Abstract
Prolonged high fat feeding is associated with myocardial contractile dysfunction in rodents. However, epidemiological data do not necessarily support the concept that fat-enriched diets adversely affect cardiac function in humans. When fed in an ad libitum manner, laboratory rodents consume chow throughout the day. In contrast, humans typically consume food only during the awake phase. Discrepancies between rodent and human feeding behaviors led us to hypothesize that the time of day at which dietary lipids are consumed significantly influences myocardial adaptation. In order to better mimic feeding behavior in humans, mice were fed (either a control or high fat diet) only during the 12-hr dark phase (i.e., no food was provided during the light phase). We report that compared to dark phase restricted control diet fed mice, mice fed a high fat diet during the dark phase exhibit: 1) essentially normal body weight gain and energy balance; 2) increased fatty acid oxidation at whole body, as well as skeletal and cardiac muscle (in the presence of insulin and/or at high workloads) levels; 3) induction of fatty acid responsive genes, including genes promoting triglyceride turnover in the heart; 4) no evidence of cardiac hypertrophy; and 5) persistence/improvement of myocardial contractile function, as assessed ex vivo. These data are consistent with the hypothesis that ingestion of dietary fat only during the more active/awake period allows adequate metabolic adaptation, thereby preserving myocardial contractile function.
Keywords: Gene expression, High fat feeding, Metabolism, Triglyceride
INTRODUCTION
Common environmental factors within Western society, such as excess caloric intake, physical inactivity, and sleep deprivation are strongly associated with the development of modern day cardiometabolic diseases, including obesity and type 2 diabetes mellitus, as well as cardiovascular disease (CVD) [1, 2]. In terms of nutritional influences, significant research efforts have focused on the quantity and/or quality (i.e., nutritional content) of calories ingested as a means of interrogating the effects of, and potential mechanisms responsible for, diet-induced alterations in heart function. Regarding macronutrients, the influence of dietary lipids/fat on myocardial function has received much attention in both human- and animal- based research, resulting in substantial controversy. It is therefore not clear whether high or low fat diets are cardioprotective or cardiotoxic in humans [3-7]. In rodents, prolonged Western/high fat feeding has often been reported to modestly depress baseline myocardial contractile function [8-12]. These effects appear to be diet composition- and duration- dependent, as well as influenced by the species and strain of the rodent. For example, feeding C57/Bl6 mice a high fat diet for up to 12 weeks does not appear to adversely affect myocardial contractile function, despite development of marked obesity [13]. Continuation of high fat feeding for 16 weeks or longer, however, has been shown by multiple laboratories to cause myocardial contractile dysfunction in several mouse strains [8, 9]. With regards to macronutrient composition, feeding rats a diet composed of 45% fat (more akin to a Western diet) attenuates myocardial contractile function to a greater extent than a 60% fat diet (more akin to a high fat/low carbohydrate Atkins diet) [11]. Similarly, we have recently reported that feeding mice a 45% high fat diet for 16 weeks in an ad libitum fashion depresses cardiac function [8]. These differences likely reflect distinct adaptation at multiple levels, including systemic and cardiac-specific.
The response of the myocardium to high fat diets is complicated further by the co-existence of additional disease/stress states. For instance, elevated fatty acid availability due to chronic high fat feeding [14] or acute increases ex vivo [15], reduces ischemia/reperfusion tolerance in rodent models, potentially due to alterations in myocardial metabolism (e.g., an uncoupling of glycolysis from glucose oxidation, causing H+ accumulation). In contrast, high fat feeding has been shown to improve myocardial contractile function during pressure overload-induced hypertrophy and permanent LAD occlusion-induced heart failure (potentially due to re-activation of fatty acid oxidation and reversal of an energy deficient state) [16-18].
The use of genetically altered mouse models has been essential for elucidation of novel mechanisms involved in the pathogenesis of human disease, including diet-induced myocardial dysfunction. It is, however, important to note that differences between rodent and human feeding behavior exist. The most common experimental approach in rodent nutritional studies typically involves continual access to a single specialized diet. Under such conditions, the rodent consumes the diet throughout the 24-hr period in a contiguous manner, although a time-of-day dependent oscillation exists with respect to the quantity of food consumed (approximately one third of daily calories during the light (less active/sleep) period and two thirds during the dark (more active/awake) period, for wild-type mice) [19]. In contrast, humans typically consume meals with often distinct caloric quantity and quality, at discrete times of the waking hours. The potential importance of these differences in feeding behavior between humans and laboratory rodents has recently been highlighted by several studies reporting that the time-of-day at which a high fat diet is consumed influences multiple cardiometabolic syndrome parameters (including adiposity and glucose tolerance) [20-22]. However, to date the impact on myocardial function is unknown.
The purpose of the present study was to investigate the adaptation of the heart to high fat feeding, when restricted only to the dark phase. The effects of dark phase restricted high fat feeding on both extra-cardiac (e.g., whole body energy balance, humoral factors, skeletal muscle metabolism) and myocardial adaptation (e.g., metabolism, gene expression, contractile function) were assessed. In marked contrast to our recently reported observations for ad libitum high fat fed mice [8], we report that restricting high fat feeding to the dark phase results in adequate adaptation at whole body and myocardial levels, which is associated with preservation of myocardial contractile function.
MATERIALS AND METHODS
Animals
Male wild-type mice (on FVB/N background) were housed under temperature-, humidity-, and light- controlled conditions either at the Children's Nutrition Research Center (Baylor College of Medicine) or at the University of Alabama at Birmingham. A strict 12-hour light/12-hour dark cycle regime was enforced (lights on at 6AM; zeitgeber time [ZT] 0). Mice received food and water ad libitum, unless otherwise specified. Mice were housed in standard micro-isolator cages, prior to initiation of feeding protocols (during which time mice were housed either in wire-bottom or CLAMS (Comprehensive Laboratory Animal Monitoring System) cages to prevent consumption of bedding or feces). All animal experiments were approved by respective Institutional Animal Care and Use Committees.
