followed by four 5-day dronabinol maintenance phases. During these phases, dronabinol was administered tid and cannabis use was restricted to a single, controlled administration on the 5th day. The 5-day duration of dronabinol maintenance was selected to capture peak withdrawal effects, which generally occur after 2–4 days of abstinence (Budney et al., 2003), and the emergence of side effects associated with chronic dosing without unnecessarily extending the duration of the study. Each dronabinol maintenance period was separated by a 9-day washout period of ad-libitum cannabis use between 12:00 and 23:00. In prior studies, separating multiple abstinence tests by 9-day, but not 5-day periods of daily cannabis smoking has been sufficient to reliably re-establish chronic exposure levels and support repeated evaluation of withdrawal symptoms without evidence of abstinence condition order effects (Budney et al., 2001, 2007, Vandrey et al., 2008).
A number of subjective assessments were conducted daily. At 9:00, participants completed 3 questionnaires based on their experience the previous day. The MWC assessed cannabis withdrawal symptoms during the prior 24 hours. A sleep diary provided estimates of sleep latency, total sleep, number of nocturnal awakenings, time awake after sleep onset, and 100 pt visual analog scale (VAS) ratings of sleep quality, mood on awakening, and alertness on awakening based on the prior night’s sleep. Participants rated medication side effects experienced during the prior 24 hours on a 4-point scale (none, mild, moderate, severe) based on a list of possible side effects listed in the package insert for dronabinol. In addition to these items, The Marijuana Craving Questionnaire (MCQ; Heishman et al., 2009) and Addiction Research Center Inventory (ARCI); Haertzen and Hickey, 1987) were used to assess participant ratings of acute cannabis craving and drug effects respectively and vital signs were obtained (via automated monitor) at 9:00, 14:00 and 19:00 each day.
2.2a. Acclimation and Training
On Day 1, participants received training on study procedures and smoked cannabis ad-libitum after training until 23:00. On Days 2 and 3, participants were trained on cognitive performance tasks. Each task was repeated until competency was demonstrated and performance was stable on primary outcome variables (within 20% on at least three consecutive trials). After training was completed on these days, ad-libitum smoking was again allowed between the hours of 12:00 and 23:00.
2.2b. Counterbalanced Dronabinol Maintenance Phases
During the dronabinol maintenance phases, oral capsules were administered at 9:00, 14:00 and 19:00 each day and cannabis smoking was restricted to a single exposure on Day 5 (See Section 2.2d). Participants received one of three doses of dronabinol: 30 mg/day (10mg tid), 60 mg/day (20mg tid), 120 mg/day (40mg tid), or placebo in a counterbalanced order. Administration of placebo dronabinol (capsules) during the ad-libitum cannabis phases and placebo cannabis during the medication maintenance phases was intentionally omitted from the study design in order to better model a cannabis quit-attempt as it would occur in a users home environment and to preclude possible placebo smoking effects on withdrawal suppression, as has been noted for denicotinized cigarettes (Buchhalter et al., 2005).
2.2c. Cognitive Performance Testing
On the last day of each dronabinol maintenance phase, participants completed a comprehensive cognitive performance assessment battery starting at 10:00 (60 min after morning dronabinol administration). Performance tasks took approximately 75 min to complete and were always administered in the same order. The timing of these procedures (60–135 min post-administration) coincides with the peak subjective and cognitive performance impairing effects of dronabinol observed in prior studies (Chait and Zacny, 1992; Curran et al., 2002; Kirk and DeWit, 1999). Cognitive performance tasks in this study included (in order of administration): Digit Symbol Substitution Test (DSST), a measure of psychomotor ability (McLeod et al., 1982); a divided attention task, a task in which participants needed to monitor and respond to peripheral stimuli while completing a central tracking task (Kleykamp et al., 2010); a simple reaction time task, a measure of attention and psychomotor speed (locally programmed); a time estimation task, a measure that assesses the participant’s ability to accurately replicate time intervals (5-, 20- and 80-sec) presented to them on a computer (Beardsley and Kelly, 1999); Trails A & B, a computerized measure of psychomotor ability and cognitive flexibility analogous to the paper/pencil Trail Making A & B tasks (Mintzer et al., 1997a; Reitan and Wolfson, 1985); the N-Back Task (0, 1, 2, and 3-Back tasks utilized), a measure of working memory (Gevins and Cutillo, 1993); the Tower of London (TOL), a measure of executive function and planning (Ramaekers et al., 2006; Shallice, 1982); and Word Memory (recognition memory and free recall assessed), a measure of episodic memory and metamemory (Mintzer et al., 1997b).
