Abstract
Objective
To evaluate the usefulness of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in the management of cervical dystonia (CD) with botulinum toxin type A (BoNT-A) injection.
Method
Thirty two subjects with CD were included. A BoNT-A injection was provided either by clinically targeting method (group 1) or by 18F-FDG PET/CT-assisted, clinically targeting method (group 2). In group 2, selection of target muscles and dosage of BoNT-A were determined according to the increased 18F-FDG uptake, in addition to physical examination and functional anatomy. The outcomes of BoNT-A injection was compared between the two groups, in terms of the number of subjects who had reinjection before and after 6 months, the number of reinjections, the interval of reinjections, the duration to the minimal Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS), the number of adverse events, the reduction rate of TWSTRS at 1-3 months and 3-6 months after injection, and the probability of reinjection-free living.
Results
The number of subjects who had reinjection within 6 months was significantly lower in group 2 than in group 1 (10 in group 1 vs. 3 in group 2). The reduction rate of TWSTRS after 3-6 months (37.8±15.7% of group 1 vs. 63.3±28.0% of group 2) and the probability of reinjection-free living were significantly higher in group 2 than in group 1.
Conclusion
These findings suggest that 18F-FDG PET/CT study could be useful in management of CD in terms of the identification of dystonic muscles if there is an increase in the 18F-FDG uptake in the cervical muscle of the images.
Keywords: Cervical dystonia, 18F-Fluorodeoxyglucose, Positron emission tomography, Botulinum toxin
INTRODUCTION
Cervical dystonia (CD) is the most common form of adult focal dystonia. The symptoms of CD include abnormal posture of the head and neck, neck pain and tremor, which are caused by involuntary and repetitive contraction of neck muscles.1-3 The prevalence of CD has been reported as 0.006-0.39% overseas,4-6 but that of South Korea has not yet been reported. A botulinum toxin (BoNT) injection has been known as the most effective treatment for CD.3,7 Other than BoNT injection, oral medication, including an anticholinergic drug, physiotherapy and relaxation techniques are used adjunctively for CD treatment.3,7,8 Deep brain stimulation can be considered if these methods are not effective.3,7,8 In 1986, Tsui et al.9 reported successful cases after the injection of BoNT into the cervical muscles to treat CD. The injection has become an innovative method for the treatment of CD. Thereafter, the United States Food and Drug Administration (US FDA) approved the use of BoNT for the treatment of CD in 1989. Kim et al.10 reported the efficacy of BoNT injection in the treatment of 2 Korean subjects with CD in 1991, and Lee et al.11 reported the clinical effectiveness after the injection of 26 subjects in 1997. Between 70-80% of subjects with CD have expressed a satisfactory therapeutic effect after BoNT injection, and the effect has been reported to continue for 3-6 months.8,12,13
To maximize the efficacy of BoNT injection in the treatment of CD, it is important to identify dystonic muscles and to inject a sufficient amount of BoNT into those muscles.3 Identification of dystonic muscles has been done by a clinical decision based on the functional anatomy and physical examination, such as inspection and palpation. Further, the dystonic activity of the identified muscles is verified by electromyography (EMG). The muscles that have been selected through these processes become the target muscles for BoNT injection.14-16
Based on the fact that contraction of skeletal muscle leads to an increase in intramuscular glucose metabolism, Sung et al.17 (2007) injected 18F-fluorodeoxyglucose (18F-FDG) to the subjects with CD and confirmed that dystonic muscles led to an increase in glucose metabolism and 18F-FDG uptake through 18F-FDG positron emission tomography/computed tomography (18F-FDG PET/CT). There were also 2 studies reporting that the deep cervical muscles, such as longus colli and obliquus capitis inferior, into which BoNT have not been frequently injected, were found to be involved in CD through 18F-FDG PET/CT. Hence, these deep cervical muscles were included into target muscles for BoNT injection and improvement of the symptoms were reported in those studies.17,18 In 2010, Choi et al.19 found that there was increased 18F-FDG uptake in the deep cervical muscles, such as semispinalis capitis, longissimus capitis and multifidi through 18F-FDG PET/CT of a subject with CD. Right torticollis of that subject had continued even after both BoNT injection and selective denervation of the branch of left accessory nerve to sternocleidomastoid (SCM). They included those 3 muscles for BoNT injection and the symptom had improved. From the result, they reported that 18F-FDG PET/CT made more accurate selection of the dystonic muscles and improved the therapeutic outcome of BoNT injection.
