Abstract
Purpose
Sarcopenia and increased fat infiltration in muscle may play a role in the functional impairment and high risk for diabetes in stroke. Our purpose was to compare muscle volume and muscle attenuation across 6 muscles of the paretic and nonparetic thigh and examine the relationships between intramuscular fat and insulin resistance and between muscle volume and strength in stroke patients.
Methods
Stroke participants (70; 39 men, 31 women) aged 40 to 84 years, BMI = 16 to 45 kg/m2 underwent multiple thigh CT scans, total body scan by DXA (dual-energy X-ray absorptiometry), peak oxygen intake (VO2peak) graded treadmill test, 6-minute walk, fasting blood draws, and isokinetic strength testing.
Results
Muscle volume is 24% lower and subcutaneous fat volume is 5% higher in the paretic versus nonparetic thigh. Muscle attenuation (index of amount of fat infiltration in muscle) is 17% higher in the nonparetic midthigh than the paretic. The semitendinosis/semimembranosis, biceps femoris, sartorius, vastus (medialis/lateralis), and rectus femoris have lower (between 9% and 19%) muscle areas on the paretic than the nonparetic thigh. Muscle attenuation is 15% to 25% higher on the nonparetic than the paretic side for 5 of 6 muscles. The nonparetic midthigh muscle attenuation is negatively associated with insulin. Eccentric peak torque of the nonparetic leg and paretic leg are associated with the corresponding muscle volume.
Conclusions
The skeletal muscle atrophy, increased fat around and within muscle, and ensuing muscular weakness observed in chronic stroke patients relates to diabetes risk and may impair functional mobility and independence.
Keywords: muscle atrophy, intramuscular fat, strength, muscle quality, hyperinsulinemia, stroke rehabilitation
Stroke is the leading cause of disability in adults; persistent neurological deficits impair functional ability and promote physical inactivity.1–3 A sedentary lifestyle magnifies the usual age-associated increase in body fat and loss of fat-free mass (FFM). We previously reported that disabling stroke accelerates sarcopenia and fat infiltration in muscle in the paretic limb,4 with additional evidence suggesting that these skeletal muscle changes may play a pivotal role in the functional aerobic impairment so prevalent in this population.5
Of the limited number of studies that have reported body composition changes and muscle atrophy after stroke,4,6–8 none have examined the precise anatomical location of the sarcopenia, nor have they examined multiple slices across the thigh to assess whole muscle volume and the relationship to muscular strength. This information may alter rehabilitation strategies if different muscles were affected after stroke. In addition, it would be relevant to study intramuscular fat distribution throughout the entire limb given our previous observation of increased intramuscular fat of a single midthigh CT slice.4 Increased fat infiltration (ie, decreased muscle attenuation) is associated with insulin resistance after stroke.9,10 Given the risk of diabetes before and after stroke, fatty infiltration in skeletal muscle should be assessed for its effect on glucose metabolism.
We tested the hypothesis that the paretic limb of stroke participants would show atrophy and decreased muscle attenuation across the entire thigh and for each of the major and minor muscle groups of the thigh. Thus, the purpose of this study was to compare muscle volume, subcutaneous fat volume, and muscle attenuation across 6 muscles of the paretic and nonparetic thigh. We also examine the relationships between intramuscular fat and insulin resistance as well as between muscle volume and muscle strength.
Methods
Participant Selection
A total of 70 individuals (39 men, 31 women) between 40 and 84 years old, with BMI between 16.5 and 45.2 kg/m2 (range underweight to morbid obesity) and residual hemi-paretic deficits >6 months after ischemic stroke participated in the study. All stroke participants had mild to moderate hemiparetic gait deficits and had completed conventional rehabilitation therapy. Evaluations included medical history, physical examination, fasting blood profile, and screening for dementia11 and depression12 to ensure adequate informed consent. Stroke participants were excluded if they had unstable angina, congestive heart failure (NYHA II), severe peripheral arterial disease, major poststroke depression, dementia, severe receptive aphasia, and orthopedic or chronic pain conditions. Stroke participants were sedentary by self-report (≤20 minutes of low-intensity aerobic exercise ≤ twice per week).
All methods and procedures were approved by the Institutional Review Board of the University of Maryland and the Baltimore VA Research & Development Committee. Each participant provided written informed consent.