Rodent Diets and Feeding Studies
A high fat diet (45% calories from fat, Research Diets, New Brunswick, NJ; catalog number D12451) and a control diet (10% calories from fat, Research Diets, New Brunswick, NJ; catalog number D12450B) were utilized for this study; these same diets were utilized in our recently published ad libitum feeding studies [8]. Diets were matched for protein content, and fat and carbohydrate were derived from the same source for each diet. Mice were randomly assigned to one of four feeding groups: 1) ad libitum control diet; 2) ad libitum high fat; 3) dark phase restricted control diet (DPCD); and 4) dark phase restricted high fat (DPHF). For the latter two groups, mice were fasted during the 12-hr light phase (which represents the less active period for these nocturnal animals). When housed within wire bottom cages (for terminal studies, such as humoral factors, skeletal and cardiac muscle incubations/perfusions, and gene expression), feeding regimes were enforced manually (i.e., addition/removal of food from the cage on a daily basis). When mice were house within the CLAMS, feeding regimes were enforced in a computer-controlled automated fashion by the CLAMS. In order to control for the potential stress associated with opening and closing of the CLAMS feeders, ad libitum fed mice were exposed to this same intervention twice daily (at ZT0 and ZT12, for only 1 minute). Feeding regimes were initiated when mice were 12 weeks of age, and were enforced for either 12 weeks or 16 weeks (depending on the endpoint measurements).
Non-invasive Mouse Monitoring
Twenty-four hour patterns of food intake, energy expenditure (indirect calorimetry), and physical activity were measured using a CLAMS (Columbus Instruments Inc., Columbus, OH). This instrument also enforced the feeding regimes in an automated, computer-controlled manner. Body weight was monitored in mice at weekly intervals.
Humoral Factor Measurement
Plasma glucose, non-esterified fatty acids, triglyceride, cholesterol, glycerol, insulin, adiponectin, and leptin concentrations were measured using commercially available kits (Thermo Scientific, Waltham, MA; Wako Diagnostics, Richmond, VA; Crystal Chem Inc., Downers Grove, IL; Thermo Scientific, Waltham, MA).
Ex Vivo Assessment of Skeletal Muscle Metabolism
Soleus muscle metabolism was assessed ex vivo using the isolated intact muscle preparation, essentially as described previously [23]. Briefly, muscles were rapidly excised from mice, tied at resting tension onto stainless steel clips, and pre-incubated in standard Krebs-Henseleit buffer supplemented with 8mM glucose, 0.4mM oleate conjugated to 3% BSA (fraction V, fatty acid-free; dialyzed), and 1μUnit/ml insulin. Following a 45 minute pre-incubation period, muscles were transferred to incubation media (Krebs-Henseleit buffer supplemented with 8mM glucose and 0.4mM oleate conjugated to 3% BSA, as well as tracer amounts of [U-14C]-glucose (0.5mCi/L) and [9,10-3H]-oleate (0.75mCi/L), in either the absence or presence of a maximal concentration of insulin (1mUnit/ml)). During both the pre-incubation and incubation periods, agitation, temperature (37°C), and gassing (95% oxygen, 5% carbon dioxide) were maintained. Following a 60 minute incubation period, muscles were briefly blotted dry, and rapidly frozen in liquid nitrogen. Rates of oleate and glucose oxidation were determined as described previously [23].
Ex Vivo Assessment of Heart Metabolism and Contractile Function
Myocardial contractile function and metabolism were determined ex vivo through isolated working mouse heart perfusions, as described previously [8, 24, 25]. All hearts were perfused in the working mode in a non-recirculating manner with a preload of 12.5mmHg and an afterload of either 50mmHg (baseline) or 80mmHg (high). Standard Krebs-Henseleit buffer was supplemented with 8mM glucose, 0.4mM oleate conjugated to 3% BSA (fraction V, fatty acid-free; dialyzed), 0.05mM L-carnitine, and 0.13mM glycerol. Radiolabeled tracers ([U-14C]-glucose (0.12mCi/L) and [9,10-3H]-oleate (0.067mCi/L)) were utilized to monitor oxidative substrate metabolism. For the initial 30 minutes, hearts were perfused under baseline conditions (i.e., 50mmHg afterload, no insulin). An insulin challenge was next performed; maximal insulin responsiveness was assessed for 30 minutes (i.e., 1mUnit/ml insulin). Finally, a workload challenge was performed; responsiveness to 1 M epinephrine plus an elevated afterload of 80mmHg was assessed for 30 minutes. Measures of cardiac metabolism (i.e., oleate and glucose oxidation) and function (i.e., cardiac power and rate pressure product) were determined as described previously [8, 24]. At the end of the perfusion period, hearts were snap-frozen in liquid nitrogen and stored at -80°C prior to analysis. Data are presented as steady state values (i.e., the mean of the last two time points during a distinct perfusion condition for each individual heart).
RNA Isolation and Gene Expression Analysis
RNA was extracted from hearts using standard procedures [26]. Microarray analysis was performed using mouse Ref-6 BeadChips and the BeadStation System (Illumina, Inc., San Diego, CA) as described previously [24]. Candidate gene expression analysis was performed by quantitative RT-PCR using previously described methods [27, 28]. Specific assays were designed for each gene from mouse sequences available in GenBank. Primer and probe sequences have been reported previously [8]. Standard RNA was made by the T7 polymerase method (Ambion, Austin, TX), using total RNA isolated from mouse hearts; the use of standard RNA allows absolute quantification of gene expression. Quantitative RT-PCR data are represented as mRNA molecules per ng total RNA.