2.2d. Smoked Cannabis Challenge Sessions
On the last day of each dronabinol maintenance phase, following completion of the cognitive performance assessment, participants were administered 5 controlled puffs of smoked cannabis. The paced puffing procedure included 5-sec inhalations, 10-sec breath holds, and 40-sec inter-puff intervals. This procedure has been shown to reliably elicit dose-sensitive subjective and physiological cannabis effects in prior laboratory research (Hart et al., 2002b). Vital signs and subjective drug effect assessments (15-item VAS drug effect questionnaire) were obtained 5 min before, immediately after the last puff, and 15, 30, 45, and 60 min following completion of the puffing procedure.
2.2e. Cannabis Cigarettes
The cannabis provided during the acclimation period (Days 1–4), the three ad-libitum cannabis-use periods, and the smoked cannabis challenge sessions was obtained from the National Institute on Drug Abuse (NIDA). The cannabis cigarettes contained approximately 5.7% THC by volume and weighed approximately 0.8 grams each. Participants were escorted, upon request, to a specially ventilated room where cannabis smoking occurred.
2.2f. Biological Specimen Collection and Testing
Blood specimens were collected at 9:00, 14:00, 19:00, and 22:00 on the 1st and 5th day of each dronabinol maintenance phase to assess plasma THC and metabolite concentrations following dronabinol and/or smoked cannabis administration. Blood was collected in 6 mL green-top (sodium heparin) Vacutainer tubes and stored on ice no more than 2 hours prior to centrifugation to separate plasma. Specimens were frozen at −20°C until analysis via solid phase extraction with Clean Screen ZSTHC020 columns (United Chemical Technologies) and subsequent two-dimensional gas chromatography-mass spectrometry with cryofocusing (Agilent 6890 GC-FID/5973 MSD; Lowe et al., 2007). Limits of quantification were 0.5 ng/mL for THC and THCCOOH, and 1.0 ng/mL for 11-OH-THC.
3. Data Analysis
Study outcome measures were analyzed using a repeated measures regression with an AR(1) covariance structure. Cannabis withdrawal effects were first assessed by comparing data from Days 1–4 of the placebo maintenance period with the 4 days of ad-libitum cannabis use that immediately preceded it (true baseline). Day 5 of the placebo maintenance period was excluded from this model due to the cannabis administered during the acute cannabis challenge session. The dose effects of dronabinol were then assessed for all measures. For daily assessments (withdrawal, side effects, sleep diary, craving, and ARCI), factors in the analyses included Dose (0, 30, 60, 120 mg/day dronabinol), and Time (Days 1–4 for each dronabinol maintenance period). For assessments obtained during the acute cannabis challenge sessions (VAS drug effect ratings and vitals), factors in the analyses included Dose (0, 30, 60, 120 mg/day dronabinol), and Time (−5, 0, 5, 15, 30, 45, and 60 min relative to cannabis exposure). Cognitive performance was only assessed once at each dose, so only Dose (not Time) was included as a Factor in analysis of cognitive performance outcomes. Planned comparisons (Student-Newman-Kuels (SNK) multiple comparison tests) were conducted to ascertain differences between dronabinol doses when main effects of dose were observed. A correlational analysis was conducted to assess the relationship between plasma levels of psychoactive cannabinoids (THC and 11-OH-THC) and withdrawal symptom severity. Data analysis was completed using SAS statistical software (Version 9.1), and α was set at 0.05 for all tests of significance.