Exercise and Functional Tests
Exercise testing with open circuit-spirometry was con- using a graded treadmill test.13 A ducted to measure VO2peak standardized 6-minute walk test recorded the distance traveled, with stroke participants walking at their comfortable self-selected walking speed using their usual assistive devices. This walking test was chosen as a functional measure of gait deficit severity.
Body Composition
Height (cm) and weight (kg) were measured. Fat mass, lean tissue mass, and percentage body fat were determined by DXA (dual-energy X-ray absorptiometry; Prodigy LUNAR GE v 7.53.002).
Thigh CT scans were performed every 4 cm starting at the patella and ending at the femoral head (Siemens Somatom Sensation 64 Scanner) to quantify skeletal muscle area, total fat area, low-density lean tissue area,4 and muscle attenuation of both the paretic and nonparetic thighs. Scans were analyzed using MIPAV (Medical Image Processing, Analysis and Visualization, v 7.0, NIH). The cross-sectional area (CSA; in cm2) of the midthigh as well as combined semitendinosis and semimembranosis, biceps femoris, gracilis, sartorius, vastus (medialis and lateralis), and rectus femoris muscles at the midthigh were manually outlined as the regions of interest by the same observer. The coefficient of variation between repeat manual outlining was <5% for muscle groups of both legs. In addition, the CSA for each axial CT slice was outlined from the superior border of the patella to a point where the proximal quadriceps muscles were no longer reliably distinguishable from the adductor and hip flexors. The CSA of each axial slice was multiplied by the distance between slices (4 cm) and summed across slices, representing volume expressed in cm3.
Blood Samples
Participants had blood drawn after a 12-hour fast. Blood samples were centrifuged at 4°C and 1 mL aliquot of plasma was frozen (−80°C). Duplicate measures of plasma glucose by the glucose oxidase method (2300 STAT Plus, YSI, Yellow Springs, Ohio) and immunoreactive insulin by RIA (Millipore Inc, St Charles, Missouri) were determined.
Kin-Com Strength Tests
A Kin-Com 125AP determined the peak torque capacity of the knee extensors for maximal isokinetic concentric and eccentric volitional contractions across the knee for the paretic and nonparetic legs at angular velocities of 90° and 120°/s. These intermediate speeds were chosen to elicit maximal dynamic torques while reducing the diminishing effects of the force–velocity relationship at higher speeds. Three passive trials were recorded, followed by 3 trials of concentric contractions at both velocities and then 3 trials of eccentric contractions, also at both velocities, with best performances used for analysis. Rest periods of 30 to 60 s were provided between trials, and the testing order of legs was randomized. Torque was also presented as adjusted for muscle volume (eg, muscle quality).
Statistical Analyses
Differences between paretic and nonparetic sides for all variables were determined using paired Student t tests. The data were analyzed for the total group as well as by sex using unpaired t tests. Relationships between variables were determined by linear regression analyses with calculation of Pearson product moment correlation coefficients. All data were analyzed using SPSS 12.0 (SPSS Inc, Chicago, Illinois). Data are presented as means ± standard error of the mean. P values <.05 are statistically significant.
Results
Physical Characteristics
Stroke participants were well represented for gender and consisted of 56% men and 44% women. The group was also racially mixed (Table 1), with 46% white (n = 32), 53% African American (n = 37), and the remaining 1% (n = 1) of other nationalities (Indian). There was a wide range of latencies since stroke (6–212 months), with an average of 39 months since the index stroke. On average, both fasting glucose and insulin concentrations were above normal levels.
Table 1.
Physical Characteristics of Stroke Participantsa
| Stroke Survivors (n = 70) | Men (n = 39) | Women (n = 31) | |
|---|---|---|---|
| Age, y | 63 ± 1 | 62 ± 2 | 64 ± 2 |
| Weight, kg | 82.4 ± 2.3 | 87.1 ± 3.1 | 76.4 ± 3.2b |
| BMI, kg/m2 | 28.8 ± 0.7 | 28.6 ± 0.9 | 29.0 ± 1.1 |
| Total body fat mass, kg | 29.7 ± 1.3 | 26.2 ± 1.3 | 33.7 ± 2.2b |
| Lean body mass, kg | 47.1 ± 1.2 | 53.1 ± 1.2 | 40.0 ± 1.3c |
| Percentage body fat | 36.9 ± 1.1 | 31.3 ± 1.1 | 43.1 ± 1.4c |
| VO2peak, mL/kg/min | 12.8 ± 0.6 | 14.0 ± 0.9 | 11.2 ± 0.7b |
| VO2peak, L/min | 1.04 ± 0.1 | 1.19 ± 0.1 | 0.86 ± 0.1b |
| Six-minute walk distance, m | 633 ± 46 | 682 ± 63 | 569 ± 66 |
| Latency since stroke, mos | 39 ± 7 | 42 ± 10 | 36 ± 10 |
| Fasting plasma glucose, mmol/L | 5.71 ± 0.14 | 5.66 ± 0.14 | 5.78 ± 0.25 |
| Fasting plasma insulin, pmol/L | 91 ± 6 | 91 ± 7 | 92 ± 11 |
Abbreviations: BMI, body mass index; VO2peak, peak oxygen consumption; SEM, standard error of the mean.
