Abstract
Background
Stress hyperglycemia and acute graft versus host disease (GVHD), the major early complication of hematopoietic stem-cell transplantation (HSCT), are both associated with excessive release of inflammatory cytokines. We investigated whether new-onset hyperglycemia immediately after HSCT predicts acute GVHD.
Methods
We studied nondiabetic adult recipients of human leukocyte antigen(HLA)-matched HSCT (peripheral blood stem-cells) for acute leukemia. Using mean morning serum glucose on Day 1–10, we classified hyperglycemia as: mild (6.11–8.33 mmol/L), moderate (8.34–9.98), severe (minimum 9.99). Subjects GVHD-free on Day 10 were followed Day 11–100 for grade II-IV acute GVHD or competing event (relapse or death). Evaluation utilized cumulative incidence-based proportional hazards regression.
Results
Subjects (n=328) were age 18–74, median 49 years. Per body mass index (BMI), 25.0% were obese (BMI 30–48), 33.8% overweight (25–<30), 30.8% normal weight (21–;<25), and 10.4% lean (18–;<21). Mild, moderate, or severe hyperglycemia occurred Day 1–10 in 50.0%, 21.3%, and 16.8% of subjects, respectively. Cumulative incidence on Day 100 was 44.8(±2.8)% acute GVHD and 7.9(±1.5)% competing event. Among normal-to-overweight subjects (n=212), severe hyperglycemia developed in 14.2% (n=30) and more than doubled the risk of acute GVHD (hazards ratio, HR, 2.71, 95% CI 1.58–4.65, adjusted for donor/recipient characteristics, prophylactic regimen, and mucositis). In contrast, among obese subjects (n=82), severe hyperglycemia developed in 30.5% (n=25) but did not significantly affect risk of GVHD. (No lean subjects (n=34) developed severe hyperglycemia.) Hyperglycemia that was less than severe had an effect indistinguishable from normoglycemia.
Conclusions
In nondiabetic patients, severe hyperglycemia immediately after allogeneic HSCT indicates increased likelihood of acute GVHD. This association is absent in obese patients, who may be primed by obesity-induced inflammation to develop severe hyperglycemia even without experiencing the cytokine storm that is essential to GVHD pathogenesis.
Keywords: Acute graft versus host disease, Biomarker, Glucose metabolism disorders, Hematopoietic stem cell transplantation, Leukemia
BACKGROUND
Acute graft-versus-host disease (GVHD), an extreme example of inflammation, is the major early complication of allogeneic hematopoietic stem-cell transplantation (HSCT) [1] yet remains difficult to predict on the basis of clinical risk factors alone. Several such factors have been identified, including recipient characteristics (age, leukemic diagnosis, Karnofsky performance score, mucositis and diarrhea associated with conditioning, immunoregulatory gene polymorphisms) and graft characteristics (source of stem-cells, cytomegalovirus(CMV) serostatus of donor and recipient, human leukocyte antigen (HLA) and minor histocompatibility antigen mismatch, conditioning regimen) [1–7]. A long-established clinical risk factor, female-to-male graft, may no longer be reliably associated with acute GVHD [2].
Biological predictors of acute GVHD are needed, and several candidates have been identified. Systemic elevation of inflammatory cytokines immediately before and after HSCT is crucial to the pathogenesis of acute GVHD. A composite biomarker panel of 4 proteins (interleukin(IL)-2-receptor-α, tumor necrosis factor(TNF)-receptor-1, IL-8, and hepatocyte growth factor) correlates with the clinical diagnosis of acute GVHD [8], and IL-2, TNF-α, and Fas have each been observed to rise prior to symptomatic acute GVHD [9–12]. However, assessment of these potential biomarkers is expensive and not routinely available.
Like acute GVHD, stress hyperglycemia in critically ill patients is closely associated with systemic elevation of inflammatory cytokines, including IL-6 and TNF-α, and is predictive of poor clinical outcome [13–16]. Unlike inflammatory cytokines, however, serum glucose is routinely monitored in the days after allogeneic HSCT, making hyperglycemia practical to consider as a potential biomarker for acute GVHD. In fact, a preliminary association was recently reported between acute GVHD grade II-IV and level of hyperglycemia during the neutropenic period [17].
However, it was unclear from the previous study [17] whether the occurrence of hyperglycemia preceded or merely coincided with acute GVHD, because acute GVHD may occur before as well as after neutrophil engraftment [18]. Moreover, the need to monitor patients throughout the variable neutropenic period would delay the calculation of mean morning serum glucose (MSG) until engraftment is confirmed, and such delay could diminish the ability to predict acute GVHD well before the onset of symptoms. Therefore, we investigated whether monitoring MSG for the 10 days immediately after HSCT would be sufficient to predict development of acute GVHD during the subsequent 90 days, independently of other risk factors. To ensure that hyperglycemia following HSCT was of new onset and hence potentially related to incipient acute GVHD, we excluded patients with preexisting diabetes mellitus or recent hyperglycemia from our study.