Because there is some overlap of known cannabis withdrawal effects and potential medication side effects (e.g., nausea), these are distinguished in the results based on the pattern of dronabinol dose effects observed. If participant ratings on an item decreased as dronabinol dose increased, it is reported as a cannabis withdrawal effect (based on prior research showing dronabinol to dose-dependently suppress withdrawal). If participant ratings on an item increased as dronabinol dose increased, it is reported as a medication side effect.
3. RESULTS
3.1 Withdrawal
Significant cannabis withdrawal effects (F1,12 from 4.97 to 27.88; p < .05) were observed during the placebo maintenance phase for subject ratings of decreased appetite, diarrhea, nausea, stomach pain, irritability, sleep difficulty, total sleep time, subjective sleep quality, mood at morning awakening, alertness at morning awakening, restlessness, nervousness/anxiety, chills, increased aggression, increased anger, headaches, difficulty concentrating, and total Withdrawal Discomfort Score (WDS).
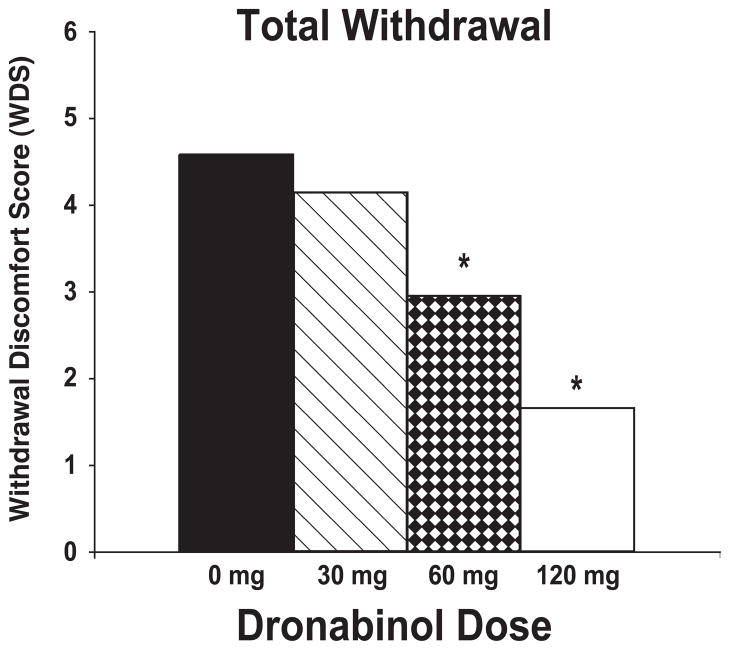
Significant dronabinol dose effects (F3,36 from 2.95 to 9.44; p < .05) were observed for each withdrawal item except “sleep difficulty” and “nervousness/anxiety.” Planned comparisons of individual dose conditions showed that 120 mg/day dronabinol significantly suppressed ratings of stomach pain, irritability, chills, headaches and total withdrawal (WDS) compared with 60 mg/day, 30 mg/day and placebo; ratings of decreased appetite, mood and alertness upon awakening, and increased aggression compared with 30 mg/day and placebo; ratings of subjective sleep quality compared with 30 mg/day; and ratings of diarrhea, nausea, increased anger, and difficulty concentrating compared with placebo. The 60 mg/day dose suppressed ratings of decreased appetite, difficulty concentrating, and WDS compared with 30 mg/day and placebo; ratings of sleep quality and mood upon awakening compared with 30 mg/day; and ratings of diarrhea and increased aggression compared with placebo. The 30 mg/day dose suppressed ratings of nausea and diarrhea compared with placebo. The effect of dronabinol dose on total withdrawal (WDS) is illustrated in Figure 1.
Figure 1.
Mean subjective withdrawal ratings (WDS) presented by dronabinol maintenance dose. Asterisks indicate significant differences between dronabinol dose conditions and placebo.