Values are mean ± SEM.
P < .05 between men and women.
P < .001 between men and women.
Gender comparisons indicate that the men and women were of similar age and latency since stroke. Despite the lack of difference in BMI between groups, the men weighed more and had 24% higher lean body mass (P < .001) and lower percentage body fat (P < .001) than women stroke participants. Although 6-minute walk distances were not significantly different between men and women, the men and 20% higher peak VO2 (measured in L/min; P < .01) had a 27% higher peak VO2 (measured in mL/kg/min; P < .05). Fasting glucose and insulin levels were not different between groups.
Paretic and Nonparetic Thigh Composition
Similar to our previous report in a smaller sample4 and now with a greater magnitude of differences between sides, the total muscle area of the paretic leg at the level of the midthigh is 26% lower than that of the nonparetic leg (Table 2; P < .001). With the larger sample size in the current study, we are able to detect differences in the subcutaneous fat areas between the paretic and nonparetic sides (Table 3). The paretic midthigh has a 6% higher subcutaneous fat area (P < .001). The low-density lean tissue area of the paretic midthigh is 4% higher than that in the nonparetic midthigh (P < .01).
Table 2.
Midthigh Cross-Sectional Area of Muscle and Knee Isokinetic Torque of Stroke Participantsa
| Area, cm2 | Paretic Thigh (n = 70) | Nonparetic Thigh (n = 70) |
|---|---|---|
| Muscle | 59.4 ± 2.5 | 74.6 ± 2.7b |
| Subcutaneous fat | 93.0 ± 6.8 | 87.4 ± 6.3b |
| Low-density lean tissue | 23.2 ± 1.0 | 22.2 ± 1.0c |
| Semitendinosis/membranosis | 24.0 ± 1.0 | 26.1 ± 0.9b |
| Biceps femoris | 14.2 ± 0.6 | 15.8 ± 0.6b |
| Gracilis | 4.0 ± 0.3 | 3.6 ± 0.2 |
| Sartorius | 4.0 ± 0.2 | 4.5 ± 0.2b |
| Vastus (lateralis, medialis) | 43.5 ± 1.6 | 51.9 ± 1.6b |
| Rectus femoris | 3.0 ± 0.2 | 3.4 ± 0.2c |
| Torque, N m | ||
| Peak 90° concentric (n = 43) | 21.9 ± 2.3 | 54.0 ± 5.2c |
| Peak 90° eccentric (n = 56) | 70.6 ± 5.1 | 120.9 ± 5.7c |
| Peak 120° concentric (n = 44) | 18.9 ± 2.1 | 42.5 ± 4.0c |
| Peak 120° eccentric (n = 55) | 68.4 ± 5.1 | 107.5 ± 5.5c |
Values are means ± standard errors of the mean.
P < .001 paretic versus nonparetic.
P < .01 paretic versus nonparetic.
Table 3.