METHODS
Eligibility
Subjects were consecutive patients, age 18 years or older who underwent primary, HLA-matched, allogeneic HSCT for acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), or acute lymphoid leukemia (ALL) at our institution between October 2003 and April 2009. Further eligibility criteria were: graft of peripheral blood stem-cells; GVHD prophylaxis that included tacrolimus with sirolimus and/or methotrexate (MTX) or a combination of cyclosporine with MTX and/or mycophenolate mofetil; and status alive and free of GVHD on Day 10 after allogeneic HSCT, when follow-up began. Patients were excluded if their conditioning regimen included rabbit antithymoglobulin or if they had a history of diabetes mellitus, MSG >5.55 mmol/L or random serum glucose >7.77 mmol/L prior to conditioning, or recent corticosteroid use prior to admission for HSCT, other than the single dose of corticosteroids routinely administered on the day of transplantation.
Study conduct
This study was approved in advance by the hospital’s institutional review board, which provided a waiver of informed consent to ensure that all eligible patients, including those lost to follow-up, would be included in the study cohort. Data were obtained from electronic medical and pharmacy records, the cancer registry, clinical trial adverse events records, and a database of standard HSCT outcomes (ie, acute GVHD, leukemic relapse, death without relapse) maintained for a federally mandated registry.
Acute GVHD
Using established criteria for GVHD [19], transplant physicians assessed patients daily during the hospitalization for HSCT and twice weekly thereafter through the study’s close on Day 100. For the study, acute GVHD was defined as cases that began during Day 11–100 after allogeneic HSCT and reached at least grade II. The competing event for acute GVHD during Day 11–100 was treatment failure (leukemic relapse or non-relapse mortality) without prior acute GVHD.
Risk factors for acute GVHD
The study’s primary risk factor, level of hyperglycemia, was derived from the mean of MSG values on Day 1–10. When present, hyperglycemia was categorized as mild (6.11–8.33 mmol/L), moderate (8.34–9.98 mmol/L), or severe (minimum 9.99 mmol/L). Serum glucose was measured between midnight and 0800 h daily as part of the routine chemistry panel. Covariates considered in the analysis included characteristics of donor, recipient, or graft: type of donor (unrelated versus sibling); sex of donor and recipient (specifically, female-to-male and female-to-female versus other graft); CMV serostatus of donor and recipient (specifically, seronegative-to-seronegative graft [2] versus other graft); underlying diagnosis (myeloid versus lymphoid); stage of disease [early (first complete response or refractory anemia), intermediate (second or third complete response or first relapse), or late (all other stages)]; conditioning (myeloablative total body irradiation(TBI)-based [6] versus other); regimen for GVHD prophylaxis; self-reported ethnicity; age and Karnofsky score at HSCT.
Additional covariates were body mass index (BMI) at admission and mucositis. BMI was categorized as obese (30–48), overweight (25–;<30), normal weight (21–;<25), and lean (18– ;<21). Had we instead defined lean as BMI 18–;<20, as is typically done, there would have been half as many lean subjects, with too little power to detect that group’s marked association with acute GVHD, and the normal weight group would then have included several lean subjects not at risk of severe hyperglycemia (observed only among subjects of BMI 21 or more). The maximum degree of mucositis (observed from start of conditioning through Day 10) was assessed separately by clinical examination and by functional/symptomatic score, each graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [20]. Mucositis was not recorded for subjects who were not participating in a clinical trial; however, such subjects were retained in all current analyses by handling “unscored for mucositis” as a separate risk category. Further covariates from Day 1–10 included: fever (>38.1°C) attributed to infection, whether suspected or confirmed; corticosteroid use; days of insulin use; and days of total parenteral nutrition (TPN), given as needed using the Harris-Benedict formula [21], 25–30 kCal per kg per day.
Statistical analysis
Proportions were compared using chi-square tests, and means were compared using t tests. Determinants of MSG during Day 1–10 were identified using a generalized linear mixed model, for which glucose values were log-transformed to normalize their skewed distribution. To estimate the occasional missing MSG value, the model shown in Table 3 was expanded to include use of insulin on the current day and on previous days.
Table 3.
Mixed Model of Daily Morning Serum Glucose (MSG), during Day 1–10 after Allogeneic HSCT.
| Estimated MSG Absent Risk Factors Below | 5.27 (4.88–5.68) mmol/L | |
|---|---|---|
| Percent Change (95% CI) in MSG per Risk Factor |
p | |
| Hispanic Recipient with Unrelated Donor | +17.15 (8.42–26.58) | <0.0001 |
| Sex of Recipient, GVHD Prophylaxis | ||
| Male, Tacrolimus-based Prophylaxis | +11.91 (4.91–19.37) | <0.001 |
| Male, Other Prophylaxis | +13.08 (4.10–22.83) | 0.004 |
| Female, Tacrolimus-Based Prophylaxis | +7.80 (1.02– 15.03) | 0.024 |
| Female, Other Prophylaxis | 0 | |
| Day 1 Post-Transplantation | +9.82 (6.72–13.01) | <0.0001 |
| TBI-based Myeloablative Conditioning | +8.60 (4.55–12.81) | <0.0001 |
| TPN, Current Day | +8.68 (4.43–13.09) | <0.0001 |
| TPN, For Each Preceding Day of Use | +1.20 (0.76–1.65) | <0.0001 |
| Per One Step Increase in Category of BMI * | +6.62 (4.53–8.76) | <0.0001 |
Categories of Body Mass Index: Lean (18–<21), Normal (21–<25), Overweight (25–<30), Obese (30–48)
The primary association under study, between level of hyperglycemia and cumulative incidence of acute GVHD, was investigated in univariate analysis using the Gray test [22] and in multivariate analysis using cumulative incidence-based proportional hazards regression [23, 24]. Covariates and interactions were retained if they improved the model’s fit to the observed data.