3.2 Acute Effects of Cannabis
Significant main effects of time were observed for subjective ratings of drug effect (F5,60 = 22.08), good effect (F5,60 = 27.03), heart racing (F5,60 = 3.95), and feeling hungry (F5,60 = 2.97) during the cannabis challenge sessions conducted on the last day of each dronabinol maintenance phase. For each of these items, ratings increased following smoked cannabis administration. However, no effects of dronabinol dose were observed on any subjective drug effect item. Figure 2a shows mean participant ratings of “Drug Effect,” which is representative of the pattern of effects observed for other items in which significant effects of time were found.
Figure 2.
Mean subjective drug effect ratings (Panel A) and heart rate assessments (Panel B) obtained during the cannabis challenge session conducted on the 5th day of each dronabinol maintenance phase. In order to account for pre-cannabis exposure (baseline) effects of oral dronabinol on these measures, data are presented as a change from baseline to better illustrate the relative effects of smoked cannabis.
Significant effects of Dose and Time were observed for heart rate during the cannabis challenge sessions (Figure 2b). The increase in heart rate following smoked cannabis exposure was significantly attenuated by both the 60 and 120 mg/day doses compared with placebo. The 120 mg/day dose also significantly attenuated the increase in heart rate compared with the 30 mg/day dose. Significant main effects of Time, but not Dose were observed for systolic and diastolic blood pressure during the cannabis challenge sessions. On average, blood pressure increased immediately following cannabis administration and then gradually declined over the course of the session, but the total mean change from baseline was relatively small and did not exceed 8 mm/Hg in either direction for any dose.
3.3 Side Effects
Significant effects of Dose were observed for ratings of dry mouth (F3,36 = 6.14), rapid heart rate (F3,36 = 3.19), and flushing in face or body (F3,36 = 6.58) on the side effect questionnaire. Planned dose comparisons indicated that subjective ratings for each of these items were significantly higher during the 120 mg/day dose compared with all three other dose conditions. There were no differences between placebo and the 30 or 60 mg/day doses. A significant effect of Dose was also observed for the Morphine-Benzedrine Group (MBG; euphoria) subscale of the ARCI (F3,36 = 3.19), in which ratings during the 60 and 120 mg/day maintenance phases were greater compared with the placebo and 30 mg/day periods. There was no effect on other ARCI sub-scales, including the Marijuana Scale.
3.4 Cognitive Performance
Significant effects of Dose were observed for the Percentage of Trials Correct on the DSST (F3,36 = 3.41) and Reaction Time for the 1-Back iteration of the N-Back task (F3,36 = 6.09). Planned dose comparisons indicated that DSST responding was less accurate during the 30 mg/day (96%) and 120 mg/day (96%) doses compared with placebo (99%). Latency to respond on the 1-Back task was delayed in the 30 mg/day condition (.733 sec) compared with the placebo (.690 sec) and 60 mg/day (.673 sec) dose conditions, and in the 120 mg/day condition (.715 sec) compared with the 60 mg/day condition.
3.5 Physiological Assessments
Significant effects of Dose were observed for diastolic blood pressure (F3,36 = 4.63) assessed daily during the dronabinol maintenance periods. Mean diastolic blood pressure was 75, 74, 70 and 71 mmHg during placebo, 30, 60 and 120 mg dosing periods, respectively. These values were significantly lower during the 60 mg/day and 120 mg/day study periods compared with the placebo and 30 mg/day periods. No significant dose effects were observed for systolic blood pressure or heart rate. Plasma analysis of THC and its metabolites (11-OH-THC, THCCOOH) demonstrated dose-related biological delivery of oral dronabinol (Figure 3). A modest inverse correlation (r2 = −0.27) was observed between peak total THC and 11-OH-THC (equipotent psychoactive metabolite) plasma concentrations and peak WDS withdrawal scores.
Figure 3.
