Abstract
Objective
To determine the prevalence of Wnt pathway activation in patients with stage I NSCLC and its influence on lung cancer recurrence.
Background
Despite resection, the 5 year recurrence with localized stage I non-small cell lung cancer (NSCLC) is 18.4–24%. Aberrant Wnt signaling activation plays an important role in a wide variety of tumor types. However, there is not much known about the role Wnt pathway plays in patients with stage I lung cancer
Methods
Tumor and normal lung tissues from 55 patients following resection for stage I NSCLC were subjected to glutathione-S-transferase (GST) E-cadherin pull-down and immunoblot analysis to assess levels of uncomplexed β-catenin, a reliable measure of Wnt signaling activation. The β-catenin gene was also screened for oncogenic mutations in tumors with activated Wnt signaling. Cancer recurrence rates were correlated in a blinded manner in patients with Wnt pathway positive and negative tumors.
Results
Tumors in twenty patients (36.4%) scored as Wnt positive with only one exhibiting a β-catenin oncogenic mutation. Patients with Wnt positive tumors experienced a significantly higher rate of overall cancer recurrence than those with Wnt negative tumors (30.0% vs. 5.7%, p=0.02), with 25.0% exhibiting distal tumor recurrence compared to 2.9% in the Wnt negative group (p=0.02).
Conclusions
Wnt pathway activation was present in a substantial fraction of Stage I NSCLCs, which was rarely due to mutations. Moreover, Wnt pathway activation was associated with a significantly higher rate of tumor recurrence. These findings suggest that Wnt activation reflects a more aggressive tumor phenotype and identifies patients who may benefit from more aggressive therapy in addition to resection.
INTRODUCTION
Lung carcinoma is the leading cause of cancer death in the United States as well as worldwide, and non small cell lung carcinoma (NSCLC) comprises 75–85% of lung cancers in the United States 1, 2. Unfortunately most patients are at an advanced stage at the time of diagnosis, and the overall 5-year survival rate for all lung cancer patients is 15% 3. Patients with Stage I NSCLC, who undergo surgical resection, have the best 5 year survival rates. However, despite surgical resection, the 5 year recurrence in this group is 18.4–24% 4–6, and the 5 year survival rate is 58–73% 7. Adjuvant chemotherapy has been investigated to improve the survival of this group, but the results have been controversial 8–10. The lack of better surgical cure for stage I patients and apparent absence of any significant benefit from adjuvant chemotherapy to improve survival has led to efforts to evaluate this group of patients for prognostic factors that may help to guide future therapy 11, 12.
Investigation of the roles of specific cellular pathways in NSCLC initiation and progression have revealed the frequent occurrence of mutations involving p53, K-ras, and EGFR 13–15. A number of studies have suggested that mutations in these genes are associated with advanced disease stage and poor prognosis. However, such findings have not been consistent 11, 14–20, 20–24. Wnt signaling plays critical roles in normal development as well as in post-natal tissue homeostasis through maintenance of stem/progenitor cells 25. Moreover, this pathway appears to regulate cell fate and differentiation in early lung development 26. A critical downstream effector in the canonical Wnt pathway is β-catenin, which is normally bound to E-cadherin at the inner surface of the cell membrane. A degradation complex comprised of glycogen synthase kinase-3β (GSK-3β), APC, and axin phosphorylates and processes unbound β-catenin for proteosomal degradation. Triggering of their cell surface receptors by secreted Wnt canonical family members leads to inhibition of the degradation complex resulting in β-catenin accumulation and translocation to the nucleus, where it participates as a transcription factor with TCF/LEF transcription factors in the activation of target genes 25, 27.
Aberrant Wnt signaling activation resulting from genetic alterations of intracellular components of this pathway plays an important role in the development of a variety of tumor types 28. Inactivating mutations of the APC gene are frequently observed in colorectal cancers, while mutations or in-frame deletions of exon 3 of the β-catenin gene, which prevent its phosphorylation and proteosome targeting have been observed in more than 90% of colon cancers and at lower frequencies in liver, endometrium, ovary, prostate, and stomach carcinomas 29–34. These genetic alterations result in constitutive activation of TCF transcription through the stabilization of β-catenin and the inappropriate activation of TCF target genes believed to be critical in tumorigenesis. More recently, Wnt canonical pathway activation has been observed in a substantial fraction of human breast, ovarian, and non-small lung carcinoma (NSCLC) cell lines. Levels of Wnt activation as measured by uncomplexed β-catenin or TCF transcriptional activation ranged from 5 to more than 100 fold higher than in normal or other tumor cells of the same tissue types 35–40. An autocrine mechanism involving over-expression of Wnt ligands and/or receptors, rather than APC or β-catenin mutations, was identified as the most frequent cause of pathway activation 39. Evidence that Wnt pathway downregulation caused specific inhibition of the proliferation of such tumor cells argues that Wnt signaling contributes importantly to the malignant phenotype 38, 39, 41. Thus, concerted efforts are underway to identify small molecule inhibitors and other approaches with which to therapeutically target this activated pathway in tumors 42.