Muscle, Subcutaneous Fat, and Attenuation Areas and Volumes in Men and Women Stroke Participantsa
| Men (n = 39) | Women (n = 31) | |
|---|---|---|
| Muscle area, cm2 | ||
| Paretic | 69.4 ± 3.0 | 46.8 ± 2.8b |
| Nonparetic | 85.7 ± 3.6 | 60.7 ± 2.0b |
| Subcutaneous fat area, cm2 | ||
| Paretic | 70.5 ± 6.8 | 121.3 ± 10.9b |
| Nonparetic | 65.3 ± 6.5 | 115.2 ± 9.7b |
| Muscle attenuation midthigh, HU | ||
| Paretic | 32.9 ± 1.4 | 28.2 ± 1.4c |
| Nonparetic | 37.8 ± 1.4 | 33.8 ± 1.3c |
| Muscle volume, cm3 | ||
| Paretic | 1482 ± 62 | 1007 ± 62b |
| Nonparetic | 1837 ± 74 | 1253 ± 52b |
| Subcutaneous fat volume, cm3 | ||
| Paretic | 1829 ± 163 | 2911 ± 244b |
| Nonparetic | 1744 ± 161 | 2781 ± 221b |
| Muscle attenuation total thigh, HU | ||
| Paretic | 747 ± 32 | 603 ± 35c |
| Nonparetic | 854 ± 31 | 729 ± 31c |
Abbreviations: HU, Hounsfield units.
Values are means ± standard errors of the mean.
P < .001 between men and women.
P < .05 between men and women.
With the multiple slices across the entire thigh, we found a 24% lower muscle volume and 5% higher subcutaneous fat volume in the paretic versus the nonparetic thigh (Figure 1; both P < .001). Low-density lean tissue volume did not differ between the paretic and the nonparetic thighs.
Figure 1.
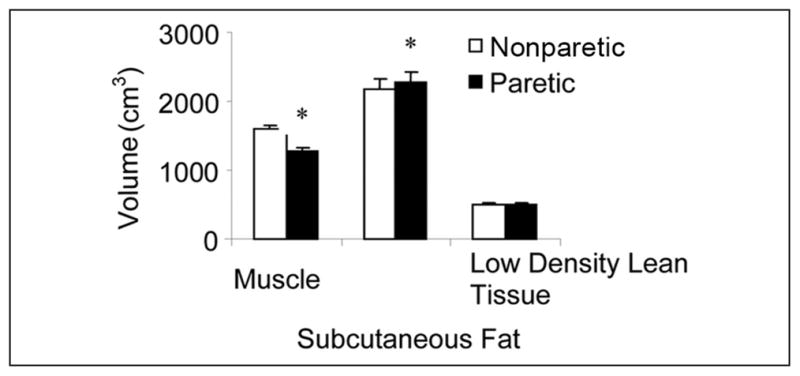
Muscle, subcutaneous fat, and low-density lean tissue volumes in the nonparetic and paretic legs of stroke participants (n = 70; *P < .001).
Representative images of midthigh areas of the 6 muscles are shown for the paretic and nonparetic thighs of a stroke patient (Figure 2). The semitendinosis/semimembranosis, biceps femoris, sartorius, vastus (medialis and lateralis), and rectus femoris all have lower muscle areas on the paretic midthigh than the nonparetic midthigh (all P < .01). Differences ranged between 9% and 19%.
Figure 2.
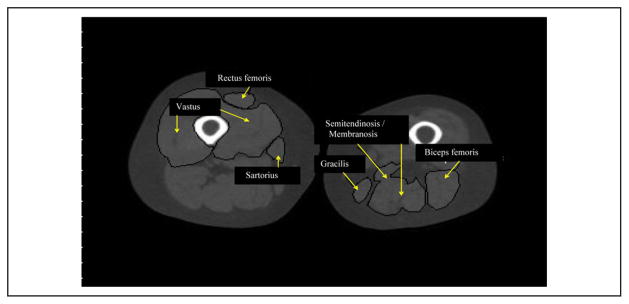
Representative bilateral midthigh slice of a hemiparetic stroke patient of specific muscles.
Significant differences were observed in muscle attenuation, which is 17% higher in the midthigh on the nonparetic side than on the paretic side (P < .001; Figure 3). Muscle attenuation was between 15% and 25% higher on the nonparetic side than the paretic side for semitendinosis/semimembranosis, biceps femoris, sartorius, vasti (medialis and lateralis), and rectus femoris muscles (P < .01). There was no difference in muscle attenuation between the paretic and nonparetic sartorius muscles.
Figure 3.