RESULTS
Sample
Subjects (n=328, Table 1) were age 46.5(±14.1) years. Virtually all subjects received TPN, usually on all or most of the 10 days (Table 2). When insulin was administered during the 10 days, the initial dose was median 10 (5th to 95th percentile range: 4–20) units, and the last dose was median 20 (5–70) units. Most subjects developed fever attributed to infection, but very few patients received corticosteroids. By Day 10, nearly all (88.1%) subjects had developed some degree of hyperglycemia, and 1 in 6 subjects had developed severe hyperglycemia (Table 2).
Table 1.
Characteristics of Allogeneic HSCT Recipients (N=328) and Their Grafts.
| Characteristics | N | (%) |
|---|---|---|
| Age at Transplantation | ||
| 18–39 Years | 108 | (32.9) |
| 40–64 Years | 191 | (58.2) |
| 65–75 Years | 29 | (8.8) |
| Stage of Disease | ||
| Early | 151 | (46.0) |
| Intermediate | 74 | (22.6) |
| Late | 103 | (31.4) |
| Karnofsky Score at HSCT | ||
| Good (90% or 100%) | 203 | (61.9) |
| Poor (70% or 80%) | 17 | (5.2) |
| Score Unavailable | 108 | (32.9) |
| Ethnicity | ||
| Non-Hispanic White | 206 | (62.8) |
| Hispanic White | 76 | (23.2) |
| Asian | 41 | (12.5) |
| African-American | 5 | (1.5) |
| Body Mass Index | ||
| 18–<21 | 34 | (10.4) |
| 21–<25 | 101 | (30.8) |
| 25–<30 | 111 | (33.8) |
| 30–48 | 82 | (25.0) |
| Type of Donor | ||
| HLA-Matched Sibling | 191 | (58.2) |
| HLA-Matched Unrelated | 137 | (41.8) |
| CMV Serostatus of | ||
| Donor (D)/Recipient (R) | ||
| D+/ R+ | 163 | (49.7) |
| D+/R− | 53 | (16.2) |
| D−/R+ | 74 | (22.6) |
| D−/R− | 38 | (11.6) |
| Sex of Donor / Recipient | ||
| D Male/ R Male | 104 | (31.7) |
| D Male / R Female | 94 | (28.7) |
| D Female / R Male | 64 | (19.5) |
| D Female / R Female | 66 | (20.1) |
| Intensity of Conditioning * | ||
| Myeloablative, TBI | 156 | (47.6) |
| Myeloablative, BuCy | 19 | (5.8) |
| Reduced Intensity | 153 | (46.7) |
| GVHD Prophylaxis † | ||
| Tacro + Siro | 179 | (54.6) |
| Tacro + Siro + Mini MTX | 50 | (15.2) |
| Tacro + Mini MTX | 33 | (10.1) |
| Cyclosporin with MTX and/or Mycophenolate Mofetil | 66 | (20.1) |
TBI = Total Body Irradiation, BuCy = Busulfan + Cyclophosphamide
Tacro = Tacrolimus, Siro = Sirolimus, MTX = methotrexate
Table 2.
Characteristics of HSCT Recipients (N=328) during the First Ten Days after Transplantation.
| Characteristics | N | (%) |
|---|---|---|
| Hyperglycemia (10-Day Mean Glucose) | ||
| None (less than 6.11 mmol/L) | 39 | (11.9) |
| Mild (6.11–8.33 mmol/L) | 164 | (50.0) |
| Moderate (8.34–9.98 mmol/L) | 70 | (21.3) |
| Severe (9.99 mmol/L or more) | 55 | (16.8) |
| Fever Attributed to Infection | 270 | (82.3) |
| Corticosteroid Use (Other than Day 1) | 20 | (6.1) |
| Total Parenteral Nutrition (TPN) | ||
| On All 10 Days | 242 | (73.8) |
| On Most (6–9) Days | 56 | (17.1) |
| On Few (1–5) Days | 12 | (3.7) |
| On None of 10 Days | 18 | (5.5) |
| Any Insulin via TPN | 129 | (39.3) |
| Mucositis on Clinical Examination | ||
| No Mucositis | 97 | (29.6) |
| Erythema of Mucosa Only | 17 | (5.2) |
| Patchy Ulceration | 34 | (10.4) |
| Confluent Ulceration | 24 | (7.3) |
| Not Examined (Non-Trial Subject) | 156 | (47.6) |
| Mucositis Symptoms, Functioning | ||
| No Symptoms | 106 | (32.3) |
| Symptoms, Adequate Normal Diet | 11 | (3.4) |
| Symptoms, Adequate Modified Diet | 22 | (6.7) |
| Symptoms, Inadequate Oral Intake | 33 | (10.1) |
| Not Examined (Non-Trial Subject) | 156 | (47.6) |
Determinants of Morning Serum Glucose Day 1–10
In 4 (1.2%) subjects, one of the 10 daily measurements of MSG was missing at random and was estimated (as described in the Methods section) to permit calculation of 10-day mean glucose for each subject. As shown in Table 3, during the 10 days following HSCT, MSG level was increased on Day 1 (when a single dose of glucocorticoid was routinely administered) and was further elevated by increasing category of BMI; by current use of TPN and by each prior day of TPN use within the 10-day period; by male recipient, tacrolimus-based prophylaxis, or both; by the combination of Hispanic recipient with unrelated donor (but not by ethnicity or unrelated donor alone); and by myeloablative TBI-based conditioning.