There is only limited evidence concerning the prevalence and clinical significance of Wnt pathway activation in tumors of NSCLC patients 40, 43. These studies have relied on the use of immunostaining for β-catenin to assess Wnt pathway activity in NSCLC tissues and have led to conflicting findings concerning both prevalence and prognostic implications. We developed a biochemical approach to identify Wnt activation in primary NSCLC by comparison of uncomplexed β-catenin levels in tumor and normal lung tissues of the same patient and utilized this methodology to determine the frequency of Wnt pathway activation in stage I NSCLC and its influence on lung cancer recurrence.
METHODS
Patients
NSCLC tumor and normal tissues were obtained from the Tisch Cancer Institute Biorepository, Mount Sinai. We selected patients who underwent resection for stage I NSCLC from June 2006 to June 2008. All patients in this study were determined to be stage I based on pathological staging, in which they underwent mediastinal lymph node sampling including N1, upper and lower mediastinal nodes. Although pathological reports did not consistently specify the station numbers for N1 nodes being evaluated, each specimen had multiple N1 lymph nodes examined by the pathologist. Patients underwent preoperative staging with CT and PET scans and were deemed to be stage I by these criteria as well. Tissue specimens were linked to each patient by use of a unique number assigned to each patient. Paired samples of tumor and normal lung were obtained from each consented patient. None of the patients in the study had been given chemotherapy or radiation prior to surgery. We used prospectively collected data linked to this tissue bank to obtain information on patient demographics, co-morbidities, operative course, pathological staging and postoperative course. Inpatient and outpatient charts were reviewed, and patients were also contacted for additional follow-up information. Pathological staging was performed in accordance with the guidelines set forth by the American Joint Committee on Cancer (AJCC) 44. The study was reviewed and approved by Mount Sinai’s Institutional Review Board.
Tissue Samples
Tissues were immersed in Optimal Cutting Temperature (OCT) compound and frozen at −70°C. Hematoxylin and eosin (H&E) stained sections were reviewed by a pulmonary pathologist to confirm the diagnosis and quality of tissue, and 20 micron thick cryosections were obtained for analysis of tumor sections exhibiting the presence of more than 50% of tumor cells. Matched normal lung tissue scored as containing 100% normal cells from each patient served as a negative control.
Protein Extraction and Western Blot Analysis
Cryo-sectioned samples were homogenized in lysis buffer as previously described 45 using mortar and pestle. Uncomplexed β-catenin, was measured by a capture assay using glutathione S-transferase (GST) fused to the β-catenin binding domain of E-cadherin synthesized in E. coli 45. Immunoblot analysis of uncomplexed and total β-catenin was performed as previously described 45 using mouse β-catenin primary antibody (BD Pharmingen, San Jose, CA, USA). Anti-mouse immunoglobulin G (IgG) secondary antibody conjugated to Alexa Fluor 680 was purchased from Amersham Bioscience (GE Healthcare, UK). Quantification of immunoreactivity was performed using the Licor Odyssey Imaging System (LI-COR, Lincoln, Nebraska, USA). For each set of paired normal and tumor samples, the capture assay and Western blot analysis were performed at least twice on consecutive sections of the same tissue with Wnt activated H23 NSCLC cells and Wnt negative 293T cells, respectively, serving as positive and negative controls.
Quantification of TCF mediated luciferase reporter activity
293T cells were infected with TOP-luciferase and renila luciferase as previously described 39. Cells were plated in 10 cm plates at 1×106 cells/plate and treated 24 hours later with increasing concentrations of Wnt3a or control conditioned medium for 48 hours. Cells were then trypsinized, washed and resuspended in 10X the cell volume prior to lysis and processing for luciferase reporter assay using the dual luciferase reporter Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Luciferase reporter activity was calculated by dividing the TOP-luciferase by renila luciferase. The rest of the cells were processed for Immunoblot analysis of uncomplexed and total β-catenin as previously described 45.
Sequencing of CTNNB1 exon 3
Genomic DNA was extracted from NSCLC tissue specimens using the DNeasy extraction kit (Qiagene, Maryland), and PCR amplified using primers flanking β-catenin (CTNNB1) exon 3 (forward 5′-TTGATGGAGTTGGACATG; reverse 5′-CAGCTACTTGTTCTTGAG). This region of the β-catenin gene corresponds to the N-terminal phosphorylation domain that contains the sites of known β-catenin mutations in tumors 46. Gel purified PCR fragments were sequenced by the Mount Sinai DNA sequencing core facility.
Statistical Analysis
The clinical characteristics of patients with Wnt positive and negative NSCLCs were compared using the Student t test, the Wilcoxon test or chi-square test, as appropriate. Disease-free survival of patients with Wnt positive and negative tumors was determined by the Kaplan-Meier method. Disease-free survival was defined as the time from surgery to the first diagnosis of local, regional or distant disease recurrence, or until the last follow-up. We used Cox Proportional Hazards Regression Analysis to assess whether Wnt status was associated with disease free survival after controlling for other prognostic factors. The SAS 9.0 statistical package (SAS Institute, Cary, NC) was used for all statistical analyses. Two-tailed p value of less than 0.05 was considered significant.