Therefore, it is postulated that additional use of 18F-FDG PET/CT, along with a clinical decision for the selection of target muscles, could result in better therapeutic outcome of BoNT injection for CD. However, there has not been relevant research yet, to the best of our literature review. Therefore, the purpose of this research is to compare the outcome of botulinum toxin type A (BoNT-A) injection for CD between one group whose dystonic muscles were selected based on 18F-FDG PET/CT along with a clinical decision and the other group where dystonic muscles were selected only by a clinical decision.
MATERIALS AND METHODS
This study is a retrospective study that analyzed the medical records of the subjects with CD, which had BoNT-A injection at the Center for Torticollis in Ajou University Medical Center from November 2009 to November 2011. This research had been approved by the Institutional Review Board at Ajou University Medical Center.
Subjects and acquisition of clinical data
CD was diagnosed if subjects had abnormal posture of the head and neck caused by involuntary contraction, which was concurrent with morning benefit, sensory trick or aggravation of symptoms by a physical or emotional stress.
The subjects were included when they met all of the following criteria: 1) Those who were 18 years old or older; 2) those who had a BoNT-A injection at the Center for Torticollis from November 2009 to November 2011; 3) those who had no previous BoNT injection or the ones whose latest botulinum toxin injection were done at least 4 months or more ago; 4) those with a Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) measured before and after the injection; and 5) those with 20 or more of the initial total TWSTRS score. We excluded subjects who had one or more of the following criteria: 1) Those who had a surgical history, such as myotomy or denervation for CD; 2) those who were suspicious of neutralizing antibody against BoNT-A as a result of the previous injections; 3) those who had cervical muscle contracture or had spondyloarthropathy that could affect the cervical range of motion; 4) those who had brain lesions demonstrated in magnetic resonance imaging (MRI) or CT; 5) those who had generalized dystonia; and 6) those who did not have cervical muscles with increased 18F-FDG uptake on 18F-FDG PET/CT.
BoNT-A injections were done only based on a clinical decision from November 2009 to December 2010, and these were called as the clinically targeting group (group 1). BoNT-A injections, based on both clinical decision and 18F-FDG PET/CT, were done from December 2010 to November 2011, and these were defined as 18F-FDG PET/CT-assisted, clinically targeting group (group 2) (Fig. 1).
Fig. 1.
The picture showing the clinically targeting group or 18F-FDG PET/CT-assisted, clinically targeting group in chronologic order.
Age, gender, duration of CD and the number of the subjects who had previous BoNT injections were collected. The severity of CD was measured before and after BoNT-A injection, using TWSTRS, which had 3 subscales, such as the severity scale (maximum value 35), disability scale (maximum value 30) and pain scale (maximum value 20).20 The dosage of BoNT-A, duration of follow-up, the number of visits, and visiting interval were reviewed, as well as the number of subjects who had reinjections, the number of reinjections for the subjects who had reinjections, the interval of reinjections for the subjects who had reinjections, the duration to the minimal TWSTRS, adverse events after BoNT-A injection and the probability of reinjection-free living.
The reduction rate of TWSTRS at 1-3 months or 3-6 months was calculated as follows : Reduction rate=(Baseline score-The follow-up score at 1-3 months or 3-6 months)/Baseline score×100.
The duration to the minimal TWSTRS was defined as the duration from the initial BoNT-A injection to the minimal TWSTRS. The probability of reinjection-free living was defined as the probability of living without reinjection.
18F-FDG PET/CT study and analysis
Group 2 had fasted more than 4 hours before taking 18F-FDG PET/CT and had 370 MBq of 18F-FDG intravenous injection. The images were obtained by a Discovery ST Scanner (GE Healthcare, Milwaukee, USA), an hour after the injection. The CT images (120 kV, 60 mA, 7.5 mm per rotation) were obtained for attenuation-correction from head to chest and the subjects were fixed not to move to obtain an emission scan within the same range with a CT image every 3 minutes per frame. The reorganization of PET data and the attenuation-correction by utilizing CT data were performed. Reorganized PET images, CT images for attenuation-correction and fusion images of PET and CT images were utilized for analysis. The standardized uptake value (SUV) was obtained by setting the globular-shaped volume of interest (VOI, 1.51 cm3) on the uptake part of each muscle, where SUV means the amount of radioactivity released per 1g of tissue divided by the amount of radioactivity administered per 1 kg of body weight. The muscles with increased 18F-FDG uptake and their maximal SUV were identified by the second author (a nuclear medicine specialist) for group 2.