Other characteristics (recipient’s age, underlying diagnosis, CMV serostatus of donor and recipient, mucositis by either scoring system, fever attributed to infection, non-routine corticosteroid use during Day 1–10) were unassociated with MSG. Same day use of insulin, being a result rather than a cause of hyperglycemia, would not have been valid to consider as a determinant of MSG.
Acute GVHD Grade II-IV during Day 11–100
Among the cases of acute GVHD (n=147), 61.9% had Grade II disease; distribution of grade did not differ by presence of severe hyperglycemia. At Day 100 post-transplantation, the cumulative incidence of acute GVHD was 44.8(±2.8)%. Another 7.9(±1.5)% of subjects developed a competing event (leukemic relapse n=19, nonrelapse mortality n=7) by Day 100.
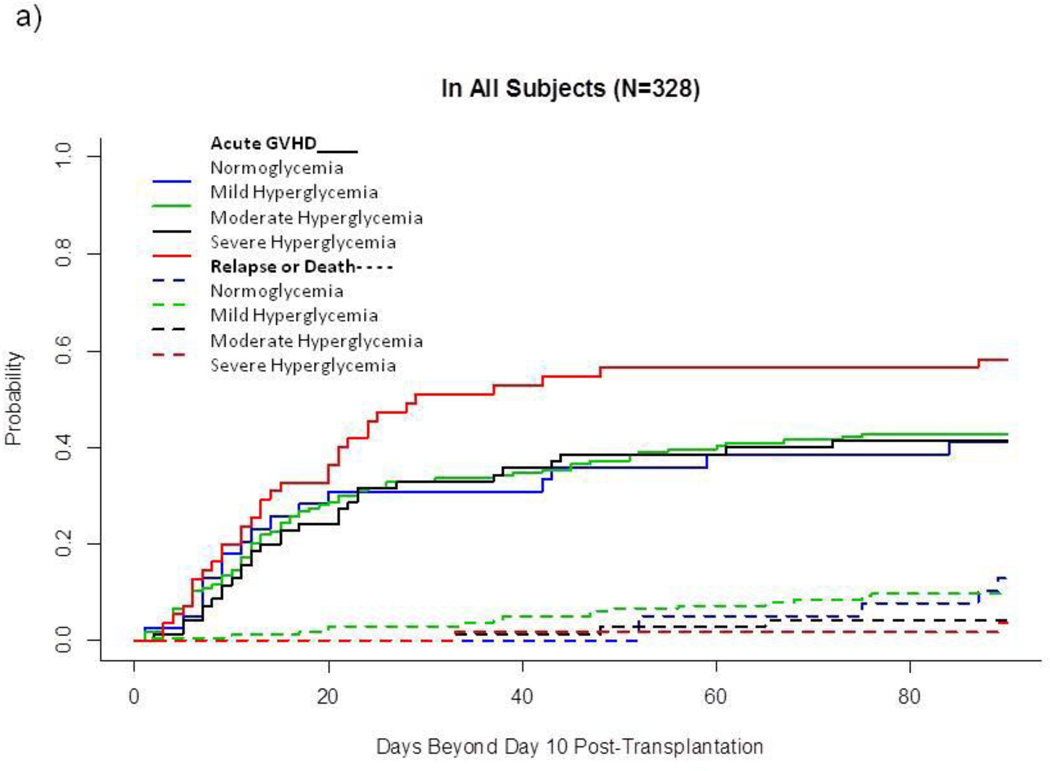
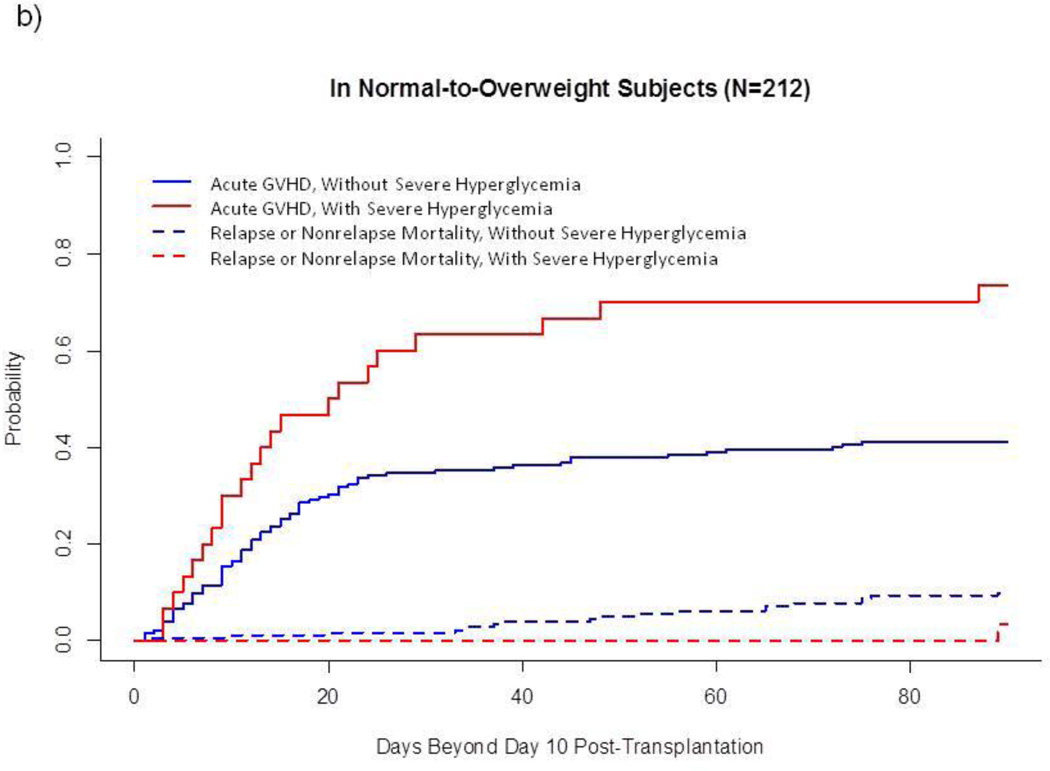
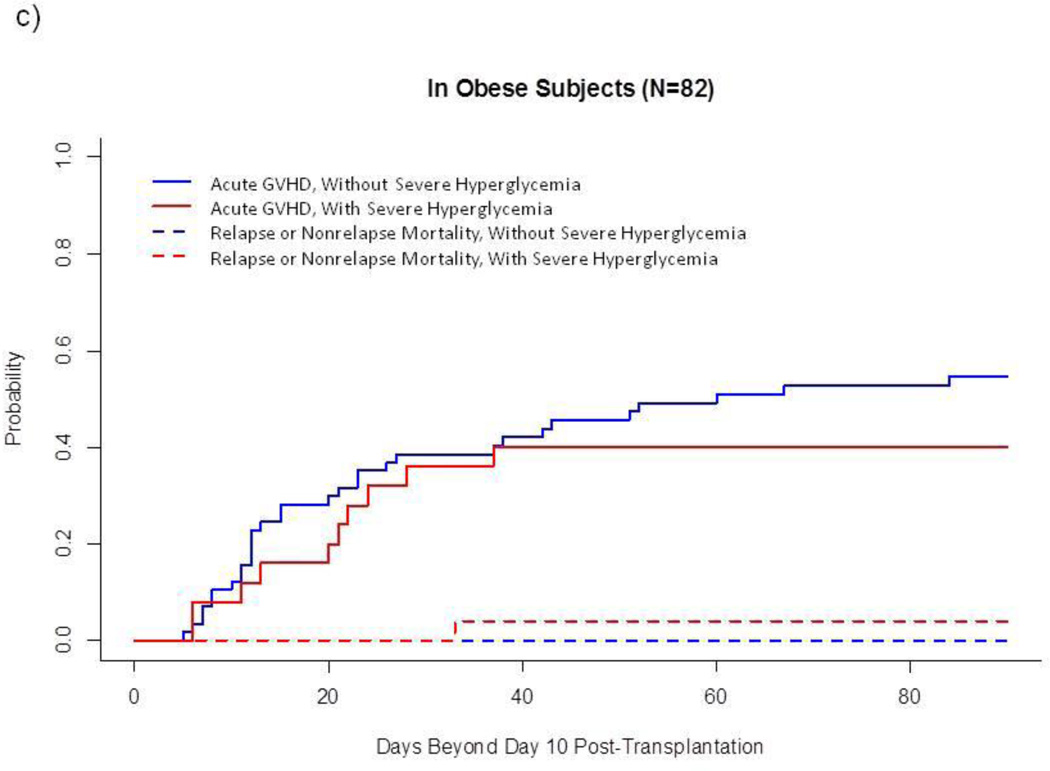
In univariate analysis of the entire sample (Fig. 1a), cumulative incidence of acute GVHD was higher after severe hyperglycemia than after the other 3 glycemic categories combined (58.2(±6.8)% vs 42.1(±3.0)%, p=0.03). However, this association between GVHD and severe hyperglycemia varied by category of BMI: Among normal-to-overweight subjects, severe hyperglycemia developed in 14.2% and was markedly associated with acute GVHD (73.3(±8.4)% vs 