RESULTS
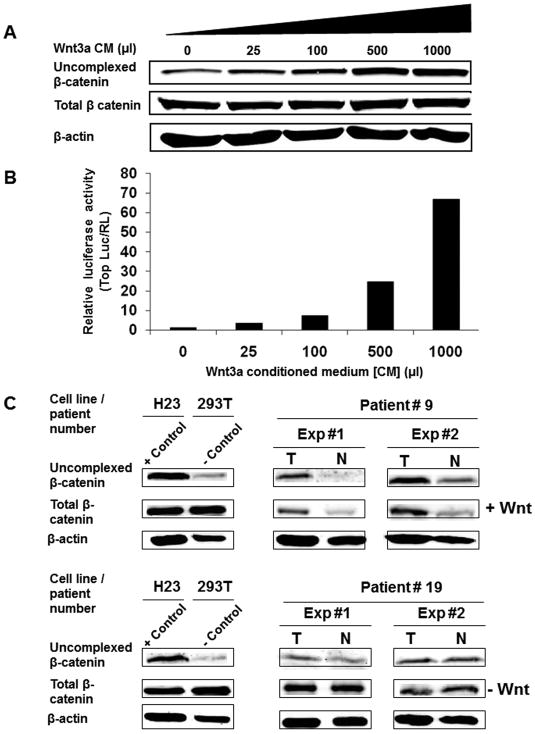
To validate the capture assay for determination of uncomplexed b-catenin levels in cells or tissues as a surrogate for detection of Wnt activation, we exposed 293T cells to increasing Wnt3a concentration and compared the resulting increase in uncomplexed β-catenin as measured by the capture assay with levels of TCF transcriptional activation using a reporter for TCF signaling. Figure 1A shows that uncomplexed β-catenin levels increased as a function of increasing Wnt concentration while total β-catenin levels were little affected. Moreover, the increased levels of uncomplexed β-catenin correlated well but with a less dynamic range compared to the increasing levels observed in TCF reporter activity (Fig. 1B), a sensitive marker for Wnt pathway activity in living cells 39.
Figure 1.
Uncomplexed b-catenin level is a valid marker for Wnt activation in non-small cell lung cancer (NSCLCs). 293T cells transduced with TOP-luciferase and renila luciferase lentiviruses were treated for 48 hours with increasing concentration of Wnt3a conditioned medium. (A) Glutathione S-transferase (GST)-E cadherin capture assay as described in the Methods was performed on the treated cells Total cell lysates (20 μg) and the GST-E-cadherin precipitates from 800 μg of cell lysate were subjected to immunoblot analysis with a mAb directed against β-catenin. (B) TCF reporter luciferase activity was performed on 1:10 of cell volume from the same Wnt3a conditioned medium treated cells as described in Methods. Luciferase reporter activity was calculated by dividing the TOP-luciferase by renila luciferase (RL). (C) β-catenin capture assay performed with two representative human paired NSCLC and normal lung tissues in two independent experiments. Total cell lysates (800 μg) were subjected to GST-E cadherin capture assay as described in Figure 1A. T denotes tumor specimen and N denotes normal lung tissue from the same patient. H23, a Wnt autocrine activated NSCLC line, and 293T, an immortalized human embryonic kidney line, were used as positive and negative controls, respectively.
We next applied the capture assay to the analysis of activated Wnt signaling in paired stage I NSCLC and normal lung tissues. Figure 1C shows results with matched tumor and normal lung tissue samples from representative Stage I NSCLC patients. H23, a Wnt autocrine activated NSCLC line, and 293T, a Wnt negative immortalized human embryonic kidney line, served as positive and negative controls, respectively, in this analysis 39. It can be readily observed in independent tests that tumor tissue from patient #9 showed increased uncomplexed β-catenin levels when compared to normal lung tissue from the same patient. In contrast, patient #19 tumor and normal tissue showed no differences in uncomplexed b-catenin expression under the same conditions. For each tumor and normal tissue pair, we also analyzed total β-catenin and actin levels as internal controls for the amount of protein utilized in the capture assay. Results of replicate analysis of the same samples were consistent (Fig. 1C, 2, and data not shown).
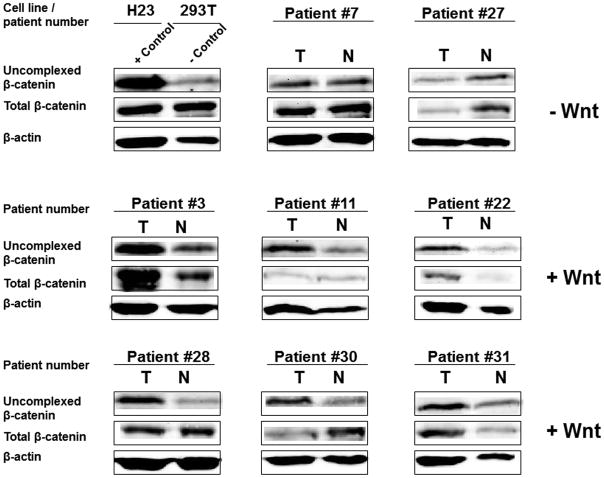
Figure 2.
Wnt pathway activation as measured by the b-catenin capture assay in NSCLCs from patients that developed recurrence. T denotes tumor specimen and N denotes normal lung tissue from the same patient. H23, a Wnt autocrine activated NSCLC line, and 293T, an immortalized human embryonic kidney line, were used as positive and negative controls, respectively.
