Abstract
The sodium channel blocker mexiletine is considered the first-line drug in myotonic syndromes, a group of muscle disorders characterized by membrane over-excitability. We previously showed that the β-adrenoceptor modulators, clenbuterol and propranolol, block voltage-gated sodium channels in a manner reminiscent to mexiletine, whereas salbutamol and nadolol do not. We now developed a pharmacological rat model of myotonia congenita to perform in vivo preclinical test of antimyotonic drugs. Myotonia was induced by i.p. injection of 30 mg/kg of anthracene-9-carboxylic acid (9-AC), a muscle chloride channel blocker, and evaluated by measuring the time of righting reflex (TRR). The TRR was prolonged from <0.5 s in control conditions to a maximum of ∼4 s, thirty minutes after 9-AC injection, then gradually recovered in a few hours. Oral administration of mexiletine twenty minutes after 9-AC injection significantly hampered the TRR prolongation, with an half-maximum efficient dose (ED50) of 12 mg/kg. Both propranolol and clenbuterol produced a dose-dependent antimyotonic effect similar to mexiletine, with ED50 values close to 20 mg/kg. Antimyotonic effects of 40 mg/kg mexiletine and propranolol lasted for 2 h. We also demonstrated, using patch-clamp methods, that both propranolol enantiomers exerted a similar block of skeletal muscle hNav1.4 channels expressed in HEK293 cells. The two enantiomers (15 mg/kg) also showed a similar antimyotonic activity in vivo in the myotonic rat. Among the drugs tested, the R(+)-enantiomer of propranolol may merit further investigation in humans, because it exerts antimyotonic effect in the rat model, while lacking of significant activity on the β-adrenergic pathway. This study provides a new and useful in vivo preclinical model of myotonia congenita in order to individuate the most promising antimyotonic drugs to be tested in humans.
Keywords: Myotonia, Over-excitability, Propranolol, Mexiletine, In vivo rat model, hNav1.4
Abbreviations: TRR, time of righting reflex; 9-AC, anthracene-9-carboxylic acid
Highlights
► An in vivo pharmacological model of myotonia congenita was developed in the rat using 9-AC i.p. injection. ► A preclinical screening of antimyotonic drugs was performed. ► Propranolol and clenbuterol exert antimyotonic activity comparable to mexiletine. ► Both propranolol enantiomers block skeletal muscle hNav1.4 sodium channels in vitro. ► Both propranolol enantiomers exert similar antimyotonic effect in vivo.
1. Introduction
The non-dystrophic myotonic syndromes are a set of disorders targeting skeletal muscles, characterized by muscle stiffness following contraction or percussion. The pathology is caused by mutations in genes encoding voltage-dependent chloride channels (CLCN1 gene, ClC-1 protein) or sodium channels (SCN4A gene, Nav1.4 protein), which are expressed exclusively in skeletal muscles (Matthews et al., 2010). Loss-of-function mutations of ClC-1 channels or gain-of-function mutations of Nav1.4 channels pathologically increase sarcolemma excitability with occurrence of abnormal action potentials runs and consequent difficulties in muscle relaxation, determining the muscle stiffness characteristic of myotonic muscle.
Today the pharmacotherapy of myotonic syndromes, resulting either from chloride or sodium channel mutations, is based essentially on the use of sodium channel blockers, which reduce sarcolemma excitability by inhibiting action potentials (Conte Camerino et al., 2007). The preferred drug is mexiletine, a class Ib antiarrhythmic, used at rather high doses (Lehmann-Horn et al., 2008; Matthews et al., 2010). Preferential binding to inactivated channels and use-dependent block are thought to constitute the basis of the selective action of mexiletine on pathologic hyperactive tissues. However, not all the patients take benefits from mexiletine, which can induce side effects limiting patient compliance, including epigastric discomfort, nausea, tremor, anxiety and headaches. Patients with cardiac diseases may avoid mexiletine. Moreover lack of mexiletine efficacy has been observed in a number of patients, most probably in relation to pharmacogenetic mechanisms (Desaphy et al., 2004). In addition, mexiletine has been withdrawn from the market in several countries. Thus there is a critical need for the individuation of new efficient and safe antimyotonic drugs.
In a previous study, we showed that drugs known as modulators of β-adrenergic receptors are able to block sodium channels in a manner reminiscent to local anesthetic drugs (Desaphy et al., 2003). The β2-agonist clenbuterol and the β-antagonist propranolol showed efficacies comparable to mexiletine in producing use-dependent inhibition of sodium currents in rat skeletal muscle fibers, as well as in tsA201 cells transiently expressing the human muscle sodium channel isoform, hNav1.4. In contrast, the β-agonist salbutamol and the β-antagonist nadolol had no effect on sodium currents at 1 mM concentration. Examination of chemical structures and physicochemical properties of these drugs revealed the presence of the two pharmacophores important for sodium channel blocking activity, the tertiary amine and the aromatic ring, which confer respectively a high pKa and a high lipophilicity. However, the two inactive compounds are characterized by the presence of two hydroxyl groups on the aromatic moiety, which appeared to be determinant for impeding sodium channel blockade (Desaphy et al., 2012). Accordingly, clenbuterol was shown to inhibit action potential firing in skeletal muscle fibers, whereas nadolol did not (Desaphy et al., 2003).
In the present study, we evaluated in vivo the antimyotonic activity of these β-adrenergic drugs compared to that of mexiletine. For this, we have developed an animal model of myotonia allowing a quantitative preclinical study. We have chosen to use a pharmacological model rather than the available genetic models, because it offers a large availability of animals, it allows to measure myotonia in a reproducible way through a non-invasive method, and it is cheaper. In this model, myotonia is induced in adult rats by a single intraperitoneal injection of anthracene-9-carboxylic acid (9-AC). By blocking muscle ClC-1 channels, 9-AC is able to induce a myotonic state similar to chloride channel myotonia (Bryant and Morales-Aguilera, 1971; Furman and Barchi, 1978; Estevez et al., 2003). The myotonic state was evaluated by calculating the time of righting reflex (TRR), that is the time taken by the rat to turn back on his four limbs after being posted in supine position. An antimyotonic drug is expected to shorten the TRR induced by 9-AC.
The results demonstrate that both clenbuterol and propranolol show a time-dependent antimyotonic effect in the dose range of 5–40 mg/kg, quite similar to that of mexiletine. In contrast, either the β2-agonist salbutamol or the β-antagonist nadolol, both unable to block sodium channels, did not show any significant antimyotonic effect. We also demonstrated that both propranolol enantiomers produced undistinguishable hNav1.4 sodium channel block in vitro and antimyotonic activity in vivo. We thus propose the R(+)-enantiomer of propranolol, which is inactive on β-adrenoreceptors, as a possible alternative to mexiletine in the treatment of myotonic syndromes.