Mean peak Day 1 plasma concentrations of THC and 11-OH-THC (an equipotent metabolite of THC) as a function of dronabinol maintenance dose.
4. DISCUSSION
The present study extends prior research examining dronabinol as a potential pharmacotherapy for treating cannabis use disorders. Consistent with other experiments, a dose-dependent suppression of cannabis withdrawal was observed when participants were maintained on dronabinol during consecutive days of abrupt cannabis abstinence (Budney et al., 2007b). Cannabis withdrawal has been associated with relapse and substance use severity outcomes among people attempting to quit use of cannabis (Budney et al., 2008; Chung et al., 2008; Cornelius et al., 2008), and two case reports suggest that carefully tailored delivery of dronabinol can contribute to cannabis cessation among those resistant to other treatments (Levin and Kleber, 2008). Thus, there is clear evidence that dronabinol can be used as a palliative tool in the treatment of cannabis use disorders in cases where withdrawal appears to be a barrier to cessation.
That being said, there is some uncertainty regarding the generality and strength of a palliative intervention approach given that dronabinol reduced subjective ratings of withdrawal without a reduction in cannabis use in a recently completed clinical trial (Levin et al., 2011). The difficulty in interpreting these apparently discordant findings is twofold. First, the laboratory studies demonstrating withdrawal suppression were conducted in non-treatment seekers during mandated periods of abstinence. Thus, it is possible there were factors besides dronabinol administration that contributed to the magnitude of withdrawal suppression observed in these studies that does not translate to outpatient clinical populations. Second, though withdrawal ratings were reduced in the dronabinol group compared with the placebo group in the clinical trial, the true magnitude of withdrawal suppression from dronabinol in that study is unknown. There was no pre-treatment assessment of withdrawal symptoms to compare to withdrawal assessed during treatment, and the comparison of dronabinol versus placebo on withdrawal included all study participants rather than being limited to those who actually abstained from cannabis. Thus, additional research is needed to examine the magnitude of withdrawal suppression that can be achieved as a function of dronabinol dose in a clinical setting, and to further explore the effect of withdrawal suppression versus other participant characteristics on cannabis cessation rates among those in treatment.
Aside from withdrawal suppression, another possible clinical benefit derived from agonist pharmacotherapies is an attenuation of the acute effects of drug use. For example, the ability of varenicline to attenuate the subjective and rewarding effects of smoking is believed to be instrumental in the improved initial abstinence success and relapse prevention rates observed among smokers using the medication during a quit attempt (McClure et al., 2012; Patterson et al., 2009; Perkins et al., 2010; West et al., 2008). In a prior study, Hart et al. (Hart et al., 2002a) showed that treatment with 80 mg/day dronabinol (but not 40 mg/day) for 3 days reduced the subjective effects (good drug effect, high) of smoked cannabis. However, in the present study, dronabinol maintenance for 5 days at doses of up to 120 mg/day did not alter the subjective effects of smoked cannabis, although it did attenuate the increase in heart rate following smoked cannabis exposure. Both studies were conducted on a residential unit with heavy, daily cannabis users who were mostly male African Americans. The primary procedural difference between the studies is that acute cannabis exposure occurred daily in the Hart et al. study, but only once after 5 days of abstinence in the present study. Thus, it is possible that dronabinol maintenance could reduce the acute positive reinforcing effects of ongoing smoked cannabis use, but not drug effects experienced initially after a period of sustained cannabis abstinence. This would suggest that dronabinol maintenance prior to a programmed quit attempt could enhance initial abstinence success in a treatment-seeking population, but is unlikely to alter relapse rates among those who lapse during a quit attempt. However, the mechanism for a differential effect of dronabinol on smoked cannabis effects in relation to periods of abstinence is uncertain and this effect needs further examination.