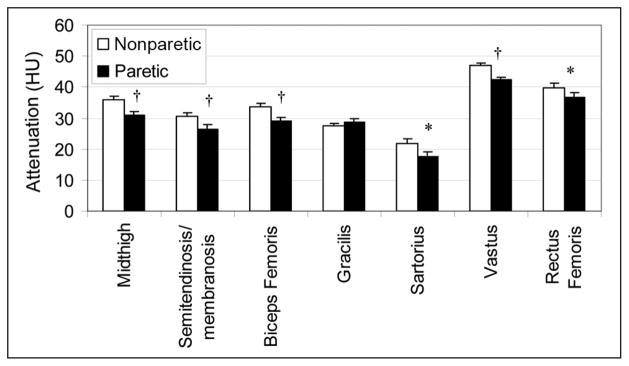
Muscle attenuation of the total midthigh and each muscle of the bilateral thighs of stroke patients (n = 70; *P < .01, †P < .001).
Gender comparisons of muscle composition indicated that men have significantly higher midthigh muscle areas than women in both the paretic and nonparetic thighs as well as higher total muscle volumes in the paretic and non-paretic thighs (all P < .001). Midthigh subcutaneous fat area of the paretic and nonparetic sides and thigh subcutaneous fat volume of the paretic and nonparetic sides are lower in men than in women, respectively (all P < .001). Muscle attenuation at the midthigh is higher in men than in women on the paretic (P < .05) and nonparetic sides (P < .05). Muscle attenuation measured across the total thigh is higher in men than in women on the nonparetic and paretic sides (both P < .01), indicating less intramuscular fat in men.
Relationships Between Muscle Attenuation and Glucose Metabolism
Nonparetic muscle attenuation at the midthigh is negatively associated with fasting insulin (r = −0.39; P < .01). Nonparetic muscle attenuation across the total thigh is associated with lower fasting insulin levels (r = −0.38; P < .01). Nonparetic muscle attenuation of individual muscles are also associated with fasting insulin (semitendinosis/semimembranosis: r = −0.30, P < .05; biceps femoris: r = −0.29, P < .05; sartorius: r = −0.50, P < .001; vastus: r = −0.39, P = .06; and rectus femoris: r = −0.33, P < .05). In contrast, neither paretic midthigh nor total thigh muscle attenuation nor the majority of specific paretic muscles are associated with fasting insulin. Paretic sartorius muscle attenuation is negatively associated with fasting insulin (r = −0.32; P < .05).
Paretic and Nonparetic Peak Torque
Isokinetic peak torques for knee extensor muscles are presented in Table 2. Nonparetic concentric torque is 147% and 125% higher than paretic concentric torque at 90°/s and 120°/s, respectively (both P < .001). Nonparetic eccentric torque is 71% and 57% higher than paretic eccentric torque at 90°/s and 120°/s, respectively (both P < .001). In addition, 90°/s torque adjusted for muscle volume is 1.9-fold lower on the paretic than on the nonparetic leg for concentric (0.019 ± 0.002 vs 0.036 ± 0.003 N m/cm3) and 1.4-fold lower for eccentric (0.058 ± 0.004 vs 0.080 ± 0.004 N m/cm3, P < .001) contractions. In contrast to the significant differences in body composition between men and women, gender differences in muscle peak torque are minimal. There are no differences in concentric or eccentric torque at 90°/s (concentric: 21 ± 3 vs 22 ± 4 N m; eccentric: 78 ± 7 vs 61 ± 6 N m) and 120°/s (concentric: 18 ± 3 vs 20 ± 4 N m; eccentric: 78 ± 7 vs 58 ± 6 N m) on the paretic side or concentric torque on the nonparetic side at 90°/s (54 ± 6 vs 44 ± 5 N m) and 120°/s (42 ± 5 vs 37 ± 4 N m) between men and women, respectively. Only nonparetic eccentric torque at 90°/s is different between groups, with men exhibiting a 27% higher torque than women (130 ± 42 vs 102 ± 36 N m; P < .05). There are no significant gender differences in muscle quality (eg, 90°/s torque adjusted for muscle volume) on the paretic (men vs women for concentric: 0.016 ± 0.003 vs 0.024 ± 0.004 N m/cm3; eccentric: 0.056 ± 0.005 vs 0.061 ± 0.006 N m/cm3) and nonparetic (concentric: 0.030 ± 0.004 vs 0.036 ± 0.004 N m/cm3; eccentric: 0.075 ± 0.004 vs 0.083 ± 0.006 N m/cm3) sides.