2. Materials and methods
2.1. Sodium current measurement in HEK293 cells
Whole-cell sodium currents (INa) were recorded with patch-clamp technique in HEK293 cells permanently transfected with the human skeletal muscle isoform of voltage-gated sodium channel, hNav1.4 (Desaphy et al., 2012). Sodium current recordings were performed at room temperature (20–22 °C) using an Axopatch 1D amplifier (Axon Instruments, Union City, CA, USA). Voltage clamp protocols and data acquisition were performed with pCLAMP 9.2 software (Axon Instruments) through a 12-bit A-D/D-A interface (Digidata 1340, Axon Instruments). Pipettes made with Corning 7052 glass (Garner Glass, Claremont, CA, USA) had resistance that ranged from 1 to 3 MW. Currents were low-pass filtered at 2 kHz (−3 dB) by the four-pole Bessel filter of the amplifier and digitized at 10–20 kHz. After the patch membrane had been ruptured, a 25-ms-long test pulse to −30 mV from a holding potential of −120 mV was applied to the cell at a low frequency until stabilization of sodium current amplitude and kinetics was achieved (typically 5 min). Only those data obtained from cells exhibiting series resistance errors <5 mV were considered for analysis. Little (<5%) or no rundown was observed within the experiments.
2.2. Animal care and in vivo experiments
The experiments have been performed in accordance with the Italian Guidelines for the use of laboratory animals, which conforms with the European Union Directive for the protection of experimental animals (2011/63/EU), and received approval from the Animal Experimentation Ethic Committee of the University of Bari-Aldo Moro (CESA). All efforts were made to minimize animal suffering and to reduce the number of animals used.
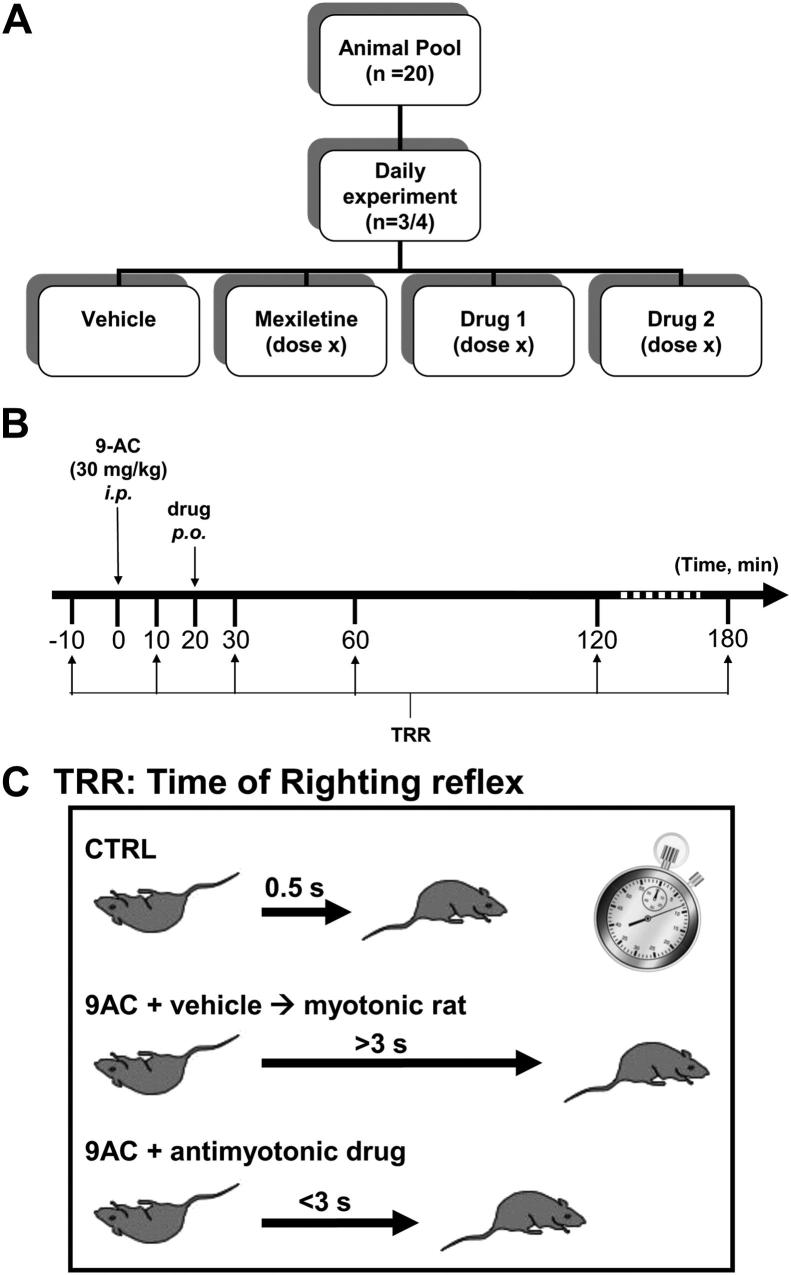
Adult WISTAR rats (350–500 g) were purchased from Charles River – Italy. A total of 20 rats were housed individually and given food and water ad libitum. In a typical daily experiment, three/four rats were randomly extracted from this pool to receive an intraperitoneal injection of 30 mg/kg anthracene-9-carboxylic acid (9-AC) (Fig. 1A). Ten minutes after 9-AC injection, the animals received drug or vehicle per os using an esophageal cannula (Fig. 1B). Myotonia state was assayed by measuring the time of righting reflex, that is the time taken by the rat to turn back on his four limbs after having been positioned in supine position. Ambient temperature was maintained to 20–22 °C. The TRR was determined 10 min before and 10, 30, 60, 120, and 180 min after 9-AC administration. At each time point, the TRR was calculated as the average of 7 determinations to obtain a S.E.M. minor than 10% of the mean. A 1-min interval was respected between two measures to avoid any warm-up phenomenon. Typically, a control rat takes less than 0.5 s to turn back on his limbs, whereas, 30 min after 9-AC injection, a myotonic rat shows a TRR greater than 3 s (Fig. 1C). A drug with antimyotonic activity is expected to reduce the TRR with respect to the TRR measured in the rat receiving drug vehicle only. Effect of 9-AC was maximal 30 min after injection and reversed spontaneously within 15 h. This protocol was used to evaluate the dose–response relationships for mexiletine, propranolol, and clenbuterol. A second protocol was used to evaluate the duration of antimyotonic effect of a single drug administration; In this case, the exploratory drug was administrated 120, 90, 60, or 30 min before 9-AC, and the TRR (mean of 7 determinations) was measured 30 min after 9-AC. This protocol was performed contemporaneously in 3 rats, one of which receiving 40 mg/kg mexiletine, an other receiving 40 mg/kg propranolol, and the third one receiving only vehicle.
Fig. 1.
Experimental design. (A) A daily experiment forecasted the use of 4 animals extracted from a reserve pool of 20 rats. The four animals received a single i.p. injection of 9-AC, 30 mg/kg, and 20 min after, a dose of either mexiletine or exploratory drugs, or the drug vehicle alone. (B) Myotonia state was assayed by measuring the time of righting reflex (TRR), that is the time taken by the rat to turn back on his four limbs, after being posted in supine position. The TRR was measured 10 min before, and 10, 30, 60, 120, and 180 min after 9-AC injection. At each time point, the TRR value was calculated as the mean of seven determinations. (C) In control condition (before 9-AC injection), the TRR is less than 0.5 s. After 9-AC and drug vehicle administration, the TRR increased up to >3 s. An antimyotonic drug is expected to reduce the TRR to less than 3 s. At the end of the experiment, the rats were allowed to rest for at least 48 h before being reintegrated in the reserve pool.
At the end of each experimental day, the tested rats were allowed a resting period of at least 48 h before to reintegrate the reserve pool. By the end of the study, animals were reinserted in the animal quarter.