Selection methods of target muscles for BoNT-A injection
Physical examinations were carried out with the subjects sitting in a chair. Abnormal posture of the head and neck was analyzed based on the following definition. Torticollis was defined as when the head was turned, which means that the chin is rotated to the right or left side on the horizontal plane. Laterocollis was defined as when the head was tilted, which means that the face is tilted to the right or left side on the coronal plane. Therefore, the ear comes closer to the ipsilateral shoulder on the coronal plane in laterocollis. Anterocollis was defined as when the head is tilted forward and the chin is lowered on the sagittal plane. Retrocollis was defined as when the head is tilted backward and the chin is elevated on the sagittal plane.21
The dystonic muscles were selected for BoNT-A injection through functional anatomy based on the subjects' abnormal posture of head and neck, as well as the inspection and palpation in group 1. From the perspective of functional anatomy of the head and neck, the ipsilateral splenius capitis, ipsilateral semispinalis capitis and contralateral SCM were considered as the target muscles if torticollis was the main symptom, while ipsilateral SCM, splenius capitis, semispinalis capitis, scalene complex and levator scapulae were considered as the target muscles if laterocollis was the main symptom. Bilateral SCM and bilateral scalene complex were selected for anterocollis, while the bilateral splenius capitis, semispinalis capitis and upper trapezius were selected for retrocollis. If there was shoulder elevation for unilateral shoulder, ipsilateral levator scapulae and upper trapezius were considered as the target muscles.22,23 EMG was done for all candidate muscles based on the above analysis. BoNT-A was injected only to the muscles where the dystonic activity was ascertained on EMG. If the subject complained of discomfort or pain over the deep cervical muscles, such as obliquus capitis inferior and rectus capitis posterior major, the BoNT-A was injected into the muscles after the dystonic activity was ascertained by EMG. In group 2, along with the above decision of the dystonic muscles, the cervical muscles with increased 18F-FDG uptake were additionally injected.
Methods of BoNT-A injection
Informed consents were taken for all subjects. Botox® (Allergan, Inc., Irvine, USA) or Dysport® (Ipsen Biopharm, Ltd., Wrexham, UK) was used. Medelec Sapphire 4ME (Vickers Healthcare Co., Woking, UK) and TECA Disposable MyoJect LuerLock needle (CareFusion, Middleton, USA) were used for the EMG-guided BoNT-A injection. The injections were done with the subjects sitting in a chair. A needle electrode was inserted into the target muscles to ascertain the dystonic activity. Dystonic activity was ascertained when there was significant increase in the interference pattern on EMG due to abnormal movement of the head and neck. The dosage of BoNT-A was determined according to the body weight and the severity of CD. In group 2, high dose of BoNT-A was injected into the muscles with increased 18F-FDG uptake in the 18F-FDG PET/CT.
Statistical analysis
IBM SPSS Statistics version 20.0 (IBM, Co., Armonk, USA) was employed for statistical analysis. The Mann-Whitney U-test and Fisher's exact test were used to compare the characteristics and outcomes of BoNT-A injection between group 1 and 2. The Kaplan-Meier plot and Log-rank test were used to ascertain the changes for the probability of reinjection-free living, according to the duration of the follow-up in both groups. A Cox regression was used for a multivariate analysis of the probability of rejection-free living. Statistical significance was set as a p-value of <0.05.
RESULTS
The characteristics of the subjects
Sixteen subjects were recruited in group 1 and 2, respectively. Thirty-one out of 32 subjects were diagnosed with idiopathic CD. The remaining 1 subject was diagnosed as tardive dystonia because the subject had been diagnosed with schizophrenia, taking resperidone for 5 years. The characteristics of the subjects were presented in Table 1. There were no significant differences in age, gender, duration of CD, the number of the subjects who had previous BoNT injection, baseline TWSTRS, duration of follow-up, number of visits and visiting interval between the groups (Table 1). Seven subjects in group 1 and 2 subjects in group 2 had Botox® injection. The remaining subjects in both groups had Dysport® injection. There was no significant difference in the dosage of BoNT-A between group 1 and group 2 (p=0.813) (Table 1).