Myocardial Triglyceride Measurement
Myocardial triglyceride contents were measured from homogenate extracts using an enzymatic spectrophotometric assay, according to the manufacturer's instructions (Wako Diagnostics, Richmond, VA).
Statistical Analysis
Statistical analysis was performed using two-way or repeated-measure ANOVA. Stata version IC10.0 (Stata Corp., San Antonio, TX) was used to perform two-way ANOVA to investigate the main effects of diet and time. Repeated-measure ANOVA was used to determine the effects of different diets across time (e.g., body weight and CLAMS data). A full model including second-order interactions was conducted for each experiment. Significant differences were determined using Type III sums of squares. The null hypothesis of no model effects was rejected at p < 0.05. Lastly, Bonferroni post hoc analyses were performed for pair-wise comparisons.
Two-way analysis of variance was performed for gene expression microarrays in which the main effects of time and diet and the interaction effect of diet-by-time were examined. All statistical analyses of gene expression data, including principal components analysis of global gene expression by group and Venn diagrams of overlapping gene lists for the main and interaction effects, were carried out using GeneSpringGX Version 11.5.1 (Agilent Technology; Santa Clara, CA).
RESULTS
Whole Body Metabolic Adaptation to Dark Phase Restricted High Fat Feeding
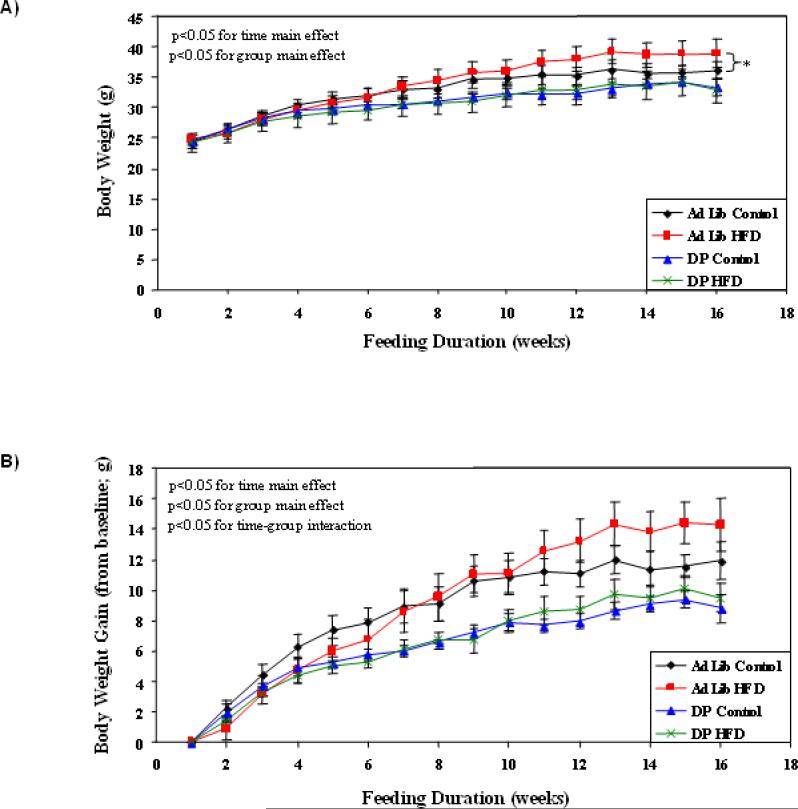
In order to gain insight regarding the impact of dark phase restricted high fat feeding, we initially assessed energy balance parameters throughout the feeding regime duration. For the sake of comparison, ad libitum fed mice were included in these initial analyses. Consistent with previously published studies, ad libitum high fat feeding resulted in increased body weight, compared to ad libitum control diet fed mice (p<0.05; Figure 1). This effect of high fat feeding was not observed in DPHF (versus DPCD) fat fed mice (Figure 1). In contrast, dark phase restricted fed mice exhibited decreased weight gain compared to ad libitum fed mice, independent of the diet consumed (p<0.05; Figure 1).
Figure 1.
Effects of ad libitum versus active phase restricted feeding on body weight (A) and body weight gain (B). Mice were randomly divided into one of four feeding groups: 1) ad libitum control diet fed; 2) ad libitum high fat fed; 3) dark phase restricted control diet (DPCD) fed; and 4) dark phase restricted high fat diet (DPHF) fed. Feeding regimes were enforced for 16 weeks (6 to 22 weeks of age) by a computer-controlled, fully automated CLAMS. Values are expressed as mean ± SEM (n=6). * denotes p<0.05 for ad libitum control versus ad libitum high fat diet (group main effect).