For each drug dose, the protocols were repeated on three-to-six days in different rats, and the experimental points are given as the mean ± S.E.M. Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by ad-hoc Bonferroni's t test. A P value minor to 0.05 was considered statistically significant.
2.3. Drugs and solutions
Patch clamp pipette solution contained in mM: 120 CsF, 10 CsCl, 10 NaCl, 5 EGTA and 5 HEPES, and the pH was set to 7.2 with CsOH. Bath solution for patch clamp recordings contained (in mM): 150 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 HEPES and 5 glucose. The pH was set to 7.4 with NaOH. All the compounds were purchased from Sigma–Aldrich (Milan, Italy).
Mexiletine hydrochloride, 9-anthracenecarboxylic acid (9-AC), salbutamol, clenbuterol hydrochloride, nadolol, DL-propranolol hydrochloride and its R(+) and S(−) enantiomers, hydrochloride salts, were purchased from Sigma-Aldrich (Milan, Italy). For patch-clamp experiments, racemic propranolol and its single enantiomers were dissolved directly in external patch solution at the desired final concentration. The patched cell was continuously exposed to a stream of control or drug-supplemented bath solution flowing out from a plastic capillary. For in vivo experiments, a solution of 2.4 g/l 9-AC was prepared each day in distilled water containing 0.3% bicarbonate; The volume of i.p. injection was adjusted to get 30 mg/kg body weight. The choice of 9-AC dose was based on preliminary experiments, indicating 30 mg/kg as the lower dose producing a reproducible myotonia, assayed by TRR, in 100% of treated rats (not shown). Exploratory drugs were diluted at the desired concentration directly in physiological 0.9% NaCl saline for oral administration of a volume close to 1 ml. The choice of drug doses was based on the usual clinical dose of mexiletine in humans, that is 3–8 mg/kg a day. Thus we first tested 5 mg/kg mexiletine and other compounds, and then increased the dose up to 40 mg/kg to draw a dose–response curve.
3. Results
3.1. Reproducible myotonia induced by 9-AC
Myotonia was induced in rats by intraperitoneal injection of 30 mg/kg 9-AC. Soon after 9-AC injection, the rats show evident stiffness and difficulties to move. Nevertheless, the animals remained fully conscious and alert. Breathing appeared normal. When hearing an unexpected noise, the animals reacted by a jump on site but had great difficulties to move away due to muscle stiffness. Myotonia was evaluated by measuring the time of righting reflex.
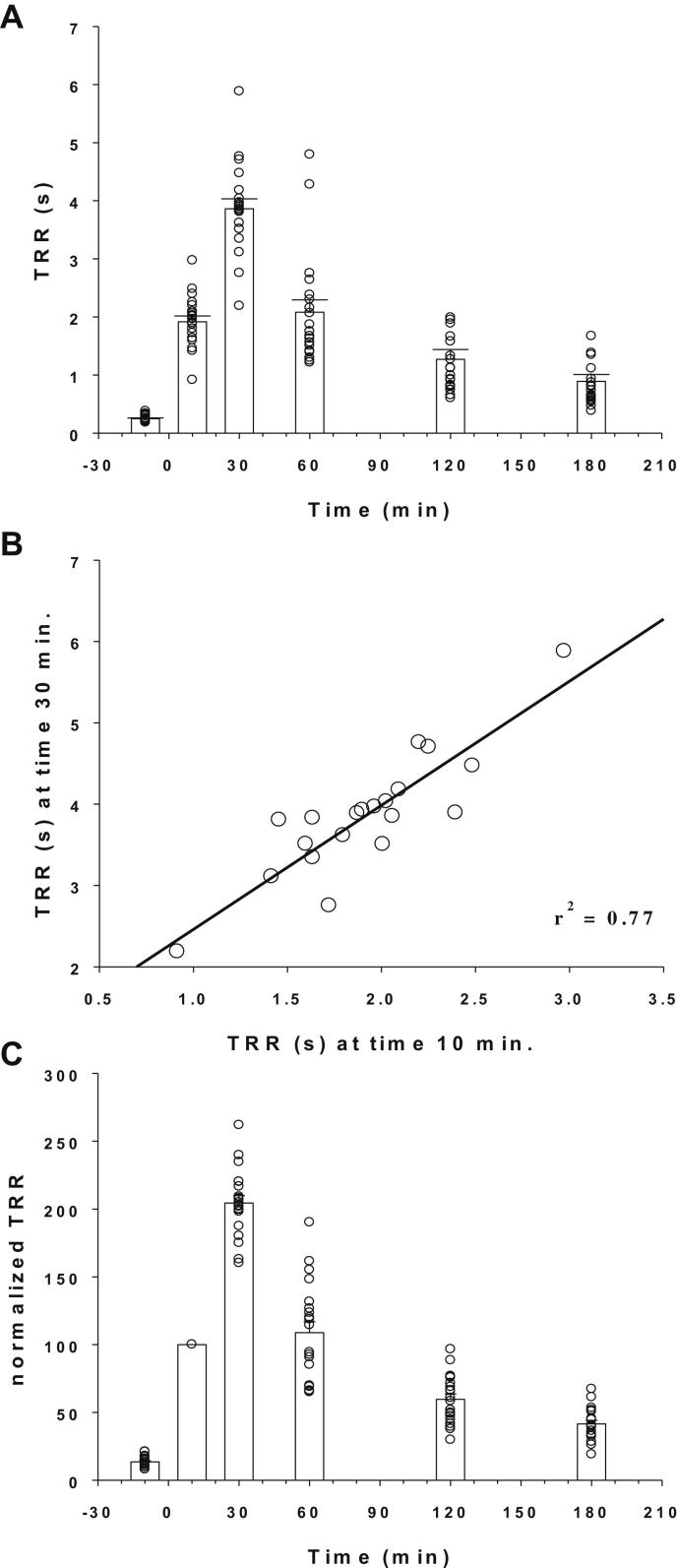
Fig. 2A shows the time course of TRR measured in a number of rats having received i.p. 9-AC and oral drug vehicle, according to the protocol described in Fig. 1B. Ten minutes before 9-AC injection the TRR was less than 0.5 s. The TRR was dramatically prolonged to ∼2 s 10 min after 9-AC, and increased further to ∼4 s 30 min after 9-AC. Then the TRR decreased gradually over time, being close to 1 s 3 h after 9-AC. Statistical comparison of TRR mean values measured at each time point was performed using one-way ANOVA followed by ad-hoc Bonferroni's t-test (ANOVA parameters: F = 96.35; k-1 = 5; N-k = 114; P < 0.0001). All TRR mean values resulted significantly different from the others (at least P < 0.04), except TRR (60) and TRR (10) that show no significant difference. The TRR was fully recovered within 15 h after 9-AC injection (not shown). Myotonia developed in all the tested rats with the same kinetics, and the individual responses of rats showed reasonable variability. Quite similar variability in peak myotonia was also observed between two 9-AC injection performed in a single rat on different days (not shown). Importantly, we observed that the peak TRR measured at time 30 min was linearly correlated to the TRR measured at 10 min (Fig. 2B), suggesting that the TRR measured at the 10 min-time point was a predictive indicator of the full response in each animal. Thus we normalized the TRR (t) as a function of TRR (10 min) as shown in Fig. 2C, as a useful maneuver to compare myotonia between rats receiving the various drugs or vehicle.