Perhaps the most important outcome from the present study was that safety and tolerability was demonstrated for high doses of chronic dronabinol administration within the context of also evaluating clinically relevant outcomes (withdrawal, acute effects of smoked cannabis). This is consistent with the research of Jones and colleagues who have previously demonstrated safe and tolerable administration of daily dronabinol up to 210 mg/day (Jones et al., 1976). Side effects in the present study were infrequent and low in magnitude, there were no concerning cardiovascular effects, nor was there substantial impairment of cognitive abilities observed in the present study. The cognitive performance assessment battery was conducted 60–135 min after dronabinol administration in order to assess performance at the expected peak level of intoxication. While statistical significance was reached for 2 of 37 cognitive performance outcomes, the true magnitude of change for these outcomes was small, and, in general, cognitive outcomes indicated high levels of performance throughout the study. The FDA recommends initial acute dosing of 2.5 – 5mg for clinical management of appetite loss and emesis in the general population. The tolerability and absence of serious side effects with much higher doses in this study suggests substantial levels of tolerance to THC among daily cannabis users, and may also reflect the fact that THC acts as a partial agonist with relatively flat dose-effect functions at high doses (Pertwee, 2008). Indeed, many volunteers in the present study verbally reported in daily interactions with study investigators a complete lack of subjective drug effects from oral medication throughout study participation.
There are some key aspects of this study that are important to note for those who may consider dronabinol as a clinical intervention for cannabis use disorders based on the present data. First, participants in this study represent the extreme end of cannabis use, typically smoking cannabis multiple times throughout the day. This is important because the safety and tolerability profile of high doses of dronabinol observed in this study may not extend to people with less frequent cannabis use patterns, or to heavy cannabis users who have already achieved a period of sustained abstinence. Second, there was no behavioral cannabis use outcome included in the present study (e.g., self-administration) due to practical limitations of the complexity and duration of the protocol. The ultimate benchmark for clinical utility of a pharmacotherapy for treating substance use disorders is a demonstration of reduced substance use following use of the medication under investigation. Third, even within this homogeneous sample of heavy cannabis users, the severity of cannabis withdrawal observed varied considerably. Some participants reported no withdrawal while others experienced severe disruption of their mood, sleep, appetite, and in some cases emesis. Withdrawal symptom expression can be influenced by subjective expectancy, the duration of cessation, and environmental cues. The present study did not control for an effect of subjective expectancy by administering placebo cannabis during the medication maintenance phases, and environmental influences were minimized because the study was conducted in a residential laboratory. Thus, withdrawal expression in this study may be an overestimate of what is due to pharmacological factors alone, but may be an underestimate of withdrawal that may occur amongst people trying to quit indefinitely in their home environment. It is also the case that the study design limited our ability to discern the specific effects of THC versus other non-THC components of cannabis on withdrawal suppression. Because the most clear clinical benefit of dronabinol appears to be withdrawal symptom suppression, a clinical approach in which dronabinol is used to alleviate demonstrated withdrawal discomfort is recommended rather than use of dronabinol as a first-line therapy.
In summary, this study adds to prior work showing a reliable suppression of cannabis withdrawal with dronabinol, and demonstrates that chronic administration of high doses of dronabinol (up to 120 mg/day) can be well tolerated and result in few adverse effects in heavy, daily cannabis users. The failure to observe a change in the subjective effects of acutely administered smoked cannabis as a function of dronabinol dose following a period of supervised abstinence suggests that any clinical benefits of dronabinol in treating cannabis use disorders are likely limited to improving rates of initial abstinence (via withdrawal suppression and/or attenuating effects of smoked cannabis prior to cessation). These data suggest that dronabinol remains a viable candidate pharmacotherapy for the treatment of cannabis use disorders, particularly cases in which severe withdrawal discomfort appears to be a barrier to cessation. However, additional evaluation of dronabinol is needed to support this argument. In particular, studies are needed to examine safety following longer periods of high dose dronabinol maintenance, effects of dronabinol on cannabis self-administration and the ability of dronabinol to improve substance use outcomes among patients in clinical settings who exhibit significant withdrawal, especially under conditions when dronabinol dose can be adjusted flexibly to maximize patient response.