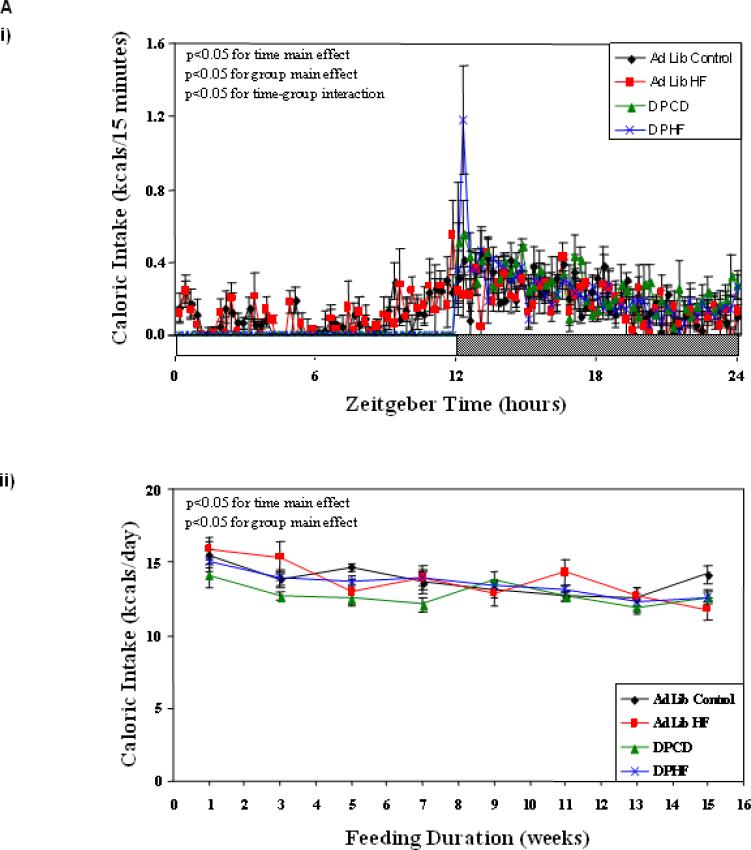
Figure 2Ai confirmed that ad libitum fed mice consume approximately 34% of their daily caloric intake during the light phase (independent of the diet provided). In contrast, dark phase fed mice consumed all calories during the dark phase. Total daily caloric intake appeared to decline steadily with respect to study duration in all four feeding groups (Figure 2Aii). When daily caloric intake was averaged across the entire study period, no significant differences were observed between the four feeding groups (Table 1). We next investigated whether energy expenditure was significantly different between the four feeding groups investigated. In all groups, energy expenditure was increased during the dark phase, relative to the light phase (Figure 2Bi). Dark phase restricted feeding decreased average daily energy expenditure, independent of the diet consumed (p<0.05; Figure 2Bii). However, no significant differences were observed in energy expenditure between DPCD and DPHF mice (Table 1). When daily energy balance (i.e., caloric intake minus energy expenditure) was estimated over the course of the entire feeding study for all mice, no significant differences were observed between the 4 feeding groups (Table 1). In addition, no differences in physical activity were observed between the groups (data not shown).
Figure 2.
Effects of ad libitum versus active phase restricted feeding on caloric intake (A), energy expenditure (B), and RER (C). Mice were randomly divided into one of four feeding groups: 1) ad libitum control diet fed; 2) ad libitum high fat fed; 3) dark phase restricted control diet (DPCD) fed; and 4) dark phase restricted high fat diet (DPHF) fed. Feeding regimes were enforced by a computer-controlled, fully automated CLAMS. Data are shown in 15 minute intervals across a 24-hour period during week 15 (i), and daily averages determined every other week (ii). Values are expressed as mean ± SEM (n=6).
Table 1.
Average daily values for energy balance parameters.
| Parameter | Ad Lib Control | Ad Lib High Fat | DPCD | DPHF |
|---|---|---|---|---|
| Caloric Intake (kcals) | 13.76 ± 0.20 | 13.66 ± 0.54 | 13.11 ± 0.33 | 13.45 ± 0.35 |
| Energy Expenditure (kcals/hr) | 0.49 ± 0.01 | 0.52 ± 0.01 | 0.45 ± 0.01* | 0.48 ± 0.01 |
| Energy Balance (net kcals/day) | 2.20 ± 0.50 | 1.52 ± 0.44 | 2.16 ± 0.29 | 2.01 ± 0.31 |
| RER | 0.93 ± 0.00 | 0.85 ± 0.00$ | 0.92 ± 0.00* | 0.85 ± 0.00#† |
Non-invasive monitoring of energy balance parameters was performed using a CLAMS, in ad libitum control fed and ad libitum high fat fed mice, as well as mice fed either a high fat diet (DPHF) or a control fat diet (DPCD) during the dark phase (whereas no food was allowed during the light phase). Data are shown as daily averages across the entire study duration. Values are expressed as mean ± SEM (n=6).
denotes p<0.05 for ad libitum high fat versus DPCD
denotes p<0.05 for ad libitum control versus ad libitum high fat
denotes p<0.05 for DPCD versus DPHF
denotes p<0.05 for ad libitum control versus DPHF.
Through calculation of the respiratory exchange ratio (RER), we next assessed alterations in whole body substrate oxidation between the feeding groups. Marked time-of-day-dependent differences were observed for RER between the 4 feeding groups (Figure 2Ci). Consistent with previously published observations [20], high fat feeding decreased RER relative to control diet fed mice, throughout the feeding protocol (indicative of increased fatty acid oxidation at the whole body level; Figure 2Cii and Table 1).
Distinct Alterations in Humoral Factors Following Chronic Dark Phase Restricted High Fat Feeding
Humoral factors (both substrates and hormones) can acutely influence metabolic homeostasis. In addition, we and others have previously shown that ad libitum high fat feeding markedly alters multiple humoral factors [8]. Accordingly, we next investigated whether dark phase restricted high fat feeding influenced distinct metabolically-relevant humoral factors. The latter were measured 4 hours into both the light phase (ZT4) and dark phase (ZT16). This analysis revealed that DPHF mice exhibit decreased plasma triglyceride levels, relative to DPCD mice, independent of the time of day (Table 2). In addition, plasma leptin and cholesterol levels were significantly increased in DPHF (versus DPCD) mice at ZT16 (Table 2). In contrast, plasma glucose, NEFA, insulin, and glycerol were not different between the two groups (Table 2). These effects of DPHF are remarkably similar to the effects of ad libitum high fat feeding [8], with one notable exception; ad libitum high fat feeding (but not DPHF) increases plasma insulin levels (a marker of impaired glucose homeostasis).
Table 2.