Fig. 2.
Time course of myotonic effect of 9-AC. (A) The myotonic effect induced by 30 mg/kg 9-AC was evaluated by measuring the TRR, as shown in Fig. 1. Time zero corresponds to 9-AC injection. Each circle represents the TRR measured in each rat (mean from seven determinations), while bars show the mean ± S.E.M. of TRR calculated from the 20 tested rats. Statistical analysis was performed with one-way ANOVA followed by ad-hoc Bonferonni's t-test (ANOVA parameters: F = 96.35; k-1 = 5; N-k = 114; P < 0.0001). All TRR mean values resulted significantly different from the others (at least P < 0.04), except TRR (60) and TRR (10) that were not significantly different. (B) The TRR value measured 30 min after 9-AC injection was linearly correlated to the TRR measured 10 min after 9-AC (r2 = 0.77). (C) The TRR values determined at each time point were normalized with respect to the TRR value measured 10 min after 9-AC injection.
3.2. Antimyotonic effects of mexiletine and beta-adrenergic drugs
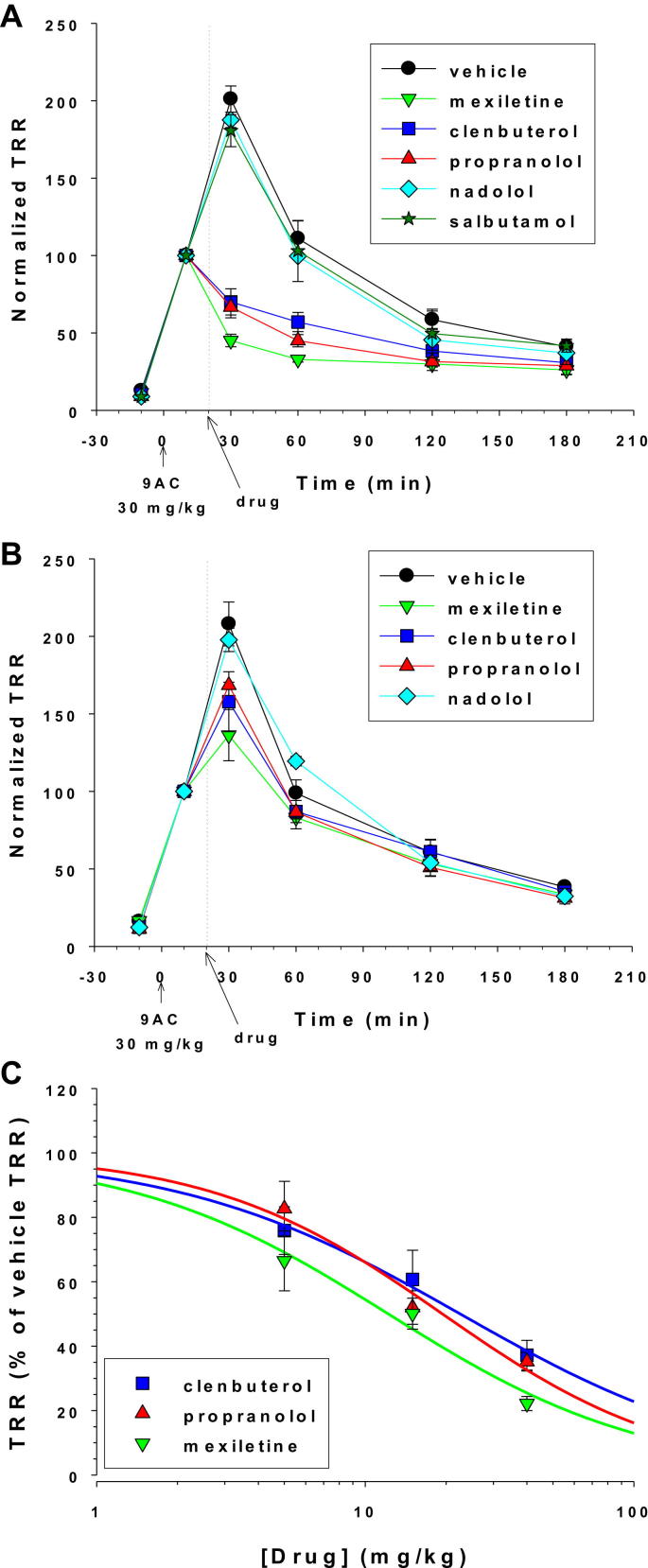
Mexiletine is today considered a first-line drug in myotonic patients (Lehmann-Horn et al., 2008), and exerted antimyotonic effect in vivo in the adr (arrested development of righting response) myotonic mouse (De Luca et al., 2004). In 9-AC treated rats, oral mexiletine, 40 mg/kg, produced a drastic reduction of TRR with respect to vehicle, as soon as 10 min after administration (Fig. 3A). Statistical comparison of all the tested drugs and vehicle was performed at each time point using one-way ANOVA followed by ad-hoc Bonferroni's t-test. Mexiletine reduced the TRR (30 min) by 77.8 ± 2.2% (n = 6, P < 0.0001). The antimyotonic effect of 40 mg/kg mexiletine was still significant after 40 (P < 0.0001) and 100 (P < 0.005) minutes, but was minimal after 160 min. Actually, the absolute TRR value remained quite constant over time in presence of mexiletine, suggesting that 40 mg/kg may be close to the maximal mexiletine efficient dose. The effects of mexiletine were dose-dependent. Effects of 5 mg/kg are shown in Fig. 3B. The reduction of TRR (30) by mexiletine with respect to vehicle was 33.5 ± 9.3% (n = 4, P < 0.005), whereas antimyotonic effect disappeared as soon as 40 min after mexiletine administration. The antimyotonic effect of 15 mg/kg mexiletine was also measured in 3 rats and was statistically significant 10 and 40 min after drug administration (not shown). The dose–response relationship was constructed for antimyotonic effect of mexiletine on TRR (30) (Fig. 3C). The fit of this relationship (see fit equation in Fig. 3 legend) indicated an half-maximal efficient dose (ED50) of 12.2 ± 2.3 mg/kg and a slope factor (nH) of 0.9 ± 0.2 (means ± S.E. of the fit).
Fig. 3.
Antimyotonic effects of exploratory drugs. The time course of normalized TRR values was determined in 9-AC treated rats receiving either 40 mg/kg (A) or 5 mg/kg (B) of exploratory drugs or relative vehicle. Each curve is the mean ± S.E.M. calculated from 3-to-9 rats. Statistical comparison was performed between all the tested drugs at each time point using one-way ANOVA followed by ad-hoc Bonferroni's t-test. At 40 mg/kg, mexiletine, propranolol, and clenbuterol significantly reduced the TRR at 30-, 60-, and 120-min time points compared to vehicle (at least P < 0.05, see Section 3.2 for details). At 5 mg/kg, mexiletine, propranolol, and clenbuterol significantly reduced the TRR only at the 30-min time point. (C) The dose–response curve were constructed for mexiletine, propranolol, and clenbuterol by reporting the value of normalized TRR (expressed as percentage of normalized TRR measured in rat receiving drug vehicle alone) measured 10 min after drug administration (i.e. 30 min after 9-AC injection). The dose–response curve were fitted with equation TRR = 100/[1 + exp ([Drug]/ED50)nH], where [Drug] is the drug dose, ED50 is the half-maximum efficient dose, and nH is the slope factor. Values of fit parameters are given in Section 3.2.