Acknowledgments
Role of Funding Source. This research was supported by grant R01 DA025044 from the National Institute on Drug Abuse and funding from the National Institutes of Health, Intramural Research Program, National Institute on Drug Abuse. The study design; collection, analysis and interpretation of data; writing of the report; and decision to submit the paper for publication were all completed at the sole discretion of the authors with no role of any funding agencies. This study was registered on clinicaltrials.gov, identifier NCT00893074.
The authors wish to thank Erin Curran, Elizabeth Girling, Linda Felch, the nursing, recruiting, and medical staff of the Behavioral Pharmacology Research Unit (BPRU), and the Chemistry and Drug Metabolism laboratory at the NIDA Intramural Research Program for their efforts and dedication in helping complete this project.
Footnotes
Contributors. All authors contributed to the design and execution of the study, analysis and interpretation of data, and preparation of the written manuscript. All authors have approved the final manuscript.
Conflict of Interest. The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AIHW. Alcohol and Other Drug Treatment Services in Australian Capital Territory 2006–07: Findings from the National Minimum Data Set. Australian Institute of Health and Welfare; Sydney: 2008. [Google Scholar]
- Beardsley PM, Kelly TH. Acute effects of cannabis on human behavior and central nervous system functions. In: Kalant H, Corrigall WA, Hall W, Smart RG, editors. The Health Effects of Cannabis. Centre for Addiction and Mental Health; Toronto: 1999. pp. 127–170. [Google Scholar]
- Benyamina A, Lecacheux M, Blecha L, Reynaud M, Lukasiewcz M. Current state of phamacotherapy and psychotherapy in cannabis withdrawal and dependence. Exp Rev Neurother. 2008;8:479–491. doi: 10.1586/14737175.8.3.479. [DOI] [PubMed] [Google Scholar]
- Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Strategies for quitting among non-treatment-seeking marijuana users. Am J Addict. 2005;14:35–42. doi: 10.1080/10550490590899835. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Acosta MC, Evans SE, Breland AB, Eissenberg T. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy P, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Novy PL. Marijuana abstinence effects in marijuana smokers maintained in their home environment. Arch Gen Psychiatry. 2001;58:917–24. doi: 10.1001/archpsyc.58.10.917. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta-9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–29. doi: 10.1016/j.drugalcdep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta-9-THC and smoked marijuana in humans. Psychopharmacology. 1992;107:255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1-year follow-up among treated adolescents. Addiction. 2008;103:787–799. doi: 10.1111/j.1360-0443.2008.02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius JR, Chung T, Martin C, Wood DS, Clark DB. Cannabis withdrawal is common among treatment-seeking adolescents with cannabis dependence and major depression, and is associated with rapid relapse to dependence. Addict Behav. 2008;33:1500–1505. doi: 10.1016/j.addbeh.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral delta-9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology. 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- deFonseca FR, Carrera MR, Navarro M, Koob GF, Weiss F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997;276:2050–2054. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- Diana M, Melis M, Muntoni AL, Gessa GL. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc Natl Acad Sci USA. 1998;95:10269–10273. doi: 10.1073/pnas.95.17.10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElSohly Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–548. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- EMCDDA. Annual Report on the State of the Drugs Problem. European Monitoring Centre for Drugs and Drug Addiction; Lisbon: 2008. [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working memory. Electroencephalogr Clin Neurophysiol. 1993;87:128–143. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Haertzen CH, Hickey JE. ARCI: measurement of euphoria and other drug effects. In: Bozarth MA, editor. Methods for Assessing the Reinforcing Properties of Abused Drugs. Springer-Verlag; New York: 1987. pp. 489–524. [Google Scholar]