Analysis of humoral factors from mice fed either a high fat diet (DPHF) or a control fat diet (DPCF) during the dark phase, whereas no food was allowed during the light phase.
| Feeding Group | DPCF | DPHF | ||
|---|---|---|---|---|
| Zeitgeber Time | ZT4 | ZT16 | ZT4 | ZT16 |
| Plasma NEFA (mM) | 0.39±0.11 | 0.29±0.02# | 0.37±0.06 | 0.30±0.03# |
| Plasma Triglyceride (mg/dl) | 76.3±9.4 | 98.6±10.6 | 42.9±4.1* | 47.0±6.2* |
| Plasma Glucose (mg/dl) | 367±15 | 308±20# | 346±13 | 259±22*# |
| Plasma Insulin (μg/l) | 1.34±0.20 | 1.49±0.12 | 1.31±0.21 | 1.55±0.43 |
| Plasma Leptin (ng/ml) | 3.45±0.67 | 7.21±1.21# | 6.02±1.4 | 12.01±2.33*# |
| Plasma Adiponectin (μg/dl) | 5.07±0.32 | 5.75±0.32 | 5.79±0.38 | 5.80±0.23 |
| Plasma Cholesterol (mg/dl) | 175±8 | 152±11# | 198±14 | 186±9* |
| Plasma Glycerol (mg/dl) | 57.0±23.3 | 49.4±7.9 | 48.2±11.2 | 57.3±11.8 |
Values are expressed as mean ± SEM (n=6).
denotes p<0.05 for control versus high fat diet within a given time
denotes p<0.05 for ZT4 versus ZT16 within a diet group.
NEFA, non-esterified fatty acids.
Dark Phase Restricted High Fat Feeding Increases Both Skeletal and Cardiac Muscle Fatty Acid Oxidation
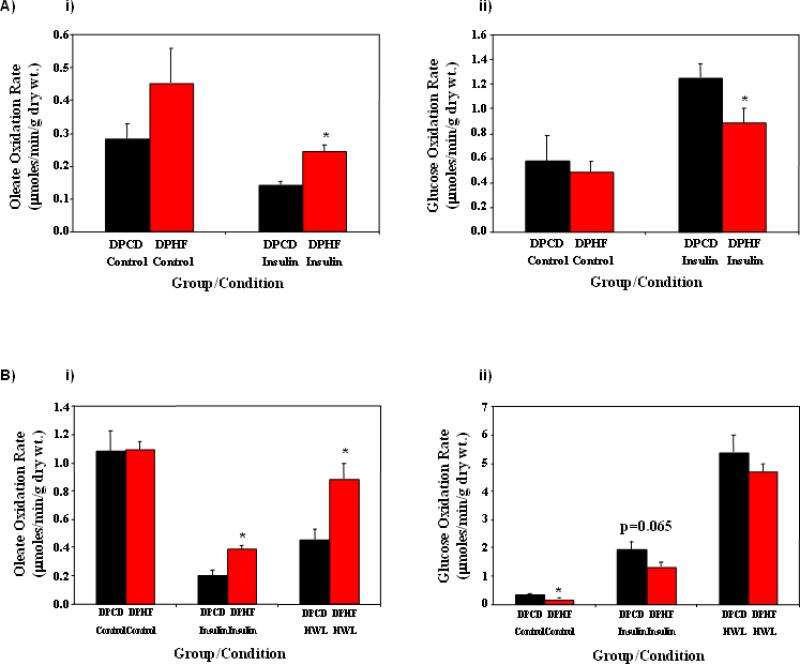
Differences in whole body substrate oxidation between DPHF and DPCD fed mice (Figure 2) were not readily explained by the humoral analysis (Table 2). This is particularly true for fatty acid oxidation, wherein the low RER value observed in DPHF (versus DPCD) fed mice occur despite equivalent NEFA levels and lower plasma TAG levels. We therefore investigated substrate utilization in relevant tissues in an ex vivo setting, in order to unmask potential metabolic adaptation. Accordingly, fatty acid (oleate) and glucose oxidation was investigated in soleus muscles isolated from DPHF versus DPCD fed mice at ZT4 (i.e., 4 hours after last food availability, to reduce acute influences of diet); half of the muscles were challenged with a maximal insulin concentration to interrogate the fed state response. Oleate oxidation rates were higher in DPHF soleus muscles during both basal and insulin-stimulated conditions (although the former condition did not reach statistical significance; Figure 3Ai). A trend (p=0.065) for decreased soleus muscle glucose oxidation rates was observed in the DPHF fed (versus DPCD) group only during maximal insulin stimulation (Figure 3Aii).
Figure 3.
Effects of active phase restricted high fat feeding on soleus muscle (A) and heart (B) substrate utilization. Mice were randomly divided into one of two feeding groups: 1) dark phase restricted control diet (DPCD) fed; and 2) dark phase restricted high fat diet (DPHF) fed. Rates of oleate (i) and glucose (ii) oxidation were determined for soleus muscles and hearts isolated 12 and 16 weeks following initiation of the feeding regimes, respectively. Values are expressed as mean ± SEM (n=6-8). * denotes p<0.05 for control versus high fat diet within a given incubation/perfusion condition. HWL, high workload.
Substrate oxidation was next investigated in hearts isolated from DPHF versus DPCD fed mice at ZT4; hearts were initially perfused under baseline conditions (30 minutes) followed by maximal insulin challenge (to interrogate the fed state response; 30 minutes) and subsequent workload challenge (to induce an energetic demand response; 30 minutes). Under baseline conditions, no significant differences in oleate oxidation rates were observed (Figure 3Bi). However, in the presence of insulin and during high workload conditions, rates of oleate oxidation were approximately 2-fold higher in DPHF hearts versus DPCF hearts (Figure 3Bi). In addition, glucose oxidation rates were lower in DPHF hearts during baseline and insulin stimulated conditions, versus DPCD hearts (Figure 3Bii). Interestingly, recent ad libitum high fat feeding studies from our laboratory failed to detect significant effects on myocardial fatty acid oxidation (in the presence of insulin) [8].