41.2(±3.7)%, p<0.001, Fig. 1b). In contrast, among obese subjects, severe hyperglycemia developed in 30.5% and was not associated with acute GVHD (Fig. 1c). Severe hyperglycemia did not develop among lean subjects (n=34), who had a relatively low cumulative incidence of acute GVHD (26.5(±7.7)%) and a relatively high incidence of competing events (17.6(±6.7)%, 5 relapse, 1 non-relapse death without prior GVHD) (data not shown).
Fig. 1.
Cumulative Incidence of Acute GVHD and Competing Events, by Glycemic Category after Allogeneic HSCT.
a) In all subjects (N=328)
b) In normal-to-overweight subjects (N=212)
c) In obese subjects (N=82)
This landmark analysis follows subjects Day 11–100 after HSCT. Unbroken lines represent cumulative incidence of acute GVHD, and dotted lines represent cumulative incidence of treatment failure without prior acute GVHD.
These univariate findings were strengthened when established risk factors for GVHD (sirolimus-based prophylactic regimen, sex and CMV serostatus of donor and recipient, mucositis, underlying diagnosis) were taken into account. As shown in Table 4, severe hyperglycemia more than doubled the risk of GVHD among normal-to-overweight subjects but did not significantly affect risk among obese subjects. Lean BMI remained associated with reduced likelihood of acute GVHD (Table 4).
Table 4.