We tested Wnt activation by this approach in paired tumor and normal lung tissue samples from 55 stage 1 NSCLC patients. For each paired sample, two different observers scored results in the absence of any knowledge of clinical data. Using this approach, tumors from twenty patients (36.4%) exhibited elevated levels of uncomplexed β-catenin. Mutations at specific serine and threonine residues within the N-terminal domain of β-catenin inhibit its phosphorylation-dependent degradation 46. To investigate the presence of mutations in this region, we performed sequence analysis on tumors, which scored positive in the capture assay. Only one (5.0%) Wnt pathway activated tumor specimen contained a β-catenin mutation. This tumor exhibited a TCT to TGT mutation changing serine to cysteine in exon 3, a common site for β-catenin oncogenic activation 46, 47. Thus, the great majority of positive tumors were likely due to Wnt pathway upregulation by an autocrine mechanism previously identified in NSCLC lines 39.
To analyze the relationship of elevated Wnt activity to lung cancer recurrence, patients were divided into Wnt positive (+Wnt) and Wnt negative (−Wnt) groups prior to decoding of the clinical data relevant to each sample. Figure 2 shows that 6 out of the 8 tumors from patients with recurrence scored as Wnt activated. Quantitative densitometry confirmed that the ratio of uncomplexed β-catenin levels in tumor as compared to normal lung tissue from the same patient was greater than 2.5 for each tumor scored as +Wnt, while the ratio was 1.0 or less for the tumors scored as −Wnt (Suppl. Fig. 1). Patient and tumor characteristics for +Wnt and −Wnt groups are summarized in Tables 1 and 2. Overall, the mean age of the patients was 67.9 years, and the median smoking history was 40 pack-years. The majority had adenocarcinoma with a median tumor size of 2.0 cm. The two groups were statistically similar in their major characteristics, including age, gender, tumor size, and type of resection (Table 1). The mean follow up length was 24.6 and 21.6 months in + Wnt and −Wnt groups, respectively. The postoperative recurrence rate was 30.0% (n=6) in the +Wnt group and 5.7% (n=2) in the −Wnt group (Table 2; p=0.02). Disease-free survival was significantly better for −Wnt compared with +Wnt patients (p=0.029; Figure 3). Analysis of recurrences revealed that patients in the +Wnt group also had a significantly higher risk of distal recurrence compared to the −Wnt group (25.0% vs. 2.9%, p=0.02). The sites of tumor recurrence are shown in Table 3. Multiple regression analysis revealed that Wnt pathway activation was the only independent risk factor for recurrence (p=0.04) after adjusting for age, gender, type of resection, and stage.
Table 1.
Demographic and clinical patient characteristics
| Variable | + Wnt (n = 20) | − Wnt (n = 35) | p Value |
|---|---|---|---|
| Age, years (median, IQ range) | 68.0 (15.5) | 70.0 (12.0) | 0.70 |
| Sex (male/female) | 8:12 | 17:18 | 0.54 |
| Race (No.) | 0.71 | ||
| White | 18 (90.0%) | 33 (94.3%) | |
| Black | 1 (5.0%) | 0 | |
| Other | 1 (5.0%) | 2 (5.7%) | |
| Smoking, pack-years (median, IQ range) | 40.0 (32.5) | 40.0 (53.0) | 0.97 |
IQ = interquartile range;
Table 2.
Tumor characteristics and surgical intervention
| Variable | + Wnt (n = 20) | − Wnt (n = 35) | p Value |
|---|---|---|---|
| Tumor size, cm. (median, IQ range) | 2.0 (1.9) | 2.1 (1.6) | 0.69 |
| Histologic type (No.) | 0.31 | ||
| Adenocarcinoma | 18 (90.0%) | 25 (71.4%) | |
| Squamous cell | 2 (10.0%) | 8 (22.9%) | |
| Other | 0 | 2 (5.7%) | |
| Differentiation* | 0.79 | ||
| Well | 6 (31.6%) | 6 (19.4%) | |
| Moderate | 7 (36.8%) | 17 (54.8%) | |
| Poor | 6 (31.6%) | 8 (25.8%) | |
| Stage (No.) | 0.96 | ||
| IA | 13 (65.0%) | 23 (65.7%) | |
| IB | 7 (35.0%) | 12 (34.3%) | |
| Resection Type | 1.00 | ||
| Lobectomy | 15 (75.0%) | 26 (74.3%) | |
| Segmentectomy | 1 (5.0%) | 3 (8.6%) | |
| Wedge | 4 (20.0%) | 6 (17.1%) | |
| # Stations examined (mean ± SD) | 4.3 ± 1.5 | 4.1 ± 1.6 | 0.68 |
| # Nodes examined (median, IQ range) | 10.0 (8.5) | 8.0 (7.0) | 0.56 |
n = 19 and n= 31 respectively; IQ = interquartile range;
Figure 3.

Disease-free survival according to Wnt pathway activity as measured by the b-catenin capture assay in tumors of 55 patients with pathologic stage I NSCLC. Included were 20 tumors scored as +Wnt and 35 tumors that scored as −Wnt in this assay.
Table 3.