Transcriptional Response of the Heart to Dark Phase Restricted High Fat Feeding
In order to gain further insight into adaptation of the heart to active phase restricted feeding, we next performed a differential gene expression analysis in hearts isolated from DPHF and DPCD fed mice at both ZT4 and ZT16. A non-biased microarray approach revealed both diet- and time-dependent differences in myocardial gene expression. A total of 2555 genes were differentially expressed across time (Supplemental Table 1), 1472 genes were differentially expressed between diet groups (Supplemental Table 2), and 622 genes showed a significant interaction in gene expression across time and diet (Supplemental Table 3). Of these three gene lists, 19 genes were present in all gene lists (Supplemental Table 4). Venn diagrams of the overlap between gene lists are depicted in Supplemental Figure 1.
Not surprisingly, a large number of fatty acid responsive genes were identified as being influenced by diet. Several of these gene expression differences were verified by RT-PCR analysis. For example, this analysis confirmed increased expression of the fatty acid responsive genes pdk4 and ucp3 in DPHF versus DPCD hearts (non-significant trend in the case of ucp3; Figure 4A). Although genes directly involved in fatty acid uptake (cd36; Figure 4Bi) and β-oxidation (mcad; Figure 4Bii) were not differentially expressed, genes promoting triglyceride synthesis (dgat2 and agpat3; Figure 4C) were induced in DPHF hearts relative to DPCD hearts. Consistent with the latter observation, myocardial triglyceride levels were higher in DPHF versus DPCD hearts (Figure 5Ai).
Figure 4.
Myocardial transcriptional adaptation to active phase restricted high fat feeding. Mice were randomly divided into one of two feeding groups: 1) dark phase restricted control diet (DPCD) fed; and 2) dark phase restricted high fat diet (DPHF) fed. Hearts were isolated 16 weeks following initiation of the feeding regimes. Quantitative RT-PCR was performed to assess expression of fatty acid responsive genes (pdk4, ucp3; A), fatty acid uptake and β-oxidation genes (cd36, mcad; B), and triglyceride synthesis genes (agpat3, dgat2; C). Values are expressed as mean ± SEM (n=6). * denotes p<0.05 for control versus high fat diet within a given time.
Figure 5.
Effects of active phase restricted high fat feeding on indices of myocardial contractile function/dysfunction. Mice were randomly divided into one of two feeding groups: 1) dark phase restricted control diet (DPCD) fed; and 2) dark phase restricted high fat diet (DPHF) fed. Hearts were isolated 16 weeks following initiation of the feeding regimes. Triglyceride content and biventricular weight to tibia length ratio (A), quantitative RT-PCR (anf, bnp; B), and contractile function (cardiac power, rate pressure product; C) were assessed. Values are expressed as mean ± SEM (n=6-8). * denotes p<0.05 for control versus high fat diet within a given incubation/perfusion condition. HWL, high workload.
Dark Phase High Fat Feeding does not Adversely Effect Cardiac Contractile Function
Previous studies have shown that chronic ad libitum high fat feeding induces cardiac hypertrophy and contractile dysfunction [8-12]. Indeed, using the same high fat diet and duration of feeding, we have recently reported that ad libitum high fat feeding results in decreased contractile function of the FVB mouse heart, as assessed ex vivo [8]. We therefore determined the effects of dark phase restricted high fat feeding on these parameters. These studies revealed no significant differences between DPHF and DPCD fed mice for bi-ventricular weight to tibia length ratio (Figure 5Aii), molecular markers of contractile dysfunction (anf and bnp mRNA; Figure 5B), or baseline contractile function ex vivo (neither cardiac power nor rate pressure product; Figure 5C). In contrast, we observed increased cardiac power for DPHF hearts (compared to DPCD hearts) when subjected to an acute workload challenge (Figure 5Ci).
DISCUSSION
The purpose of the present study was to investigate the impact of consumption of a high fat diet during the dark (more active/awake) phase on the heart. The results show that restricting high fat diet consumption to only the dark phase is associated with normal weight gain, metabolic adaptation at whole body and tissue-specific levels, transcriptional adaptation of the myocardium, and preserved/improved cardiac contractile function. These data are consistent with the hypothesis that consumption of a high fat diet during the awake period allows adequate adaptation, thus preserving myocardial function in the face of a potentially “lipotoxic” environment. These observations may also help to explain differences observed for associations between dietary lipids and heart function in humans versus experimental rodent models.
Humans and rodents exhibit marked differences in feeding behavior. Humans tend to consume the majority of daily calories in discrete meals typically during the waking hours. In contrast, when laboratory rats/mice are provided food in an ad libitum fashion, they consume the food throughout the 24-hr period; approximately one third of calories during the less active/sleep phase and two thirds of calories during the more active/wake phase (Figure 2Ai) [19]. The potential importance of this discrepancy has been highlighted recently, wherein consumption of a high fat diet by rodents at the end of the dark phase and/or during the light phase is associated with detrimental effects on metabolic homeostasis [21]. Indeed, a study published during preparation of this manuscript confirms the findings presented in Figure 1, that consumption of a high fat diet in an ad libitum fashion is associated with obesity, while restricting the same number of calories to the dark phase is not [22]. Whole body metabolic adaptation was further evident in DPHF fed mice, as exhibited by decreased RER (indicative of increased fatty acid oxidation reliance; Figure 2C and Table 1). Increased whole body fatty acid oxidation in DPHF fed mice was mirrored by increased skeletal muscle fatty acid oxidation rates in the presence of insulin (due to its mass and metabolic activity, skeletal muscle plays a significant role in whole body energy balance; Figure 3A). Whole body metabolic adaptation was associated with decreased plasma triglyceride levels, as previously reported for humans consuming a low carbohydrate (Atkins-like) diet. Prolonged ad libitum high fat feeding results in glucose intolerance and elevated plasma levels of insulin [20]. In contrast, we have previously reported that DPHF mice exhibit normal glucose tolerance [20], with normal plasma insulin levels (as observed presently; Table 2). Collectively, these data suggest that in contrast to ad libitum high fat feeding, restricting high fat diet consumption to the active phase confers adequate whole body metabolic homeostasis. The precise mechanism(s) for this observation is(are) currently unknown. Indeed, DPHF and ad libitum high fat fed mice consume the same number of calories and do not exhibit differences in energy expenditure (Table 1). One possible explanation involves digestion/absorption, processes known to exhibit diurnal rhythms [29]. Accordingly, although calories consumed are identical between the investigated feeding groups, calories absorbed may not be.