Table 1.
Characteristics of Subjects
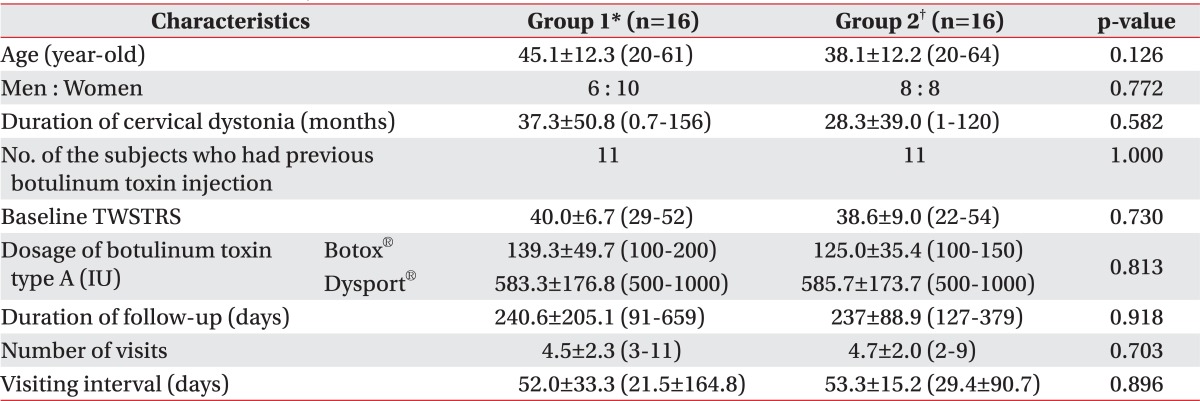
All values are expressed as means±standard deviation or numbers
*Group 1: Clinically targeting group, †Group 2: 18F-FDG PET/CT-assisted, clinically targeting group
Findings of 18F-FDG PET/CT imaging in group 2
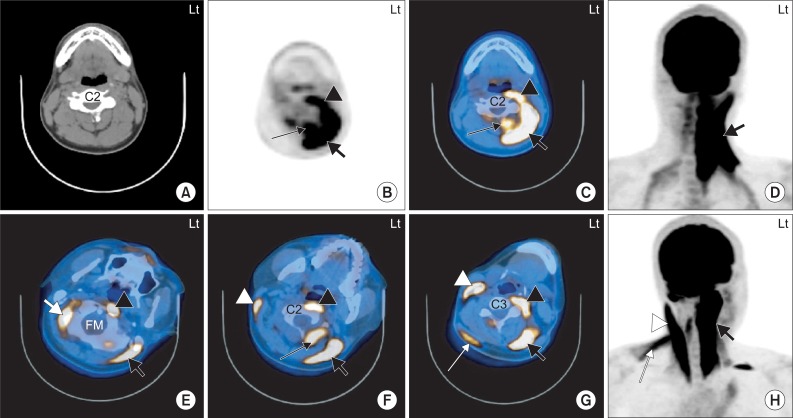
Supplement Table S1 shows the muscles with increased 18F-FDG uptake in group 2, along with their symptoms. Fig. 2 shows the 18F-FDG PET/CT images of 2 independent subjects with main symptoms of left torticollis. One subject shows that there was increased 18F-FDG uptake in the left splenius capitis, semispinalis capitis, longus capitis and longus colli, and the other subject shows that there was increased 18F-FDG uptake in the right SCM, obliquus capitis superior, upper trapezius, left longus capitis, splenius capitis and rectus capitis posterior major (Fig. 2). According to Supplement Table S1 and Fig. 2 we found that there was increased 18F-FDG uptake in different muscles although the subjects had the same abnormal posture of head and neck.
Supplement Table S1.