Long-term outcome
| Variable | + Wnt (n = 20) | − Wnt (n = 35) | p Value |
|---|---|---|---|
| Mean follow-up (m., mean ± SD) | 24.6 ± 8.3 | 21.6 ± 7.4 | 0.17 |
| Recurrence (No.) | 6 (30.0%) | 2 (5.7%) | 0.02 |
| Distant recur. (No.) | 5 (25.0%) | 1 (2.9%) | 0.02 |
SD = standard deviation;
A minority of NSCLCs were squamous cell carcinomas, and Wnt activation in these tumors occurred at lower frequency (2 out of 10 tumors) than was observed for adenocarcinomas (18 out of 43 tumors) as indicated in Table 2. We then performed statistical analysis of recurrence frequency in those patients with Wnt positive versus Wnt negative adenocarcinomas. The results also showed a higher rate of recurrence in patients with Wnt+ versus Wnt− adenocarcinomas (33.3% vs. 8.0%, p=0.05).
DISCUSSION
The present study analyzed the frequency of Wnt pathway activation in stage I NSCLC, the mechanisms involved, and the relationship of Wnt pathway activation to disease free survival. This is the first study to utilize uncomplexed β-catenin levels as a means to measure Wnt activation in this way. This biochemical assay is only semi-quantitative but improves on immunostaining, which cannot discriminate uncomplexed (active) from complexed beta-catenin (inactive) and, thus, cannot quantify Wnt activation. When we quantified immunoreactivity of uncomplexed beta-catenin in tissue lysates by use of the Licor Odyssey Imaging System, densitometric analysis revealed that the ratio uncomplexed β-catenin levels in tumor as compared to normal lung tissue of the same patient was greater than 2.5 for tumors scored as +Wnt, while the ratio was 1.0 or less for those tumors scored as −Wnt. Using this approach, Wnt pathway activation occurred frequently in patients with stage I NSCLC and was found to potentially be useful as a prognostic factor for cancer recurrence.
For surgically resected stage I NSCLC the 5 year recurrence is 18.4–24% 4–6. To date there has not been consistent evidence indicating a benefit of chemotherapy after surgery in patients with early stage NSCLC. Because of heterogeneity in recurrence rates among patients within the same stage, a reliable tumor molecular or genetic marker that predicted those patients likely to develop recurrent disease might identify a subset that would benefit from adjuvant chemotherapy. Several studies, based on microarray technology, have been performed to determine genetic profiles predictive of survival in NSCLC and to develop genomic approaches for stratifying risk 48–50. However, the identified survival-related genes have lacked consistency among these studies. This may be due to differences in microarray technique, samples analyzed, and/or statistical methods applied. Also, mRNA expression cannot always indicate which proteins are expressed or how their activities may be modulated.
Mutations of K-ras, EGFR and p53 are frequently observed in NSCLC and have been analyzed as predictive markers 13–15. Some studies have suggested that mutations which activate K-Ras or EGFR and inactivate p53 are associated with advanced disease stage and poor prognosis in NSCLC 16–20. However, such findings have not been consistent 11, 14, 15, 21–24. Moreover, most of these studies have involved analysis of heterogeneous groups of patients across different stages of disease.
Studies evaluating the role of these pathways as prognostic markers in early NSCLC have also resulted in conflicting findings (11, 18, 21. Silini and colleagues reported that K-ras mutation has an adverse effect on survival in patients with stage I lung adenocarcinoma 51. However, other studies showed no statistically significant difference in overall or cancer-specific survival in patients with stage I NSCLC 52, 53. Similarly, conflicting outcomes have been reported on the roles of EGFR and p53 as potential prognostic markers in early NSCLC 11, 15, 24, 54. Whereas K-ras mutations tend to occur most commonly in the adenocarcinomas of the lung in smokers, EGFR mutations appear to target adenocarcinomas arising in individuals who have never smoked and women 55, 56. These findings suggest that K-ras and EGFR mutations may each exclude substantial numbers of patients. The problems associated with use of p53 mutations as a prognostic marker are due to the heterogeneity in methodology for detecting the presence of p53 mutations, which have been reported in terms of both gene mutations and detection of protein expression. Also, a large spectrum of ‘hot spots’ makes mutational analysis of p53 gene difficult in the clinical testing.
There is a growing body of evidence for activation of Wnt pathway in NSCLC 39, 40, 43. Efforts to detect evidence of Wnt pathway activation in lung tumor tissues by immunostaining revealed different patterns of b-catenin expression including membranous, membranous-cytoplasmic, and cytoplasmic-nuclear, at frequencies, which were difficult to reconcile with the lack of b-catenin mutations detected in the same large series 57. The mechanism responsible for a much higher frequency of Wnt pathway activation in NSCLC than accounted for by pathway activating mutations in known intracellular components, β-catenin or APC 58, 59 derives from demonstration of autocrine mechanism in as many as 40–50% of NSCLC tumor lines 39. Of note, treatment of such tumor cells with Wnt antagonists, DKK1 or FRP, which act at the cell surface to inhibit Wnt/receptor interaction, resulted in downregulation of activated Wnt signaling associated with inhibition of NSCLC proliferation and acquisition of a more differentiated phenotype 39. In our present study, primary NSCLCs showed a high prevalence of Wnt pathway activation as measured by elevated levels of uncomplexed β-catenin with 36.4% exhibiting increases compared to matched normal lung tissues. Yet, only 1 patient out of 20 with activated Wnt signaling exhibited a β-catenin oncogenic mutation, consistent with previous findings that such mutations are rare events in NSCLC 39, 59, 57. The high prevalence of Wnt pathway activation in NSCLC tissues analyzed by us strongly supports the conclusion that a Wnt autocrine mechanism is responsible in the large majority of Wnt activated primary NSCLCs.