The antimyotonic activity of β2-adrenoceptor agonists, salbutamol and clenbuterol, and β-antagonists, nadolol and propranolol, were tested in parallel to mexiletine (Fig. 3). At 40 mg/kg, both salbutamol (n = 3) and nadolol (n = 3) did not show any significant antimyotonic effect. In contrast clenbuterol and propranolol induced antimyotonic effects quite similar to mexiletine, although with a reduced efficiency. The TRR (30) was reduced by 64.8 ± 2.9% with propranolol (n = 4, P < 0.0001) and 62.8 ± 2.9% with clenbuterol (n = 4, P < 0.0001), and significant antimyotonic effect was also observed after 40 (P < 0.0001 for propranolol and P < 0.001 for clenbuterol) and 100 (P < 0.01 for propranolol and P < 0.05 for clenbuterol) minutes. At 5 mg/kg, antimyotonic activities on TRR (30) of propranolol and clenbuterol were lower than that of mexiletine but still statistically significant (P < 0.05 for both drugs). The ED50 values were 22.6 ± 2.4 mg/kg (nH = 0.8 ± 0.1) for clenbuterol and 19.4 ± 3.2 mg/kg (nH = 1.0 ± 0.2) for propranolol (Fig. 3C).
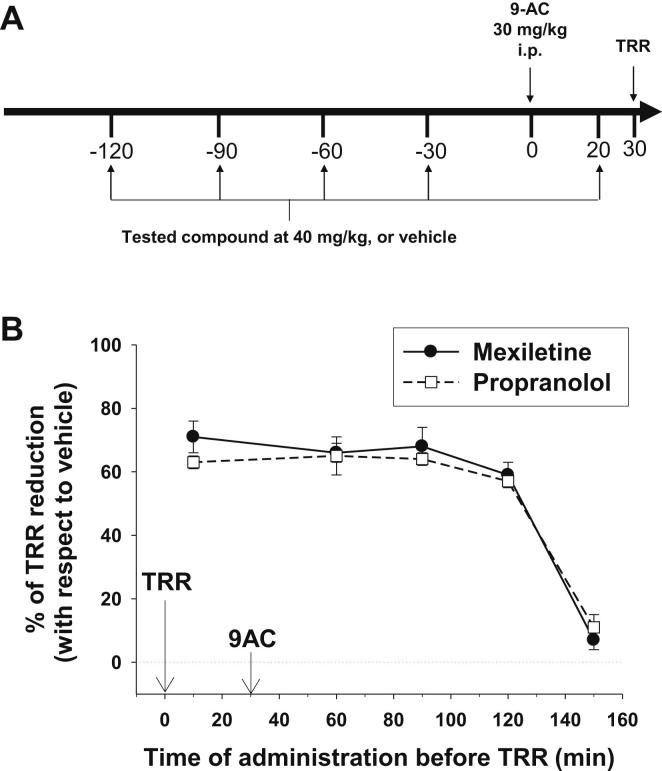
To compare the duration of antimyotonic effect of mexiletine and propranolol, we modified the protocol as shown in Fig. 4A. The TRR was measured only once, 30 min after 9-AC injection, but either drug (40 mg/kg) or vehicle were orally administrated to different groups of animals at various time points from 150 to 10 min before TRR measurement. The TRR values measured in drug-treated rats were normalized with respect to the TRR measured in rat receiving the vehicle alone and reported as a function of time (Fig. 4B). The time courses for propranolol and mexiletine were quite similar. Effect was maximum 10 min after drug administration, remained quite constant for 2 h, and dropped drastically after 150 min.
Fig. 4.
Duration of antimyotonic effect of mexiletine and propranolol. (A) The drug (40 mg/kg) or relative vehicle were administrated to different groups of animals at 120, 90, 60, 30 min before or 20 min after 9-AC injection. Myotonia was assayed by measuring the TRR 30 min after 9-AC injection. (B) The percentage of TRR reduction obtained with 40 mg/kg mexiletine or propranolol with respect of the TRR measured in rats receiving the vehicle alone is plotted against the time of drug/vehicle administration before TRR measurement. Each experimental point is the mean ± S.E.M. from 3 to 6 rats.
3.3. Sodium channel blockade and antimyotonic effects of propranolol enantiomers
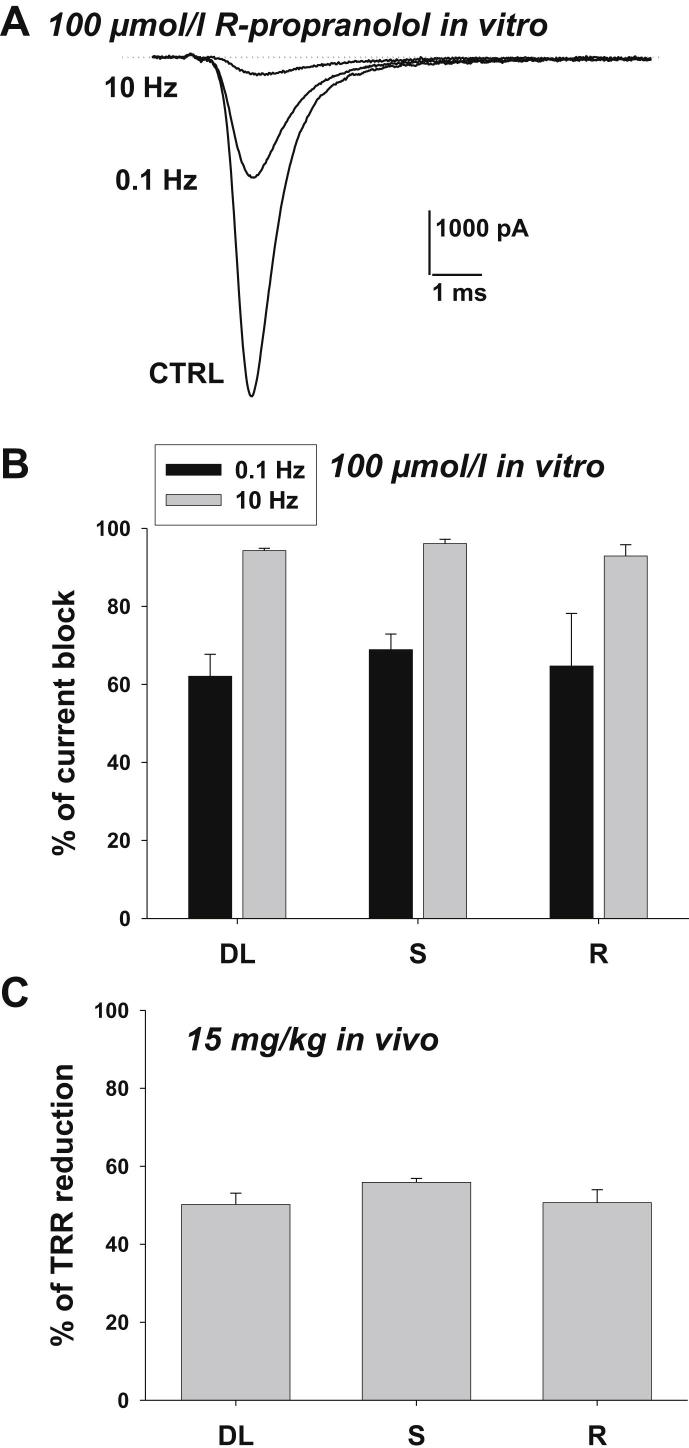
Although propranolol is clinically used as a racemic mixture, a number of evidences indicate that only the S(−)-enantiomer displays β-adrenoceptor antagonism and exerts hemodynamic effects in humans (Stoschitsky et al., 1989; Mehvar and Brocks, 2001). Sodium channel blockade by propranolol has been demonstrated by us and others (Fischer, 2002; Desaphy et al., 2003; Wang et al., 2010; Bankston and Kass, 2010), and both enantiomers appeared to exert a similar effect at least on cardiac sodium channels (Fischer, 2002; Wang et al., 2010). Here we compared the effects of racemic propranolol and single enantiomers (100 μmol/l) on the skeletal muscle hNav1.4 sodium channel isoform expressed in HEK293 cells. Representative sodium current traces in Fig. 5A illustrate the huge use-dependent block of hNav1.4 channels by R-propranolol. The drug blocked sodium currents by 64.7 ± 13.5% at 0.1 Hz stimulation frequency and 92.9 ± 2.9% at 10 Hz (n = 3). Similar results were obtained with the 3 compounds, showing the lack of stereoselectivity for propranolol in blocking sodium channels (Fig. 5B). The two single propranolol enantiomers were also tested in vivo for antimyotonic activity in the 9-AC treated rat. We tested the dose of 15 mg/kg, which is close to the ED50 of racemic propranolol, in order to detect any potential difference between drugs efficacies. Both enantiomers reduced the TRR (30) to the same extent of the racemic mixture.