Relationships Between Peak Torque, Muscle Composition and Quality, and Function
Peak torque values from the 90°/s trials are correlated with muscle composition and gait function because these represent the highest torque outputs measured. Eccentric peak torque of the nonparetic leg is associated with nonparetic muscle volume (r = 0.50; P < .001) and nonparetic attenuation volume (r = 0.29; P < .05). Likewise, eccentric torque of the paretic leg is associated with paretic muscle volume (r = 0.40; P < .01) but not with paretic attenuation volume (r = 0.24; P = .07). Concentric torque on the nonparetic leg is associated with nonparetic muscle volume (r = 0.28; P < .05) and muscle attenuation (r = 0.31; P < .05). In contrast, there are no relationships between concentric torque of the paretic leg and any of the muscle areas of the 6 muscle groups. In the examination of specific muscles and their relationship to torque, on the paretic side, only the vastus lateralis muscle area and biceps femoris were associated with eccentric torque (r = 0.36 and r = 0.31, respectively; P < .05). However, on the nonparetic side, the area of all muscles except the sartorius muscle is associated with eccentric torque (range r = 0.25 to 0.53; P < .05). Similar to the paretic leg, there are no significant relationships between nonparetic concentric torque and any of the muscle areas of the 6 muscles.
Paretic and nonparetic eccentric torques are associated with VO2peak (r = 0.26, P < .05 and r = 0.41, P < .01, respectively). Paretic and nonparetic eccentric torques are also associated with 6-minute walk distances (paretic: r = 0.55, P < .001; nonparetic: r = 0.33, P < .05). Paretic concentric torque is not associated with VO2peak or 6-minute walk distance, but nonparetic concentric torque is associated with VO2peak (r = 0.48; P < .001) and 6-minute walk distance (r = 0.34; P < .05). Muscle quality was also associated with fitness, including significant relationships between concentric 90°/s torque adjusted for muscle volume and VO2peak (r = 0.26; P < .05) and eccentric 90°/s torque adjusted for muscle volume with 6-minute walk distance (r = 0.43; P < .005).
Discussion
The present study is the first to show that the muscle atrophy in stroke patients occurs in every muscle measured except the gracilis in the thigh. The decreased muscle area, muscle volume, and muscle quality in the paretic thigh is accompanied by increased thigh subcutaneous fat as well as reduced muscle attenuation, indicating increased fat both surrounding the muscle and inside the muscle. Negative associations between nonparetic muscle attenuation and fasting insulin levels indicate that increased fat infiltration may have deleterious effects on glucose homeostasis. Further findings of associations between muscle volume, VO2peak, and eccentric torque indicate that muscle atrophy may have negative functional ramifications in stroke.
Our findings of muscle-specific atrophy and increased intramuscular fat across multiple muscles are novel and may have special relevance for stroke patients based on their medically induced propensity for a sedentary lifestyle. This further accelerates the loss of FFM and may increase the risk for diabetes. In these stroke patients, we also report that lower eccentric torques of the nonparetic and paretic legs are associated with lower muscle volumes on the respective sides as well as reduced function and lower fitness levels, thus substantiating that the loss of muscle results in reductions in muscular strength and aerobic fitness similar to that observed in healthy individuals. Because stroke patients begin with a much lower level of functional ability, our findings imply that the consequences of sarcopenia in stroke survivors may be even more severe than in healthy older people.
The few reports that examine differences in paretic versus nonparetic leg composition by DXA are inconsistent. In a longitudinal follow-up study, there were no changes reported in total body lean mass measured at 3 weeks compared with that measured at 12 months after hospital admission for a stroke.6 Furthermore, lean mass of the hemiparetic leg is lower at 3 weeks but not at the 12-month follow-up in this small sample (n = 12) of stroke patients.6 It is possible that the follow-up was not of sufficient duration to detect a loss of muscle mass after a stroke and that several years of follow-up are necessary to detect overall atrophy. Our observed 24% difference between paretic and nonparetic leg muscle volumes in older stroke patients with an average latency of 3 years is considerable, representing atrophy that is as much as 8% greater per year on the paretic than the nonparetic side.
In another study, patients with stroke who had previously participated in an exercise recovery program were compared with controls without a history of stroke, and no differences in percentage body fat and FFM by bioelectrical impedance and skin fold thicknesses were observed.7 These results might suggest that the recovery program prevented significant atrophy, but this should be interpreted with caution because it was a cross-sectional analysis. Moreover, it is possible that the body composition analyses used are not sensitive enough to detect differences between the paretic and nonparetic limbs, such as by CT as used here.