Using immunostaining approaches, previous studies indicated that increased expression of β-catenin predicted a favorable prognosis in patients with NSCLC after resection 60–62. Hommura and colleagues reported a trend toward improved survival in patients with tumors having higher β-catenin expression. Multivariate analysis also showed that high β-catenin expression was a significant and independent favorable prognostic factor (p=0.007) 60. Another report indicated that reduced β-catenin expression in surgically treated NSCLC was associated with lymph node metastasis and an unfavorable prognosis 61. In contrast, other studies have suggested that overexpression of the Wnt pathway was associated with poor tumor differentiation and tumor progression 40, 43. Immunostaining for Wnt 1 ligand expression led to the conclusion that Wnt1 positive status was a significant prognostic factor for recurrence in NSCLC patients (p=0.013). Wnt1 expression was also found to correlate with the expression of reported Wnt pathway targets, including c-Myc, vascular endothelial growth factor-A (VEGF-A), and matrix metalloproteinase-7 (MMP-7) 43. All of these studies evaluated the level of the Wnt pathway activity using immunohistochemical staining for beta-catenin protein, which is not quantitative and may be confounded by the presence of inactive, complexed β-catenin. In the present study, those patients identified as having increased Wnt pathway activity by the capture assay exhibited a higher rate of recurrence than those whose tumors showed no Wnt activation. Moreover, the pattern of recurrence in the +Wnt group favored distal metastasis with 5 patients (25.0%) in this group developing metastases distally compared to 1 patient (2.9%) in the −Wnt group. These results are consistent with a study by Nguyen and colleagues, who analyzed the role of Wnt pathway in determining distal NSCLC metastasis to the brain and bone. They found that Wnt activation, through HOXB9 and LEF1 target genes, was associated with metastasis to multiple organs in mouse models and humans. Also, reduction of Wnt activity attenuated the ability to form brain or bone metastasis in a mouse model 63.
There are some limitations in our study. Although our analysis showed that Wnt pathway activation significantly increases the risk of recurrence after resection of stage I NSCLC, sample size used in the study was relatively small. Thus, larger studies will be necessary to confirm our observations. Also, follow up of the patients potentially was not long enough to detect all recurrences despite the fact that the mean follow up time of around 2 years should capture most recurrences. In fact, new localized malignant lesions more than 2 years after resection of early lung cancers are more likely due to new primary malignancies than recurrences 4, 64.
Adjuvant chemotherapy has been shown to benefit patients with stage II-III NSCLC. However, the benefit for stage IB patients is controversial 8–10. The Stage IA patient group does not appear to benefit from adjuvant therapy after surgical resection 65. Our study is the first utilizing a quantitative means of identifying Wnt activated primary tumors to measure the level of Wnt pathway activity and correlate it with clinical outcomes. An increased level of Wnt pathway activation was associated with a statistically significant increased risk of tumor recurrence after surgical resection. Thus, Wnt pathway activation may have an important role in guiding adjuvant therapy in stage I patients. Investigating potential benefits of adjuvant chemotherapy in such patients with stage I NSCLC could be an important step in efforts to improve outcomes of stage I NSCLC patients.
Supplementary Material
Table 4.
Postoperative recurrence pattern
| Recurrence Pattern | + Wnt (n=6) | − Wnt (n=2) |
|---|---|---|
| Locoregional | ||
| Lung | 1 | 1 |
| Distant | ||
| Brain | 2 | 0 |
| Adrenal glands | 0 | 1 |
| Lung | 1 | 0 |
| Bone | 2 | 0 |
Acknowledgments
Source of Funding: This study is supported in part by NIH and NYSTEM grants.
Footnotes
Conflicts of Interest: None of the authors have conflicts of interest to declare.