Fig. 5.
Sodium channel blockade and antimyotonic effect of propranolol enantiomers. (A) The racemic DL-propranolol and single propranolol S(−) and R(+) enantiomers (100 μmol/l) were tested on sodium currents measured in HEK293 cells permanently transfected with the human skeletal muscle hNav1.4 isoform of sodium channels. Sodium currents were elicited by depolarizing the cells for 20 ms at −30 mV from a holding potential of −120 mV every 10 or 0.1 s (i.e. 0.1 Hz or 10 Hz stimulation frequency, respectively). Sodium current traces recorded in a representative cell are shown in control condition (CTRL) and after acute application of R-propranolol at 0.1 and 10 Hz stimulation frequency. (B) Percentage of sodium current block by DL-propranolol and single propranolol enantiomers (100 μmol/l) at 0.1 or 10 Hz stimulation frequency. Each bar is the mean ± S.E.M. from at least 3 cells. (C) In vivo antimyotonic effect in 9-AC treated rats of DL-propranolol and single propranolol enantiomers (15 mg/kg) expressed as the percentage of TRR reduction with respect to rats receiving drug vehicle alone. Each bar is the mean ± S.E.M. from at least 3 animals. No significant difference in sodium channel blockade and in vivo antimyotonic effects was found between the two single enantiomers and racemic propranolol (P > 0.05 with one-way ANOVA).
4. Discussion
In this study, we developed a new pharmacological in vivo rat model of myotonia congenita to evaluate the antimyotonic activity of mexiletine and β-adrenergic drugs.
Genetic animal models of myotonia include adr or mto (a myotonic mutant mouse arisen spontaneously at the Jackson laboratories; Heller et al., 1982) mice, dogs, and goats (Matthews et al., 2010). Although these models have proven very useful for the understanding of the physiopathology of myotonia, their use for preclinical screening of drugs shows obvious limitations. Dogs and goats are difficult and expensive to breed in the laboratory and their use raises ethical issues. In the adr or mto mice, myotonia is inherited in a recessive mode and is very severe. Myotonia that develops very early after birth hampers normal animal growth, induces skeletal malformation, and significantly reduces life expectancy. The myotonia severity can vary substantially among individuals and worsens with age. The myotonic mice are very delicate to manipulate and support repetitive exercise with great difficulty. Nonetheless, myotonic mice were successfully used, with many precautions, by us and others to test some antimyotonic drugs administrated by i.p. injection (Aichele et al., 1985; De Luca et al., 2004). Because of their small size and extreme fragility, the use of an esophageal cannula for oral administration of drugs is quite difficult in myotonic mice. One of the advantages of the rat model used in this study is indeed the possibility to use oral administration of drugs, which is the common way used by patients. Other advantages include the very limited stress held to rats, the easy standardization of protocols, the reduced number of animals needed to obtain experimental points, and the reduced costs of experimentation. The aromatic monocarboxylic acids have been shown to produce myotonia in mammalian skeletal muscle by blocking muscle chloride channels, 9-AC being the most potent (Bryant and Morales-Aguilera, 1971; Palade and Barchi, 1977). Single i.p. doses of 9-AC, 5 or 8 mg/kg, in rats induces electromyographic myotonia as early as a few minutes after administration, including action and percussion myotonia as well as a warm-up phenomenon (Mrozek et al., 1974; Conte Camerino et al., 1989). A dose–response curve was only performed in a single anesthetized goat, suggesting an ED50 close to 4 mg/kg 9-AC for percussion myotonia (Bryant and Morales-Aguilera, 1971). In preliminary experiments, we tested 5, 10, and 30 mg/kg 9-AC using the TRR test in a larger number of rats (not shown). Although myotonia was observed at all the three doses, a consistent myotonic response (maximal TRR 30 min after 9-AC administration observed in 100% of the tested animals) was observed only at the higher dose, that is less than 8 times the estimated ED50 in goats. Thus 30 mg/kg was the lowest dose inducing reproducible behavioral myotonia allowing an easy quantification by means of the non-invasive TRR test. The effects of 9-AC were fully reversible within 15 h, and no side effects were observed even after repeated administration.
We can note here that a myotonia-like state can be also obtained in vivo with chemicals affecting sodium channels, including veratrinic agents. Nevertheless, such a model may not be adapted to the current study because the veratrinic agents acts on both nerve and muscle sodium channels and may generate drug interaction with the exploratory compounds (Ulbricht, 1998). Importantly, no toxic effect of 9-AC has been observed on action potentials recorded in isolated rat diaphragm muscle fibers at concentrations up to 25 times the Ki for reduction of sarcolemma chloride conductance, indicating lack of direct effects of the drug on sodium channels as well as on resting membrane potential (Furman and Barchi, 1978). From these results, we can conclude that an effect of 9-AC on nerve excitability is very unlikely in our experimental conditions.
Up today, the study of potential countermeasures against myotonia induced by 9-AC has been performed only in vitro (Dengler and Rudel, 1979; van Lunteren et al., 2011; Su et al., 2012). Therefore one important novelty of the model described here is that drugs are tested in vivo in conscious animals. The model as shown here may present some limitations, especially regarding long term studies aimed at testing repeated administration of drugs or sustained-release drug formulations, which would merit to be addressed in future experiments. Nonetheless, we were able to perform a quantitative analysis of dose-dependent acute effects of antimyotonic drugs in vivo.