The Muscles with Increased 18F-FDG Uptake in Group 2*
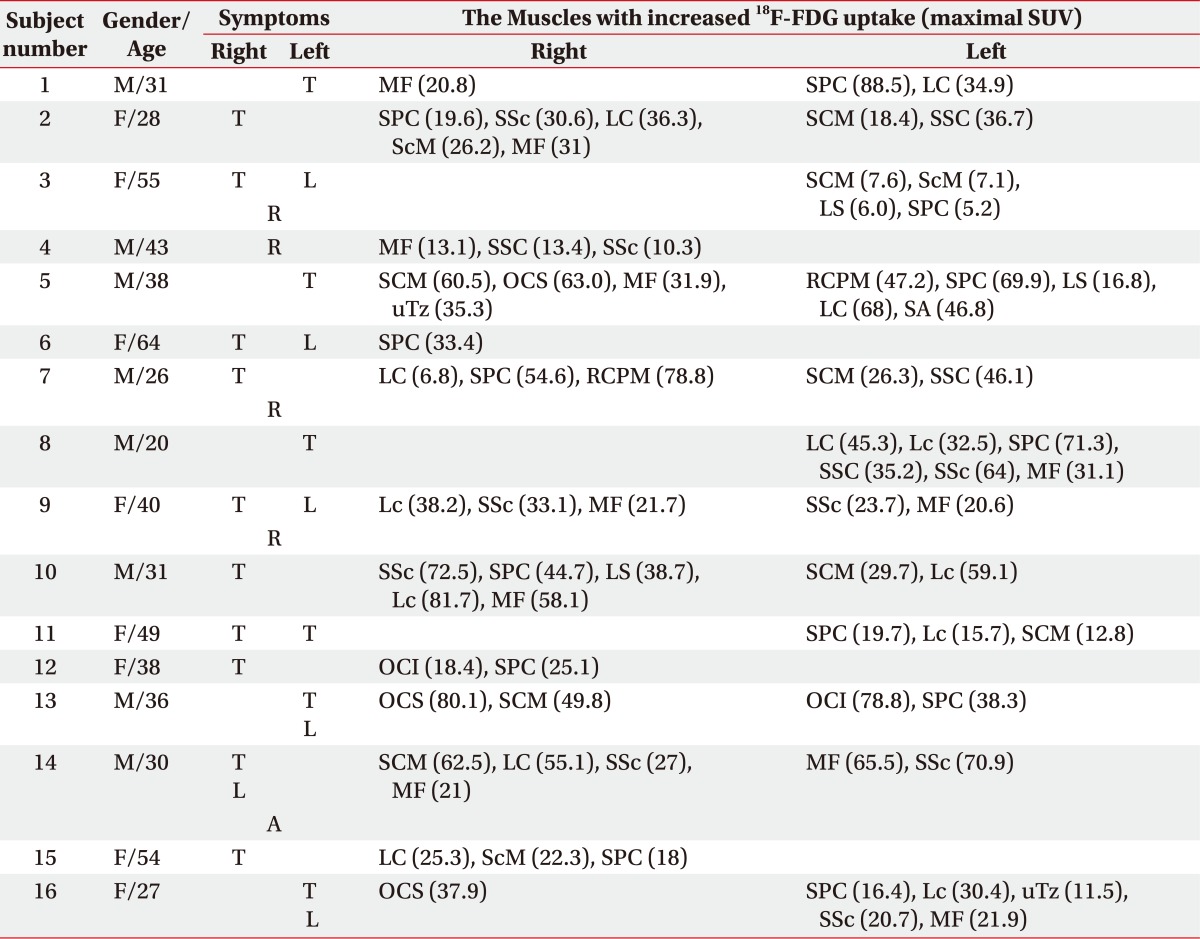
*Group 2: 18F-FDG PET/CT-assisted, clinically targeting group
SUV: Standardized uptake value, T: Toricollis, L: Laterocollis, R: Retrocollis, A: Anterocollis, MF: Multifidi, SPC: Splenius capitis, LC: Longus capitis, SSc: Semispinalis cervicis, ScM: Scalenus medius, SCM: Sternocleidomastoid, SSC: Semispinalis capitis, LS: Levator scapulae, OCS: Obliquus capitis superior, uTz: Upper trapezius, RCPM: Rectus capitis posterior major, SA: Serratus anterior, Lc: Longus colli, OCI: Obliquus capitis inferior
Fig. 2.