Cumulative Incidence-Based Proportional Hazards Model of Acute GVHD (N=328).
| Risk Factors | N | Hazards Ratio (95% CI) | p* |
| Glycemic Category Day 1–10, By Body Mass | |||
| Normal or Overweight | |||
| Without Severe Hyperglycemia | 182 | 1.00 | |
| With Severe Hyperglycemia | 30 | 2.71 (1.58–4.65) | 0.0003 |
| Obese | |||
| Without Severe Hyperglycemia | 57 | 1.14 (0.70–1.85) | NS |
| With Severe Hyperglycemia | 25 | 0.82 (0.44–1.52) | NS |
| Lean | |||
| Without Severe Hyperglycemia | 34 | 0.30 (0.10–0.86) | 0.0249 |
| With Severe Hyperglycemia | 0 | ----- | |
| GVHD Prophylaxis | |||
| Sirolimus-Based Regimen | 229 | 0.44 (0.29–0.67) | |
| Other Regimen | 99 | 1.00 | |
| Mucositis on Clinical Examination | |||
| Patchy or Confluent Ulceration | 58 | 2.80 (1.59–4.95) | |
| No Mucositis or Erythema Only | 114 | 1.00 | |
| Not Examined | 156 | 1.45 (0.93–2.28) | |
| CMV Serostatus of Donor (D) and Recipient (R) | |||
| D−,R+ | 74 | 2.60 (1.27–5.32) | |
| D+, R− | 53 | 1.88 (0.89–3.99) | |
| D+, R+ | 163 | 1.47 (0.75–2.89) | |
| D−, R− | 38 | 1.00 | |
| Female to Female Graft | |||
| Yes | 66 | 2.53 (1.67–3.84) | |
| No | 262 | 1.00 | |
| Underlying Diagnosis | |||
| Acute Lymphoid Leukemia (ALL) | 104 | 0.60 (0.39–0.93) | |
| Acute Myeloid Disease (AML/MDS) | 224 | 1.00 | |
Covariates were not evaluated for statistical significance; they were included in the model solely to control potential confounding of the primary association. Interaction between glycemic category and body mass was statistically significant (p=0.004). NS= not statistically significant (p>0.50).
Covariates excluded from the final model for lack of effect on fit were: age, ethnicity, unrelated versus sibling donor, stage of disease, Karnofsky score, and Day 1–10 factors (fever attributed to infection, corticosteroid use, days of TPN, and days of insulin). Because the 2 mucositis scores were highly correlated, the final model retained only the more significant one, clinical examination score. Subjects not scored for mucositis due to nonparticipation in a clinical trial had a risk of acute GVHD that fell between those of subjects with and without mucositis, as would be expected from a mixture of these 2 risk groups.
DISCUSSION
In contrast to a previous report [17] that associated acute GVHD with moderate-to-severe hyperglycemia prior to neutrophil engraftment, the current study has narrowed the GVHD-associated signal to severe hyperglycemia evident during the first 10 days after allogeneic HSCT. We found that while lesser degrees of hyperglycemia are unassociated with acute GVHD, severe hyperglycemia immediately after transplantation indicates an increased likelihood of developing this complication. Among patients who are obese, however, severe hyperglycemia has no prognostic value, for reasons explored below. Finally, level of blood glucose does not correlate with grade of acute GVHD: the severity distribution of GVHD was alike in current subjects who did and did not experience severe hyperglycemia. The latter finding suggests that the pathogenesis of GVHD is the same regardless of its severity and that the ultimate grade of GVHD may be determined by a different set of factors than those that initiate the disease.
That severe hyperglycemia after transplantation is predictive of acute GVHD in non-obese subjects but not in obese patients may result from the chronic inflammation triggered by obesity [25]. In brief, overnutrition results in recruitment of monocytes into the enlarging adipose tissue. There, the cells differentiate into classically activated macrophages that encircle necrotic adipocytes and produce pro-inflammatory molecules such as TNF and IL-1β. Crosstalk between the classically activated macrophages in obese adipose tissue and pathogenic CD4+ and CD8+ T cells further amplifies the inflammatory milieu. The inflammation associated with obesity exceeds the control of antiinflammatory mechanisms resident in alternatively activated macrophages, found mainly in lean adipose tissue. The result of this chronic inflammation is insulin resistance, in which impaired glucose disposal leads to hyperinsulinemia, hyperglycemia, and hyperlipidemia.[25]
Thus primed by obesity-induced chronic inflammation, obese subjects might develop severe hyperglycemia as a consequence of HSCT-related stress alone, even without the inflammatory cytokine storm associated with GVHD pathogenesis. In contrast, non-obese HSCT recipients may be unlikely to develop severe hyperglycemia without also experiencing cytokine storm. To learn whether correlation between severe hyperglycemia and excessive cytokine levels differs between obese and non-obese HSCT recipients, future studies should track glucose in tandem with inflammatory cytokines during pretransplantation conditioning and the first 10 days after HSCT, analyzing the data with attention to category of BMI.
Whether tighter control of serum glucose before and after allogeneic HSCT might reduce the incidence of acute GVHD, particularly among normal-to-overweight recipients, awaits a clinical trial. Previously, a pilot trial of intensive glucose management after allogeneic HSCT did not detect a reduced incidence of acute GVHD [26]. However, that small, non-randomized trial must be regarded as inconclusive, especially because its eligibility criteria did not target normal-to-overweight subjects at high risk of severe hyperglycemia, the patient group our study suggests would be most likely to benefit from the intervention.