We previously described significant muscular atrophy and increased low-density lean tissue in the paretic leg of stroke patients using a single midthigh CT cross section with no difference in subcutaneous midthigh fat between legs.4 However, in this study involving a much larger sample size and more extensive CT analysis in a new group of stroke patients, the subcutaneous fat is significantly higher on the paretic than the nonparetic limb. Moreover, the amount of subcutaneous fat is ~55% greater than the amount of muscle at the level of the midthigh. In striking contrast, in healthy older men, the amount of muscle in the midthigh is approximately 2.5-fold greater than midthigh fat illustrating the substantial muscle atrophy and increased thigh subcutaneous fat in our stroke patients. Our gender findings also provide new information that women stroke patients with lower muscle area and volume and greater subcutaneous and fat infiltration in the muscle than men may be at heightened risk for sarcopenic obesity and could benefit from interventions targeted to alter muscle composition.
We previously reported increased low-density lean tissue (eg, greater proportion of fat within the muscle area) by a single-slice CT scan of the midthigh in the paretic than in the nonaffected limb.4 In the present study, we added the measurement of muscle attenuation, and the results confirm increased fat infiltration in the paretic side. This was observed across the total thigh (volume) as well as in 5 of the 6 muscles studied. The significance of this finding is underscored by the reports that intramuscular fat is associated with insulin resistance and dyslipidemia14,15 and that stroke patients have a 70% prevalence of type 2 diabetes and insulin resistance16 with reports of an association of insulin resistance with risk for stroke.17,18 It is unclear whether the stroke precedes the diabetes or is a consequence of the stroke. Our study shows that muscle attenuation is higher (less intramuscular fat) in those stroke individuals with the lowest insulin levels. These observations are generally consistent across each of the 6 muscles on the nonparetic side. The associations were less significant on the paretic side despite greater fat infiltration, which suggests that it is the sedentary lifestyle of a stroke survivor, secondary to the paresis, which leads to the increase in fat within the muscle to affect systemic insulin sensitivity, thereby increasing the risk for type 2 diabetes.
Our data on strength demonstrate a consistently higher muscle torque on the nonparetic side, although the differences between the legs are more dramatic in the concentric mode. In addition, we found that muscle quality (concentric torque adjusted for muscle volume) is almost 2-fold lower in the paretic leg than the nonparetic leg. Of note, knee extensor torque is only associated with muscle volume and muscle attenuation in the eccentric mode. Because concentric torque is not associated with muscle volume, this suggests that the muscle atrophy on the paretic side has a more modest effect on lifting than lowering of a load. Our findings of reduced torque associated with lower VO2peak and 6-minute walk distances indicate that a reduction in strength limits fitness and function in participants with stroke and confirms our earlier report.19 We also add new data indicating that higher muscle quality was associated with higher VO2peak and 6-minute walk distances. Unexpectedly, we also observe no differences in concentric muscle torque between men and women despite the higher total muscle volumes and areas in each muscle in the men. Women may activate and sustain the neural drive to the quadriceps differentially in the concentric mode, but electromyography would be necessary to confirm this. Neural factors such as motor unit firing rates may also contribute to these gender-based observations in concentric versus eccentric torque. The greater eccentric muscle torque in men than women supports our observation of the association between eccentric torque and muscle volume. The reduced muscle volumes associated with muscle weakness may lead to functional consequences, including the risk of falls and injury.
The skeletal muscle atrophy, increased fat around and in muscle, ensuing muscular weakness, and reduced muscle quality that we observe in stroke participants may increase diabetes risk and impair functional mobility and independence. Exercise and other interventions designed to increase muscle area, reduce fat infiltration, and improve muscular strength are needed to alleviate these problems.20,21
Acknowledgments
Our appreciation is extended to those stroke patients who participated in this study. We are grateful to the nurses, research assistants, and laboratory staff in the Geriatrics Services at the Baltimore Veterans Administration (VA) Medical Center for technical assistance.
Funding
This study was supported by funds from the following sources: VA Research Career Scientist Award; VA Merit Awards; NIH grants R01-AG19310 and KO1-AG019242, R29 AG14487; Claude D. Pepper Older Americans Independence Center (P60AG12583); the Baltimore VA Geriatric Research, Education, and Clinical Center (GRECC); VA Rehabilitation Research & Development Maryland Exercise and Robotics Center of Excellence (B3688R; RR &D MERCE); and VA Stroke Research Enhancement Award Program.