- Haney M, Comer SD, Ward AS, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999;14:385–394. doi: 10.1007/s002130050848. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Reed SC, Foltin RW. Effects of THC and lofexidine in a human laboratory model of marijuana withdrawal and relapse. Psychopharmacology. 2008;197:157–168. doi: 10.1007/s00213-007-1020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Nasser J, Bennett A, Zubaran C, Foltin RW. Marijuana withdrawal in humans: effects of oral THC or Divalproex. Neuropsychopharmacology. 2004;29:158–170. doi: 10.1038/sj.npp.1300310. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002a;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral delta-9-tetrahydrocannabinol in humans. Psychopharmacology. 2002b;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug Alcohol Depend. 2009;102:35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Hudziak J, Helzer JE, Wetzel MW, Kessel KB, McBee B, Janca A, Przybeck P. The use of the DSM-III-R Checklist for initial diagnostic assessments. Compr Psychiatry. 1993;34:375–383. doi: 10.1016/0010-440x(93)90061-8. [DOI] [PubMed] [Google Scholar]
- Jones RT, Benowitz N, Bachman J. Clinical studies of cannabis tolerance and dependence. Ann NY Acad Sci. 1976;282:221–239. doi: 10.1111/j.1749-6632.1976.tb49901.x. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Higgins ST, Hughes JR. The effects of delta-9-tetrahydrocannabinol on repeated acquisition and performance of response squences and on self-reports in humans. Behav Pharmacol. 1994;5:71–78. doi: 10.1097/00008877-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Kirk JM, DeWit H. Responses to oral delta-9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav. 1999;63:137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp Clin Psychopharmacol. 2010;18:1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Kleber HD. Use of dronabinol for cannabis dependence: two case reports and review. Am J Addict. 2008;17:161–164. doi: 10.1080/10550490701861177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2011;116:142–150. doi: 10.1016/j.drugalcdep.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe RH, Karschner EL, Schwilke EW, Barnes AJ, Huestis MA. Simultaneous quantification of Delta9-tetrahydrocannabinol, 11-hydroxy-Delta9-tetrahydrocannabinol, and 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in human plasma using two-dimensional gas chromatography, cryofocusing, and electron impact-mass spectrometry. J Chromatogr. 2007;1163:318–327. doi: 10.1016/j.chroma.2007.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Vandrey R, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine Tob Res. 2012 doi: 10.1093/ntr/nts101. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behav Res Methods. 1982;14:463–466. [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997a;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Frey JM, Yingling JE, Griffiths RR. Triazolam and zolpidem: a comparison of their psychomotor, cognitive, and subjective effects in healthy volunteers. Behav Pharmacol. 1997b;8:561–574. doi: 10.1097/00008877-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Nordstrom BR, Levin FR. Treatment of cannabis use disorders: a review of the literature. Am J Addict. 2007;16:331–342. doi: 10.1080/10550490701525665. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry. 2009;65:144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Mercincavage M, Fonte CA, Lerman C. Varenicline’s effects on acute smoking behavior and reward and their association with subsequent abstinence. Psychopharmacology. 2010;210:45–51. doi: 10.1007/s00213-010-1816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: D9-tetrahydrocannabinol, cannabidiol and D9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, Van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31:2296–2303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Raupach T, van Schayck CP. Pharmacotherapy for smoking cessation: current advances and research topics. CNS Drugs. 2011;25:371–382. doi: 10.2165/11590620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson E. The Halstead-Reitan Neuropsychological Test Battery. Neuropsychology Press; Tuscon: 1985. [Google Scholar]
- SAMHSA. Treatment episode data set (TEDS) 1995–2007: National Admissions to Substance Abuse Treatment Services. US Department of Health and Human Services; Rockville, MD: 2008. [Google Scholar]
- Shallice T. Specific impairments of planning. Phil Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Allen JP, Litten RZ, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Human Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Stotts AL, Dodrill CL, Kosten TR. Opioid dependence treatment: options in pharmacotherapy. Expert Opin Pharmacother. 2009;10:1727–1740. doi: 10.1517/14656560903037168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World Drug Report - 2007. United Nations Office on Drugs and Crime; New York, NY: 2007. [Google Scholar]
- Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]