Despite growing evidence that the time of day at which a high fat diet is consumed markedly influences multiple cardiometabolic syndrome parameters, to date no studies have reported the impact on cardiac adaptation and/or contractile function. However, indirect evidence suggests that the impact may be significant. For example, the transcriptional responsiveness of the rodent heart to fatty acids is increased during the dark phase, such that fatty acid-responsive genes (including ucp3 and pdk4) are induced to a greater extent by fatty acids at this time [30]. In addition, fatty acids acutely depress contractile function and efficiency of the rat heart to a greater extent during the light phase [31]. More recently, we have shown that the mouse heart channels fatty acids into triglyceride to a greater extent during the dark phase, which might help to sequester excess, potentially “lipotoxic”, fatty acid species at this time [8]. Indeed, the current study shows that restricting high fat feeding to the dark phase, a time at which capacity for triglyceride synthesis is highest, is associated with elevated myocardial steatosis (Figure 5Ai). Collectively, these observations indirectly suggest that fatty acids may exert a greater “lipotoxic” effect on the heart during the less active/sleep phase. In the present study, we investigated the effects of feeding mice a high fat diet only during the dark (more active/awake) phase for 16 weeks on cardiac adaptation at transcriptional, metabolic, and functional levels. We report that, relative to DPCD fed mice, hearts from DPHF fed mice exhibit induction of genes promoting β-oxidation (e.g., ucp3) and triglyceride synthesis (e.g., dgat2 and agpat3), which are mirrored by increases in myocardial fatty acid oxidation and triglyceride levels (Figures 3-5). In the case of fatty acid oxidation, rates were elevated in DPHF hearts in the presence of insulin and increased workload (Figure 4B). Importantly, these transcriptional and metabolic changes are not associated with indices of contractile dysfunction. In contrast, bi-ventricular weight and anf/bnp mRNA were comparable between DPHF and DPCD hearts, as was cardiac power and rate pressure product under baseline conditions (Figure 5). Furthermore, under high workload conditions, DPHF hearts exhibit greater cardiac power, relative to DPCD (Figure 5C). As a point of reference, we have recently shown that feeding the same background strain of mice a high fat diet in an ad libitium fashion for the same duration (16 weeks) results in an approximate 33% decrease in cardiac power, when assessed ex vivo under the same perfusion conditions [8]. These data therefore suggest that restricting high fat feeding to the active phase allows adequate adaptation of the myocardium, for preserved/improved contractile function.
The purpose of the present study was to investigate the impact of restricting high fat feeding to the dark phase on multiple aspects of whole body and cardiac adaptation. Regarding the latter, we observed that adequate cardiac adaptation results at multiple levels (including transcriptional and metabolic), which is associated with preserved/improved contractile function. However, the experimental design of the present study did not afford direct comparisons regarding the effects of ad libitum versus restricted feeding regimes on cardiac adaptation parameters; instead comparisons were made to our recently published studies investigating the effects of ad libitum high fat feeding (45% calories from fat for 16 weeks in FVB mice) on cardiac metabolism and function [8]. In addition, the present study did not investigate heart function in vivo (e.g., through echocardiography), nor elucidate the exact nature of the mechanism(s) by which time-of-day-dependent high fat feeding exerts its effects on contractile function. Clearly additional studies are required to address these important questions.
In conclusion, we report that restricting high fat diet consumption to the more active/awake/dark phase affords adequate whole body and myocardial adaptation in mice, thus preserving/improving cardiac function. These findings will likely improve our understanding of the influence of dietary lipids on heart function, and highlight a need for future animal-based studies to appreciate differences in human and rodent feeding behavior.
Supplementary Material
Highlights.
High fat feeding during the dark phase results in whole body metabolic adaptation
High fat feeding during the dark phase results in cardiac and skeletal muscle metabolic adaptation
High fat feeding during the dark phase does not negatively impact heart function
ACKNOWLEDGEMENTS
This work was supported by Kraft Foods Inc., the USDA/ARS (6250-51000-046 and 6250-51000-044) and the National Heart, Lung, and Blood Institute (HL-074259). Ju-Yun Tsai was supported by the DeBakey Heart Fund at Baylor College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None declared.