Mexiletine, the preferred drug in myotonic humans, produced a dose-dependent antimyotonic effect in the 9-AC treated rat, as expected. The ED50 value for oral mexiletine in the myotonic rat (12 mg/kg) is comparable to that measured in the adr mouse for i.p. mexiletine, in which 5 mg/kg produced a ∼50% reduction of TRR (De Luca et al., 2004). In humans, when needed, treatment for myotonia relief may require up to 800 mg a day mexiletine (Dr. Mauro LoMonaco, Catholic University, Rome, personal communication), which closely corresponds to the mexiletine ED50 found in the animal models. The dose of 40 mg/kg appeared likely close to the maximal efficient mexiletine dose in the rat; At this dose, mexiletine effects developed very rapidly and lasted for 2 h. Higher doses were not tested because of risks of undesirable life-threatening side effects.
A number of evidences suggest that mexiletine effects are related to sodium channel blockade in vivo, thereby reducing over-excitability in myotonic fibers independently of the genetic origin. Our results confirm such hypothesis since clenbuterol, propranolol, and single propranolol enantiomers, which all display sodium channel blockade and action potential firing inhibition in vitro, exerted antimyotonic effects in vivo, whereas nadolol and salbutamol did not. Clearly, the antimyotonic effects of these compounds were not related to modulation of the β-adrenergic pathway. Despite clenbuterol and propranolol showed greater efficiency in blocking hNav1.4 sodium channels in HEK cells (Desaphy et al., 2003), their antimyotonic efficacy was slightly lower than that of mexiletine. Such an apparent discrepancy may be due to differences in pharmacokinetics or to the inappropriateness of voltage clamp protocols used in cell lines. Indeed sodium currents were recorded in HEK cells using a holding potential of −120 mV, which is more negative than muscle cell membrane potential, and a stimulation frequency of 10 Hz, which is likely less than the frequency of action potential runs in the myotonic muscle fiber. Future studies will be performed to assess sodium channel inhibition in HEK cells using more physio/pathological conditions.
In our hands, the β2-agonist clenbuterol displayed more variable antimyotonic efficiency at lower doses and undesirable side effects at the highest dose, including generalized weakness and breathing difficulties (not shown). Propranolol was safe of side effects and produced constant antimyotonic activity lasting two hours, such as mexiletine. The propranolol ED50 was 19 mg/kg compared to 12 mg/kg for mexiletine. In the clinical setting, propranolol is used essentially for cardiovascular diseases at doses ranging between 80 and 320 mg a day, corresponding to a maximal dose of ∼5 mg/kg. It is possible that antimyotonic effect in humans may thus require dosages in the higher range. It is noteworthy that sporadic cases of myotonia aggravation have been reported in humans with the use of β2-agonists as well as with β-blocking adrenergic agents (Blessing and Walsh, 1977; Ricker et al., 1978), and use of such drugs is generally not recommended (Chrestian et al., 2006; Duno and Colding-Jorgensen, 2011). Regarding propranolol, the inhibition of β-adrenoceptor would favor muscle membrane depolarization, that is thought to facilitate myotonia. Although we never observed myotonia exacerbation by propranolol in the rat model, a valuable approach to avoid undesirable β-adrenergic effects in the myotonic patients would be to use the single R(+)-enantiomer of propranolol, instead of the racemic mixture. Indeed the R(+)-enantiomer is ∼100 times less active on β-adrenoceptor pathway than the S(−)-enantiomer (Barrett and Cullum, 1968), but both stereoisomers share a similar action on skeletal muscle sodium channels (this study). It should be noted that the side effects of R(+)-propranolol may be similar to those of mexiletine, including CNS troubles or cardiotoxicity in patients with heart disease, since these effects may result from sodium channel blockade. Nevertheless, mexiletine use is commonly associated with gastrointestinal disturbances, especially nausea (see for instance Duff et al., 1983), that can significantly limit patient compliance or even fully exclude mexiletine therapy. This latter effect may not be related to sodium channel blockade, since it is not consistently observed with other class I antiarrhythmic drugs. Interestingly, nausea has been only reported in a very limited number of patients receiving propranolol (Stephen, 1966). An other limit of mexiletine therapy may be the lack of response in a number of patients, which might stem from individual variations in drug pharmacokinetics or mutation-induced sodium channel mutation/polymorphism. In this case, other sodium channel blockers may be useful. For instance, flecainide has been shown to be efficient in a myotonic patient carrying the sodium channel V445M mutation and resistant to mexiletine (Rosenfeld et al., 1997). Thus, R(+)-propranolol might be useful as an alternative to mexiletine in a number of unsatisfied myotonic patients.
In conclusion, a recent systematic review has underscored the lack of sufficient randomized controlled trials of treatments for the non-dystrophic myotonias (Trip et al., 2006). A controlled trial of mexiletine versus placebo started in late 2008, and results are expected soon (clinicaltrials.gov). Such trials are difficult to perform, being hampered by the difficulty in quantifying myotonia and in recruiting adequate number of patients to reach statistical power (Matthews et al., 2010). In this context, a preclinical model appears of great interest allowing a screening of compounds aimed at individuating the most promising drug to be tested in humans. The pharmacological model described in this study may encounter all the requisites for such preclinical studies. Although mexiletine is considered as a first-line treatment in both sodium and chloride channel myotonias, a number of patients cannot use this drug because of contraindications, side effects, or lack of efficacy. Among the drugs tested in this study, the R(+)-enantiomer of propranolol may thus merit further investigation in humans with the aim of increasing the arsenal of available antimyotonic drugs. Last but not least, the ability of propranolol to reduce cell over-excitability suggests a possible therapeutic value of this drug in various conditions of neuron excitability disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Acknowledgment
The financial contribution of Telethon-Italy (grant GGP10101 to DCC) and Association Française contre les Myopathies (grant #15020 to DCC) is gratefully acknowledged. The authors thank Sabata Pierno, Michela De Bellis, and Gianluca Gramegna for their contribution to preliminary experiments, and Maria Giovanna Pagano for her technical help during the study.