The 18F-FDG PET/CT images of 2 independent subjects with left torticollis. 1. A 20-year-old man with cervical dystonia whose main symptom was left torticollis (A-D). The 18F-FDG uptake is increased at the anterior and posterior neck muscles on the left side at the level of the C2 vertebral body. The hot uptake of 18F-FDG was seen in the left LC/Lc (black arrow head), left SPC (thick black arrow) and left SSC (thin black arrow) (B-D). 2. A 38-year-old man with cervical dystonia whose main symptom was left torticollis (E-H). The 18F-FDG uptake is increased at the anterior and posterior neck muscles at the levels of foramen magnum (FM; E), C2 vertebral body (F) and C3 vertebral body (G). The hot uptake of 18F-FDG was seen in the right OCS (thick white arrow), left LC (black arrow head) and left SPC (thick black arrow) (E). Increased 18F-FDG uptake was also seen in the right SCM (white arrow head), left LC (black arrow head), left SPC (thick black arrow) and left RCPM (thin black arrow) (F). The hot uptake of 18F-FDG was showed in the right SCM (white arrow head), right uTz (thin white arrow), left LC (black arrow head) and left SPC (thick black arrow) (G). LC: Longus capitis, Lc: Longus colli, SPC: Splenius capitis, SSC: Semispinalis capitis, OCS: Obliquus capitis superior, SCM: Sternocleidomastoid, RCPM: Rectus capitis posterior major, uTz: Upper trapezius.
Comparison of the outcomes of BoNT-A injection between group 1 and 2
The injected muscles were presented in Table 2 according to the two groups. While 10 out of 16 subjects in group 1 had reinjection within 6 months since the initial injection (62.5%), and 3 out of 16 subjects (18.8%) in group 2 had reinjection. This shows significant difference in the number of subjects who received reinjection within 6 months since the initial injection between the two groups (p=0.029) (Table 3). Additional 3 independent subjects