Footnotes
Reprints and permission: http://www.sagepub.com/journalsPermissions.nav
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.American Heart Association (AHA) Heart and Stroke Facts. Dallas, TX: AHA; 2010. [Google Scholar]
- 2.Stuart M, Benvenuti F, Macko R, et al. Community-based adaptive physical activity program for chronic stroke: feasibility, safety, and efficacy of the Empoli model. Neurorehabil Neural Repair. 2009;23:726–734. doi: 10.1177/1545968309332734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broderick J, Brott T, Kothari R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- 4.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 5.Ryan AS, Dobrovolny CL, Silver KH, Smith GV, Macko RF. Cardiovascular fitness after stroke: role of muscle mass and gait deficit severity. J Stroke Cerebro Dis. 2000;9:185–191. doi: 10.1053/jscd.2000.7237. [DOI] [PubMed] [Google Scholar]
- 6.Carin-Levy G, Greig C, Young A, Lewis S, Hannan J, Mead G. Longitudinal changes in muscle strength and mass after acute stroke. Cerebrovasc Dis. 2006;21:201–207. doi: 10.1159/000090792. [DOI] [PubMed] [Google Scholar]
- 7.Sotillo C, Lopez-Jurado M, Martin E, Mataix J, Llopis J. Body composition assessed by anthropometry and bioelectric impedance analysis in older persons recovering from cerebro-vascular accident. Int J Vitam Nutr Res. 2003;73:32–38. doi: 10.1024/0300-9831.73.1.32. [DOI] [PubMed] [Google Scholar]
- 8.Pang MY, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos Int. 2005;16:1769–1779. doi: 10.1007/s00198-005-1925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes. 2004;5:219–226. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 10.Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity. 2006;14:1064–1072. doi: 10.1038/oby.2006.122. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Radloff LS, Rae DS. Susceptibility and precipitating factors in depression: sex differences and similarities. J Abnorm Psychol. 1979;88:174–181. doi: 10.1037//0021-843x.88.2.174. [DOI] [PubMed] [Google Scholar]
- 13.Macko RF, DeSouza CA, Tretter LD, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients: a preliminary report. Stroke. 1997;28:326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 14.Ryan AS, Nicklas BJ. Age-related changes in fat deposition in mid-thigh muscle in women: relationships with metabolic cardiovascular disease risk factors. Int J Obes Relat Metab Disord. 1999;23:126–132. doi: 10.1038/sj.ijo.0800777. [DOI] [PubMed] [Google Scholar]
- 15.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 16.Kernan WN, Inzucchi SE. Type 2 diabetes mellitus and insulin resistance: stroke prevention and management. Curr Treat Options Neurol. 2004;6:443–450. doi: 10.1007/s11940-004-0002-y. [DOI] [PubMed] [Google Scholar]
- 17.Shinozaki K, Naritomi H, Shimizu T, et al. Role of insulin resistance associated with compensatory hyperinsulinemia in ischemic stroke. Stroke. 1996;27:37–43. doi: 10.1161/01.str.27.1.37. [DOI] [PubMed] [Google Scholar]
- 18.Folsom AR, Rasmussen ML, Chambless LE, et al. Prospective associations of fasting insulin, body fat distribution, and diabetes with risk of ischemic stroke: The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Diabetes Care. 1999;22:1077–1083. doi: 10.2337/diacare.22.7.1077. [DOI] [PubMed] [Google Scholar]
- 19.Patterson SL, Forrester LW, Rodgers MM, et al. Determinants of walking function after stroke: differences by deficit severity. Arch Phys Med Rehabil. 2007;88:115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Cooke EV, Tallis RC, Clark A, Pomeroy VM. Efficacy of functional strength training on restoration of lower-limb motor function early after stroke. Neurorehabil Neural Repair. 2010;24:88–96. doi: 10.1177/1545968309343216. [DOI] [PubMed] [Google Scholar]
- 21.Ryan AS, Ivey FM, Prior S, Li G, Hafer-Macko C. Skeletal muscle hypertrophy and muscle myostatin reduction after resistive training in stroke survivors. Stroke. 2011;42:416–420. doi: 10.1161/STROKEAHA.110.602441. [DOI] [PMC free article] [PubMed] [Google Scholar]