Our study also observed that the leanest patients do not develop severe hyperglycemia following allogeneic HSCT and that acute GVHD develops less often in lean patients, apparently preempted by competing events, chiefly leukemic relapse. Recently, underweight (BMI <18), a degree of leanness not found in the current study, was associated with lower risk of acute GVHD among recipients of allogeneic HSCT, but only among subjects with a related donor [27]. In our study, however, type of donor had no effect on the association between BMI and acute GVHD. Elsewhere, a potential association has been reported between increasing category of BMI and risk of acute GVHD [28]. In contrast, our study detected no greater risk in obese or overweight subjects than in normal weight subjects.
To better understand hyperglycemia following allogeneic HSCT, we identified factors independently associated with increase in blood glucose during the first 10 days after allogeneic HSCT. Two of these risk factors (male gender and the combination of Hispanic ethnicity with unrelated donor) are novel, while the other risk factors for hyperglycemia are well established, including: greater BMI, which promotes insulin resistance [25]; tacrolimus [29], which impairs insulin secretion [30]; glucocorticoids (administered on Day 1), which cause impaired glucose uptake, enhanced gluconeogenesis, and increased glycogenolysis [31]; myeloablative conditioning with total body irradiation, which like chemotherapy, may damage islet beta cells directly [32,33]; and TPN, which is associated with profound hyperglycemia even when used per clinical guidelines in HSCT recipients [34]. From Table 3, it is clear that a patient would have needed nearly all of these risk factors to develop severe hyperglycemia, which corresponds to nearly double (190%) the MSG level estimated for a patient with none of the factors.
Although conceived and executed after all subjects had completed their first 100 days of care, our study was nevertheless free of the recall and selection biases common to retrospective studies, because we were able to study 100% of eligible patients and because our data had been recorded as measurements were made and as events occurred, i.e., prospectively. The fact that clinicians were unaware of hyperglycemia as a potential predictive factor at the time they were monitoring or prophylaxing subjects for acute GVHD was a strength of the study, because it ensured against diagnostic bias.
A limitation of the study was that only routinely recorded data were available for study. Thus we had access to data on MSG but not on cytokines such as IL-6, IL-18, and TNF-α, which have been shown to rise after induction of hyperglycemia [16]. Specimens for MSG testing were collected in the early morning hours, when fasting was presumed but not formally documented. Data on one of the covariates, mucositis [3], had been recorded only for subjects who were clinical trial participants. Nonetheless, we were able to include subjects who lacked mucositis evaluations in all current analyses, detecting increased risk of acute GVHD in subjects found to have ulcerations on clinical examination, whether or not symptoms interfered with oral intake. This finding is consistent with a previous report [3] that painful mucositis requiring TPN or opiate analgesics precedes acute GVHD, although direct comparison between our finding and the previous one is precluded by non-comparable grading of mucositis.
Another limitation of our study is that data were unavailable on diarrhea related to conditioning, a GVHD risk factor independent of mucositis [4, 5]. In addition, our study was uninformative about hyperacute GVHD, because our eligibility criteria excluded from the cohort those patients who developed GVHD before Day 11 after allogeneic HSCT. Finally, because the majority of GVHD cases were Grade II, there were not sufficient numbers of Grade III-IV cases to support analyzing these separately.
In conclusion, severe hyperglycemia in the first 10 days after allogeneic HSCT is a novel predictor of acute GVHD among non-obese patients. This prognostic signal is detectable well before onset of symptoms, is independent of established risk factors for GVHD, and requires only short-term monitoring of a marker (glucose) that is routinely measured in clinical practice.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Fouad Kandeel for helpful comments on the manuscript, to Chakriya D. Anunta for editorial assistance, and to Eric Huang, Vanetta Onwochei, Cindy Stahl, Blanca Herrera, and Abayneh Shitahun for assistance with data collection and management.
This project was supported in part by Grant Number P30 CA033572 from the National Cancer Institute, U.S. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or National Institutes of Health.
Footnotes
Disclosures: A preliminary analysis of this study was presented at the annual meeting of the Endocrine Society, San Diego, California, June 2010.
DISCLOSURE STATEMENT
The authors state that they have no potential conflicts of interest to declare.
AUTHORS’ CONTRIBUTIONS
All authors participated in designing the study, interpreting the data, and writing the manuscript. EG and CEB conducted the data collection and analysis.