References
- 1.Spira A, Ettinger DS. Multidisciplinary management of lung cancer. N Engl J Med. 2004;350:379–392. doi: 10.1056/NEJMra035536. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;355:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Chu KC, Tarone RE. Recent trends in lung cancer mortality in the United States. J Natl Cancer Inst. 2001;93:277–283. doi: 10.1093/jnci/93.4.277. [DOI] [PubMed] [Google Scholar]
- 4.Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 5.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:15–22. doi: 10.1016/0003-4975(95)00537-u. discussion 622-3. [DOI] [PubMed] [Google Scholar]
- 6.Goodgame B, Viswanathan A, Zoole J, et al. Risk of recurrence of resected stage I non-small cell lung cancer in elderly patients as compared with younger patients. J Thorac Oncol. 2009;4:1370–1374. doi: 10.1097/JTO.0b013e3181b6bc1b. [DOI] [PubMed] [Google Scholar]
- 7.Tanoue LT, Detterbeck FC. New TNM classification for non-small-cell lung cancer. Expert Rev Anticancer Ther. 2009;9:413–423. doi: 10.1586/era.09.11. [DOI] [PubMed] [Google Scholar]
- 8.Solomon B, Mitchell JD, Bunn PA., Jr Adjuvant chemotherapy for resected non-small-cell lung cancer. Oncology (Williston Park) 2005;19:1685–97. discussion 1698-700, 1705. [PubMed] [Google Scholar]
- 9.Visbal AL, Leighl NB, Feld R, et al. Adjuvant Chemotherapy for Early-Stage Non-small Cell Lung Cancer. Chest. 2005;128:2933–2943. doi: 10.1378/chest.128.4.2933. [DOI] [PubMed] [Google Scholar]
- 10.Tiseo M, Franciosi V, Grossi F, et al. Adjuvant chemotherapy for non-small cell lung cancer: ready for clinical practice? Eur J Cancer. 2006;42:8–16. doi: 10.1016/j.ejca.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 11.Tsao MS, Aviel-Ronen S, Ding K, et al. Prognostic and predictive importance of p53 and RAS for adjuvant chemotherapy in non small-cell lung cancer. J Clin Oncol. 2007;25:5240–5247. doi: 10.1200/JCO.2007.12.6953. [DOI] [PubMed] [Google Scholar]
- 12.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 13.Rodenhuis S, Slebos RJ. The ras oncogenes in human lung cancer. Am Rev Respir Dis. 1990;142:S27–30. doi: 10.1164/ajrccm/142.6_Pt_2.S27. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch FR, Varella-Garcia M, Bunn PA, Jr, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 15.Ahrendt SA, Hu Y, Buta M, et al. p53 mutations and survival in stage I non-small-cell lung cancer: results of a prospective study. J Natl Cancer Inst. 2003;95:961–970. doi: 10.1093/jnci/95.13.961. [DOI] [PubMed] [Google Scholar]
- 16.Huncharek M, Muscat J, Geschwind JF. K-ras oncogene mutation as a prognostic marker in non-small cell lung cancer: a combined analysis of 881 cases. Carcinogenesis. 1999;20:1507–1510. doi: 10.1093/carcin/20.8.1507. [DOI] [PubMed] [Google Scholar]
- 17.Rosell R, Li S, Skacel Z, et al. Prognostic impact of mutated K-ras gene in surgically resected non-small cell lung cancer patients. Oncogene. 1993;8:2407–2412. [PubMed] [Google Scholar]
- 18.Nelson HH, Christiani DC, Mark EJ, et al. Implications and prognostic value of K-ras mutation for early-stage lung cancer in women. J Natl Cancer Inst. 1999;91:2032–2038. doi: 10.1093/jnci/91.23.2032. [DOI] [PubMed] [Google Scholar]
- 19.Volm M, Rittgen W, Drings P. Prognostic value of ERBB-1, VEGF, cyclin A, FOS, JUN and MYC in patients with squamous cell lung carcinomas. Br J Cancer. 1998;77:663–669. doi: 10.1038/bjc.1998.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsudomi T, Hamajima N, Ogawa M, et al. Prognostic significance of p53 alterations in patients with non-small cell lung cancer: a meta-analysis. Clin Cancer Res. 2000;6:4055–4063. [PubMed] [Google Scholar]
- 21.Mascaux C, Iannino N, Martin B, et al. The role of RAS oncogene in survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2005;92:131–139. doi: 10.1038/sj.bjc.6602258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller JH, Adak S, Feins RH, et al. Lack of prognostic significance of p53 and K-ras mutations in primary resected non-small-cell lung cancer on E4592: a Laboratory Ancillary Study on an Eastern Cooperative Oncology Group Prospective Randomized Trial of Postoperative Adjuvant Therapy. J Clin Oncol. 2001;19:448–457. doi: 10.1200/JCO.2001.19.2.448. [DOI] [PubMed] [Google Scholar]
- 23.Greatens TM, Niehans GA, Rubins JB, et al. Do molecular markers predict survival in non-small-cell lung cancer? Am J Respir Crit Care Med. 1998;157:1093–1097. doi: 10.1164/ajrccm.157.4.9707108. [DOI] [PubMed] [Google Scholar]
- 24.Steels E, Paesmans M, Berghmans T, et al. Role of p53 as a prognostic factor for survival in lung cancer: a systematic review of the literature with a meta-analysis. Eur Respir J. 2001;18:705–719. doi: 10.1183/09031936.01.00062201. [DOI] [PubMed] [Google Scholar]
- 25.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 27.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 28.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 29.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 30.Fukuchi T, Sakamoto M, Tsuda H, et al. Beta-catenin mutation in carcinoma of the uterine endometrium. Cancer Res. 1998;58:3526–3528. [PubMed] [Google Scholar]
- 31.Miyoshi Y, Iwao K, Nagasawa Y, et al. Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res. 1998;58:2524–2527. [PubMed] [Google Scholar]
- 32.Palacios J, Gamallo C. Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 1998;58:1344–1347. [PubMed] [Google Scholar]
- 33.Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–2523. [PubMed] [Google Scholar]
- 34.Park WS, Oh RR, Park JY, et al. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res. 1999;59:4257–4260. [PubMed] [Google Scholar]