References
- Aichele R., Paik H., Heller A.H. Efficacy of phenytoin, procainamide, and tocainide in murine genetic myotonia. Exp. Neurol. 1985;87:377–381. doi: 10.1016/0014-4886(85)90228-6. [DOI] [PubMed] [Google Scholar]
- Bankston R.S., Kass R.S. Molecular determinants of local anesthetic action of beta-blocking drugs: implications for therapeutic management of long QT syndrome variant 3. J. Mol. Cell. Cardiol. 2010;48:246–253. doi: 10.1016/j.yjmcc.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A., Cullum V. The biological properties of the optical isomers of propranolol and their effects on cardiac arrhythmias. Br. J. Pharmacol. 1968;34:43–55. doi: 10.1111/j.1476-5381.1968.tb07949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing W., Walsh J.C. Myotonia precipitated by propranolol therapy. Lancet. 1977;1:73–74. doi: 10.1016/s0140-6736(77)91083-2. [DOI] [PubMed] [Google Scholar]
- Bryant S.H., Morales-Aguilera A. Chloride conductance in normal and myotonic muscle fibers and the action of monocarboxylic acids. J. Physiol. 1971;219:367–383. doi: 10.1113/jphysiol.1971.sp009667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrestian N., Puymirat J., Bouchard J.P., Dupré N. Myotonia congenita – a cause of muscle weakness and stiffness. Nat. Clin. Pract. Neurol. 2006;2:393–399. doi: 10.1038/ncpneuro0239. [DOI] [PubMed] [Google Scholar]
- Conte Camerino D., De Luca A., Mambrini M., Ferrannini E., Franconi F., Giotti A., Bryant S.H. The effects of taurine on pharmacologically induced myotonia. Muscle Nerve. 1989;12:898–904. doi: 10.1002/mus.880121105. [DOI] [PubMed] [Google Scholar]
- Conte Camerino D., Tricarico D., Desaphy J.-F. Ion channel pharmacology. Neurotherapeutics. 2007;4:184–198. doi: 10.1016/j.nurt.2007.01.013. [DOI] [PubMed] [Google Scholar]
- De Luca A., Pierno S., Liantonio A., Desaphy J.-F., Natuzzi F., Didonna M.P., Ferrannini E., Jockusch H., Franchini C., Lentini G., Corbo F., Tortorella V., Conte Camerino D. New potent mexiletine and tocainide analogues evaluated in vivo and in vitro as antimyotonic agents on myotonic ADR mouse. Neuromuscul. Disord. 2004;14:405–416. doi: 10.1016/j.nmd.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Dengler R., Rudel R. Effects of tocainide on normal and myotonic mammalian skeletal muscle. Arzneimittelforschung. 1979;29:270–273. [PubMed] [Google Scholar]
- Desaphy J.-F., De Luca A., Didonna M.P., George A.L., Jr., Conte Camerino D. Different flecainide sensitivity of hNav1.4 channels and myotonic mutants explained by state-dependent block. J. Physiol. 2004;554:321–334. doi: 10.1113/jphysiol.2003.046995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J.-F., Dipalma A., Costanza T., Carbonara R., Dinardo M.M., Catalano A., Carocci A., Lentini G., Franchini C., Conte Camerino D. Molecular insights into the local anesthetic receptor within voltage-gated sodium channels using hydroxylated analogs of mexiletine. Front. Pharmacol. 2012;3:17. doi: 10.3389/fphar.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaphy J.-F., Pierno S., De Luca A., Didonna P., Conte Camerino D. Different ability of clenbuterol and salbutamol to block sodium channels predicts their therapeutic use in muscle excitability disorders. Mol. Pharmacol. 2003;63:659–670. doi: 10.1124/mol.63.3.659. [DOI] [PubMed] [Google Scholar]
- Duff H.J., Roden D.M., Primm R.K., Oates J.A., Woosley R.L. Mexiletine in the treatment of resistant ventricular arrhythmias: enhancement of efficacy and reduction of dose-related side effects by combination with quinidine. Circulation. 1983;67:1124–1128. doi: 10.1161/01.cir.67.5.1124. [DOI] [PubMed] [Google Scholar]
- Duno M., Colding-Jorgensen E. Myotonia congenita. In: Pagon R.A., Bird T.D., Dolan C.R., Stephens K., editors. Gene Reviews [internet] University of Washington; Seattle (WA): 2011. Seattle, 1993–2005 Aug 03 (updated 2011 Apr 12) [Google Scholar]
- Estevez R., Schroeder B.C., Accardi A., Jentsch T.J., Pusch M. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron. 2003;38:47–59. doi: 10.1016/s0896-6273(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Fischer W. Anticonvulsivant profile and mechanism of action of propranolol and its two enantiomers. Seizure. 2002;11:285–302. doi: 10.1053/seiz.2001.0644. [DOI] [PubMed] [Google Scholar]
- Furman R.E., Barchi R.L. The pathophysiology of myotonia produced by aromatic carboxylic acids. Ann. Neurol. 1978;4:357–365. doi: 10.1002/ana.410040411. [DOI] [PubMed] [Google Scholar]
- Heller A.H., Eicher E.M., Hallett M., Sidman R.L. Myotonia, a new inherited muscle disease in mice. J. Neurosci. 1982;2:924–933. doi: 10.1523/JNEUROSCI.02-07-00924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann-Horn F., Jurkat-Rott K., Rudel R. Diagnostics and therapy of muscle channelopathies – guidelines of the Ulm muscle centre. Acta Myol. 2008;27:98–113. [PMC free article] [PubMed] [Google Scholar]
- Matthews E., Fialho D., Tan S.V., Venance S.L., Cannon S.C., Sternberg D., Fontaine B., Amato A.A., Barohn R.J., Griggs R.C., Hanna M.G., the CINCH investigators The non-dystrophic myotonias: molecular pathogenesis, diagnosis and treatment. Brain. 2010;133:9–22. doi: 10.1093/brain/awp294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehvar R., Brocks D.R. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Pharm. Sci. 2001;4:185–200. [PubMed] [Google Scholar]
- Mrozek K., Kwiecinski H., Kaminska A. Experimental myotonia. Myotonic activity of the fast and slow muscles. Acta Physiol. Pol. 1974;25:321–327. [PubMed] [Google Scholar]
- Palade P.T., Barchi R.L. On the inhibition of muscle membrane chloride conductances by aromatic carboxylic acids. J. Gen. Physiol. 1977;69:875–896. doi: 10.1085/jgp.69.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricker K., Haass A., Glotzner F. Fenoterol precipitating myotonia in a minimally affected case of recessive myotonia congenita. J. Neurol. 1978;219:279–282. doi: 10.1007/BF00312981. [DOI] [PubMed] [Google Scholar]
- Rosenfeld J., Sloan-Brown K., George A.L., Jr. A novel muscle sodium channel mutation causes painful congenital myotonia. Ann. Neurol. 1997;42:811–814. doi: 10.1002/ana.410420520. [DOI] [PubMed] [Google Scholar]
- Stephen S.A. Unwanted effects of propranolol. Am. J. Cardiol. 1966;18(3):463–472. doi: 10.1016/0002-9149(66)90071-3. [DOI] [PubMed] [Google Scholar]
- Stoschitsky K., Lindner W., Rath M., Leitner C., Uray G., Zernig G., Moshammer T., Klein W. Stereoselective hemodynamic effects of (R)- and S-propranolol in man. Naunyn Schmiedebergs Arch. Pharmacol. 1989;339:474–478. doi: 10.1007/BF00736064. [DOI] [PubMed] [Google Scholar]
- Su T.R., Zei W.S., Su C.C., Hsiao G., Lin M.J. The effects of the KCNQ Openers Retigabine and Flupirtine on myotonia in mammalian skeletal muscle induced by a chloride channel blocker. Evid. Based Complement. Alternat. Med. 2012 doi: 10.1155/2012/803082. (Epub 2012 Mar 25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trip J., Drost G., van Engelen B.G., Faber C.G. Drug treatment for myotonia. Cochrane Database Syst. Rev. 2006;1:CD004762. doi: 10.1002/14651858.CD004762.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W. Effects of veratridine on sodium currents and fluxes. Rev. Physiol. Biochem. Pharmacol. 1998;133:1–54. doi: 10.1007/BFb0000612. [DOI] [PubMed] [Google Scholar]
- van Lunteren E., Spiegler S.E., Moyer M. Fatigue-inducing stimulation resolves myotonia in a drug-induced model. BMC Physiol. 2011;11:5. doi: 10.1186/1472-6793-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.W., Mistry A.M., Kahlig K.M., Keamey J.A., Xiang J., George A.L., Jr. Propranolol blocks cardiac and neuronal voltage-gated sodium channels. Front. Pharmacol. 2010;1:144. doi: 10.3389/fphar.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]