REFERENCES
- 1.Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn T, McCarthy PL, Jr, Zhang MJ, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol. 2008;26:5728–5734. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Au WY, Wong RWM, Wong BCY, et al. Reduced incidence of acute graft versus host disease (GVHD) of the gut in Chinese carriers of Helicobacter Pylori during allogeneic bone marrow transplantation. Ann Hematol. 2004;83:34–37. doi: 10.1007/s00277-003-0780-4. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg J, Jacobsohn DA, Zahurak ML, et al. Gastrointestinal toxicity from the preparative regimen is associated with an increased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:101–107. doi: 10.1016/j.bbmt.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Liu D, Yan C, Xu L, et al. Diarrhea during the conditioning regimen is correlated with the occurrence of severe acute graft-versus-host disease through systemic release of inflammatory cytokines. Biol Blood Marrow Transplant. 2010;16:1567–1575. doi: 10.1016/j.bbmt.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Hill GR, Crawford JM, Cooke KR, et al. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–3213. [PubMed] [Google Scholar]
- 7.Conway SE, Abdi R. Immunoregulatory gene polymorphisms and graft-versus-host disease. Expert Rev Clin Immunol. 2009;5:523–534. doi: 10.1586/eci.09.44. [DOI] [PubMed] [Google Scholar]
- 8.Paczesny S, Krijanovski OI, Braun TM, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009;113:273–278. doi: 10.1182/blood-2008-07-167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamoto T, Akashi K, Hayashi S, et al. Serum concentration of the soluble interleukin- 2-receptor for monitoring of acute graft-versus-host disease. Bone Marrow Transplant. 1996;17:185–190. [PubMed] [Google Scholar]
- 10.Kayaba H, Hirokawa M, Watanabe A, et al. Serum markers of graft-versus-host disease after bone marrow transplantation. Allergy Clin Immunol. 2000;106:40–44. doi: 10.1067/mai.2000.106060. [DOI] [PubMed] [Google Scholar]
- 11.Remberger M, Ringden O, Markling M. TNF-α levels are increased during bone marrow transplantation conditioning in patients who develop acute GVHD. Bone Marrow Transplant. 1995;15:99–104. [PubMed] [Google Scholar]
- 12.Choi SW, Kitko CL, Braun T, et al. Change in plasma tumor necrosis factor receptor 1 levels in the first week after myeloablative allogeneic transplantation correlates with severity and incidence of GVHD and survival. Blood. 2008;112:1539–1542. doi: 10.1182/blood-2008-02-138867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–982. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 14.McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001;17:107–124. doi: 10.1016/s0749-0704(05)70154-8. [DOI] [PubMed] [Google Scholar]
- 15.Boura-Halfon S, Zick Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am J Physiol Endocrinol Metab. 2009;296:E581–E591. doi: 10.1152/ajpendo.90437.2008. [DOI] [PubMed] [Google Scholar]
- 16.Esposito K, Nappo F, Marfella R, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 17.Fuji S, Kim SW, Mori SI, et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation. 2007;84:814–820. doi: 10.1097/01.tp.0000296482.50994.1c. [DOI] [PubMed] [Google Scholar]
- 18.Saliba RM, Lima MD, Giralt S, et al. Hyperacute GVHD: risk factors, outcomes and clinical implications. Blood. 2007;109:2751–2758. doi: 10.1182/blood-2006-07-034348. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 20.Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. assessed at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf on 11 January 2012.
- 21.Harris JA, Benedict FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 23.Klein JP, Andersen PK. Regression modeling of competing risks data based on pseudovalues of the cumulative incidence function. Biometrics. 2005;61:223–229. doi: 10.1111/j.0006-341X.2005.031209.x. [DOI] [PubMed] [Google Scholar]
- 24.Klein JP, Gerster M, Andersen PK, et al. SAS and R functions to compute pseudo-values for censored data regression. Comput Methods Programs Biomed. 2008;89:289–300. doi: 10.1016/j.cmpb.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuji S, Kim SW, Yoshimura K, et al. Possible association between obesity and posttransplantation complications including infectious diseases and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:73–82. doi: 10.1016/j.bbmt.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 27.Fuji S, Kim SW, Mori SI, et al. Intensive glucose control after allogeneic hematopoietic stem cell transplantation: a retrospective matched-cohort study. Bone Marrow Transplant. 2009;44:105–111. doi: 10.1038/bmt.2008.431. [DOI] [PubMed] [Google Scholar]
- 28.Navarro WH, Agovi MA, Logan BR, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myeloid leukemia (AML) in adults. Biol Blood Marrow Transplant. 2010;16:1442–1450. doi: 10.1016/j.bbmt.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo M, Przepiorka D, Ippoliti C, et al. Toxicities of tacrolimus and cyclosporin A after allogeneic blood stem cell transplantation. Bone Marrow Transplant. 1997;20:1095–1098. doi: 10.1038/sj.bmt.1701027. [DOI] [PubMed] [Google Scholar]
- 30.Øzbay LA, Smidt K, Mortensen DM, et al. Cyclosporin and tacrolimus impair insulin secretion and transcriptional regulation in INS-1E beta-cells. Br J Pharmacol. 2011;162:136–146. doi: 10.1111/j.1476-5381.2010.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandit MK, Burke J, Gustafson AB, et al. Drug-induced disorders of glucose tolerance. Ann Intern Med. 1993;118:529–539. doi: 10.7326/0003-4819-118-7-199304010-00008. [DOI] [PubMed] [Google Scholar]
- 32.Tsubouchi S, Suzuki H, Ariyoshi H, et al. Radiation-induced acute necrosis of the pancreatic islet and the diabetic syndrome in th golden hamster (Mesocricetus auratus) Int J Radiat Biol Relat Stud Phys Chem Med. 1981;40:95–106. [PubMed] [Google Scholar]
- 33.Feng JP, Yuan XL, Li M, et al. Secondary diabetes associated with 5-Fluorouracil-based chemotherapy regimens in non-diabetic patients with colorectal cancer: results from a single centre cohort study. Colorectal Dis. 2012 doi: 10.1111/j.1463-1318.2012.03097.x. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Sheean P, Braunschwieg C. The incidence and impact of dextrose dose on hyperglycemia from parenteral nutrition (PN) exposure in hematopoietic stem cell transplant (HSCT) recipients. JPEN J Parenter Enteral Nutr. 2006;30:345–350. doi: 10.1177/0148607106030004345. [DOI] [PubMed] [Google Scholar]