REFERENCES
- 1.U.S. Centers for Disease Control and Prevention . Preventing Obesity and Chronic Diseases through Good Nutrition and Physical Activity. US Department of Health and Human Services; http://www.cdc.gov/nccdphp/publications/factsheets/Prevention/obesity.htm. [Google Scholar]
- 2.Magee CA, Iverson DC, Huang XF, Caputi P. A link between chronic sleep restriction and obesity: methodological considerations. Public Health. 2008 Dec;122(12):1373–81. doi: 10.1016/j.puhe.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein AH. Dietary fat and cardiovascular disease risk: quantity or quality? J Womens Health (Larchmt) 2003 Mar;12(2):109–14. doi: 10.1089/154099903321576493. [DOI] [PubMed] [Google Scholar]
- 4.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial Fatty Acid metabolism in health and disease. Physiol Rev. 2010 Jan;90(1):207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 5.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006 Feb 8;295(6):655–66. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB. Diet and cardiovascular disease prevention the need for a paradigm shift. J Am Coll Cardiol. 2007 Jul 3;50(1):22–4. doi: 10.1016/j.jacc.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Hu FB, Manson JE, Willett WC. Types of dietary fat and risk of coronary heart disease: a critical review. J Am Coll Nutr. 2001 Feb;20(1):5–19. doi: 10.1080/07315724.2001.10719008. [DOI] [PubMed] [Google Scholar]
- 8.Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, et al. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem. 2010 Jan 29;285(5):2918–29. doi: 10.1074/jbc.M109.077800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SY, Cho YR, Kim HJ, Higashimori T, Danton C, Lee MK, et al. Unraveling the temporal pattern of diet-induced insulin resistance in individual organs and cardiac dysfunction in C57BL/6 mice. Diabetes. 2005 Dec;54(12):3530–40. doi: 10.2337/diabetes.54.12.3530. [DOI] [PubMed] [Google Scholar]
- 10.Aasum E, Khalid AM, Gudbrandsen OA, How OJ, Berge RK, Larsen TS. Fenofibrate modulates cardiac and hepatic metabolism and increases ischemic tolerance in diet-induced obese mice. J Mol Cell Cardiol. 2008 Jan;44(1):201–9. doi: 10.1016/j.yjmcc.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CR, Tran MK, Salazar KL, Young ME, Taegtmeyer H. Western diet, but not high fat diet, causes derangements of fatty acid metabolism and contractile dysfunction in the heart of Wistar rats. Biochem J. 2007 Sep 15;406(3):457–67. doi: 10.1042/BJ20070392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang CX, Dong F, Thomas DP, Ma H, He L, Ren J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: role of suppression of forkhead transcription factor and atrophy gene transcription. Am J Physiol Heart Circ Physiol. 2008 Sep;295(3):H1206–H15. doi: 10.1152/ajpheart.00319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, et al. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes. 2009 Aug;58(8):1766–75. doi: 10.2337/db09-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panagia M, Gibbons GF, Radda GK, Clarke K. PPAR-alpha activation required for decreased glucose uptake and increased susceptibility to injury during ischemia. Am J Physiol Heart Circ Physiol. 2005 Jun;288(6):H2677–83. doi: 10.1152/ajpheart.00200.2004. [DOI] [PubMed] [Google Scholar]
- 15.Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Circ Res. 1996 Nov;79(5):940–8. doi: 10.1161/01.res.79.5.940. [DOI] [PubMed] [Google Scholar]
- 16.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006 Dec;48(6):1116–23. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 17.Rennison JH, McElfresh TA, Okere IC, Patel HV, Foster AB, Patel KK, et al. Enhanced acyl-CoA dehydrogenase activity is associated with improved mitochondrial and contractile function in heart failure. Cardiovasc Res. 2008 Jul 15;79(2):331–40. doi: 10.1093/cvr/cvn066. [DOI] [PubMed] [Google Scholar]
- 18.Berthiaume JM, Young ME, Chen X, McElfresh TA, Yu X, Chandler MP. Normalizing the metabolic phenotype after myocardial infarction: Impact of subchronic high fat feeding. J Mol Cell Cardiol. 2012 Apr 20; doi: 10.1016/j.yjmcc.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turek F, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, et al. Obesity and metabolic syndrome in Clock mutant mice. Science. 2005;308:1043–5. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray MS, Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, et al. Time-of-day-dependent dietary fat consumption influences multiple cardiometabolic syndrome parameters in mice. Int J Obes (Lond) 2010 Nov;34(11):1589–98. doi: 10.1038/ijo.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009 Nov;17(11):2100–2. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatori M, Vollmers C, Zarrinpar A, Ditacchio L, Bushong EA, Gill S, et al. Time-Restricted Feeding without Reducing Caloric Intake Prevents Metabolic Diseases in Mice Fed a High-Fat Diet. Cell Metab. 2012 May 16; doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young ME, Radda GK, Leighton B. Nitric oxide stimulates glucose transport and metabolism in rat skeletal muscle in vitro. Biochem J. 1997 Feb 15;322(Pt 1):223–8. doi: 10.1042/bj3220223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray M, Shaw C, Moore M, Garcia R, Zanquetta M, Durgan D, et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function; metabolism; and gene expression. Am J Physiol Heart Circ Physiol. 2008;294:H1036–H47. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 25.Belke D, Betuing S, Tuttle M, Graveleau C, Young M, Pham M, et al. Insulin signaling coordinately regulates cardiac size; metabolism; and contractile protein isoform expression. J Clin Invest. 2002;109:629–39. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996 Oct;6(10):995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 28.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996 Oct;6(10):986–94. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 29.Pan X, Hussain MM. Clock is important for food and circadian regulation of macronutrient absorption in mice. J Lipid Res. 2009 Sep;50(9):1800–13. doi: 10.1194/jlr.M900085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durgan D, Trexler N, Egbejimi O, McElfresh T, Suk H, Petterson L, et al. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem. 2006;281:24254–69. doi: 10.1074/jbc.M601704200. [DOI] [PubMed] [Google Scholar]
- 31.Durgan D, Moore M, Ha N, Egbejimi O, Fields A, Mbawuike U, et al. Circadian rhythms in myocardial metabolism and contractile function: influence of workload and oleate. Am J Physiol Heart Circ Physiol. 2007;293:H2385–H93. doi: 10.1152/ajpheart.01361.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.