- 35.Kim J, You L, Xu Z, et al. Wnt inhibitory factor inhibits lung cancer cell growth. J Thorac Cardiovasc Surg. 2007;133:733–737. doi: 10.1016/j.jtcvs.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 36.You L, He B, Uematsu K, et al. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res. 2004;64:3474–3478. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- 37.You L, He B, Xu Z, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004;23:6170–6174. doi: 10.1038/sj.onc.1207844. [DOI] [PubMed] [Google Scholar]
- 38.Bafico A, Liu G, Goldin L, et al. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 39.Akiri G, Cherian MM, Vijayakumar S, et al. Wnt pathway aberrations including autocrine Wnt activation occur at high frequency in human non-small-cell lung carcinoma. Oncogene. 2009;28:2163–2172. doi: 10.1038/onc.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei Q, Zhao Y, Yang ZQ, et al. Dishevelled family proteins are expressed in non-small cell lung cancer and function differentially on tumor progression. Lung Cancer. 2008;62:181–192. doi: 10.1016/j.lungcan.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 41.van de Wetering M, Sancho E, Verweij C, et al. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241–250. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 42.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 43.Huang CL, Liu D, Ishikawa S, et al. Wnt1 overexpression promotes tumour progression in non-small cell lung cancer. Eur J Cancer. 2008;44:2680–2688. doi: 10.1016/j.ejca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 44.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 45.Bafico A, Gazit A, Wu-Morgan SS, et al. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- 46.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 47.Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 48.Beer DG, Kardia SL, Huang CC, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 49.Wigle DA, Jurisica I, Radulovich N, et al. Molecular profiling of non-small cell lung cancer and correlation with disease-free survival. Cancer Res. 2002;62:3005–3008. [PubMed] [Google Scholar]
- 50.Garber ME, Troyanskaya OG, Schluens K, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silini EM, Bosi F, Pellegata NS, et al. K-ras gene mutations: an unfavorable prognostic marker in stage I lung adenocarcinoma. Virchows Arch. 1994;424:367–373. doi: 10.1007/BF00190558. [DOI] [PubMed] [Google Scholar]
- 52.Graziano SL, Gamble GP, Newman NB, et al. Prognostic significance of K-ras codon 12 mutations in patients with resected stage I and II non-small-cell lung cancer. J Clin Oncol. 1999;17:668–675. doi: 10.1200/JCO.1999.17.2.668. [DOI] [PubMed] [Google Scholar]
- 53.Lu C, Soria JC, Tang X, et al. Prognostic factors in resected stage I non-small-cell lung cancer: a multivariate analysis of six molecular markers. J Clin Oncol. 2004;22:4575–4583. doi: 10.1200/JCO.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 54.Pastorino U, Andreola S, Tagliabue E, et al. Immunocytochemical markers in stage I lung cancer: relevance to prognosis. J Clin Oncol. 1997;15:2858–2865. doi: 10.1200/JCO.1997.15.8.2858. [DOI] [PubMed] [Google Scholar]
- 55.Ahrendt SA, Decker PA, Alawi EA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 56.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 57.Kotsinas A, Evangelou K, Zacharatos P, et al. Proliferation, but not apoptosis, is associated with distinct beta-catenin expression patterns in non-small-cell lung carcinomas: relationship with adenomatous polyposis coli and G(1)-to S-phase cell-cycle regulators. Am J Pathol. 2002;161:1619–1634. doi: 10.1016/s0002-9440(10)64440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sunaga N, Kohno T, Kolligs FT, et al. Constitutive activation of the Wnt signaling pathway by CTNNB1 (beta-catenin) mutations in a subset of human lung adenocarcinoma. Genes Chromosomes Cancer. 2001;30:316–321. doi: 10.1002/1098-2264(2000)9999:9999<::aid-gcc1097>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 59.Shigemitsu K, Sekido Y, Usami N, et al. Genetic alteration of the beta-catenin gene (CTNNB1) in human lung cancer and malignant mesothelioma and identification of a new 3p21. 3 homozygous deletion. Oncogene. 2001;20:4249–4257. doi: 10.1038/sj.onc.1204557. [DOI] [PubMed] [Google Scholar]
- 60.Hommura F, Furuuchi K, Yamazaki K, et al. Increased expression of beta-catenin predicts better prognosis in nonsmall cell lung carcinomas. Cancer. 2002;94:752–758. doi: 10.1002/cncr.10213. [DOI] [PubMed] [Google Scholar]
- 61.Retera JM, Leers MP, Sulzer MA, et al. The expression of beta-catenin in non-small-cell lung cancer: a clinicopathological study. J Clin Pathol. 1998;51:891–894. doi: 10.1136/jcp.51.12.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choi YS, Shim YM, Kim SH, et al. Prognostic significance of E-cadherin and beta-catenin in resected stage I non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;24:441–449. doi: 10.1016/s1010-7940(03)00308-7. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen DX, Chiang AC, Zhang XH, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009;138:51–62. doi: 10.1016/j.cell.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawachi R, Tsukada H, Nakazato Y, et al. Early recurrence after surgical resection in patients with pathological stage I non-small cell lung cancer. Thorac Cardiovasc Surg. 2009;57:472–475. doi: 10.1055/s-0029-1185734. [DOI] [PubMed] [Google Scholar]
- 65.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.