were given a reinjection after 6 months in group 1. Therefore, a total of 13 subjects were given a reinjection in group 1. There were no significant differences in the number and interval of reinjection for the subjects who had reinjection, the duration of minimal TWSTRS and the number of adverse events between the two groups (Table 3). Regarding the adverse events, there were 3 cases of neck muscle weakness and 1 case of dysphagia in group 1. Group 2 had 1 case of dizziness and 1 case of dysphagia. However, all the symptoms disappeared over time.
Table 2.
The Injected Muscles according to the Groups
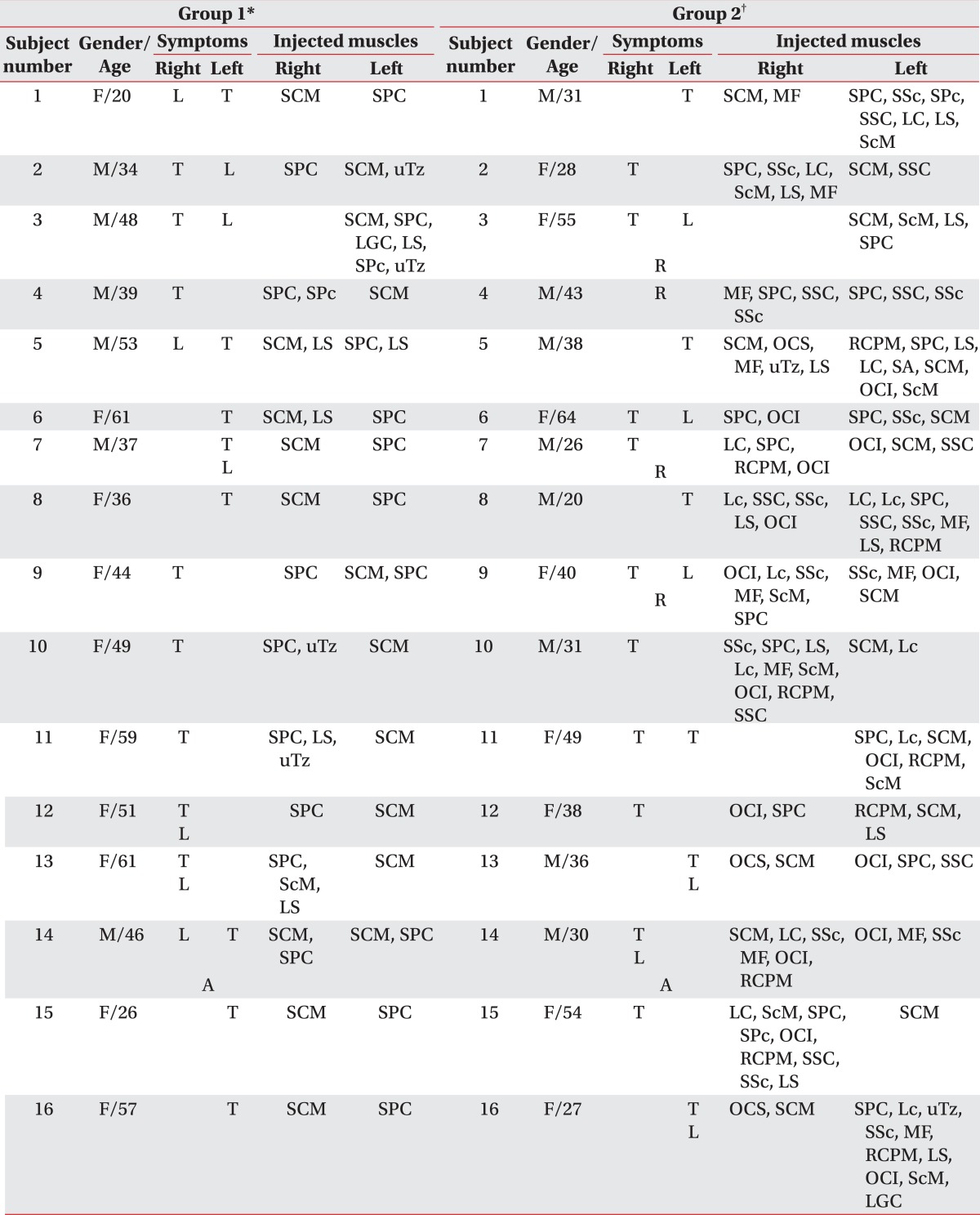
T: Toricollis, L: Laterocollis, A: Anterocollis, R: Retrocollis, SCM: Sternocleidomastoid, SPC: Splenius capitis, MF: Multifidi, SSc: Semispinalis cervicis, SPc: Splenius cervicis, SSC: Semispinalis capitis, LC: Longus capitis, LS: Levator scapulae, ScM: Scalenus medius, uTz: Upper trapezius, LGC: Longissimus capitis, OCS: Obliquus capitis superior, RCPM: Rectus capitis posterior major, SA: Serratus anterior, OCI: Obliquus capitis inferior, Lc: Longus colli
*Group 1: Clinically targeting group, †Group 2: 18F-FDG PET/CT-assisted, clinically targeting group
Table 3.
Comparison of the Outcomes of BoNT-A Injection between Group 1 and 2
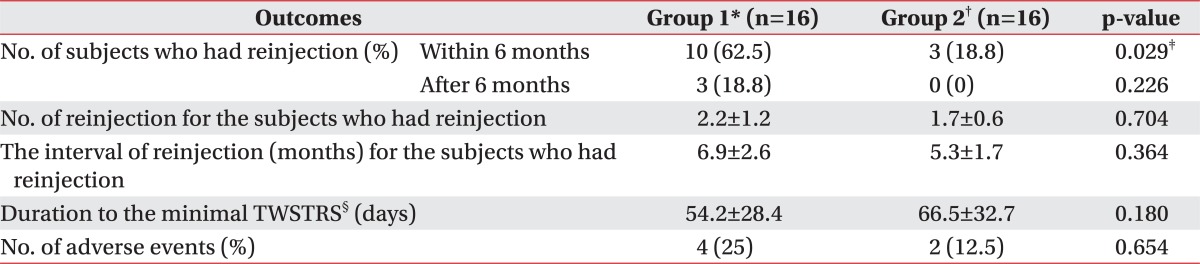
All values are expressed as means±standard deviation or numbers (%)
BoNT-A: Botulinum toxin type A, TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale
*Group 1: Clinically targeting group, †Group 2: 18F-FDG PET/CT-assisted, clinically targeting group, ‡p<0.05, §The duration from the initial BoNT-A injection to the minimal TWSTRS
Comparison of reduction rate of TWSTRS ac cording to groups in reference to the duration since the initial BoNT-A Injection
There was no significant difference in the reduction rate of the TWSTRS at 1-3 months after the initial BoNT-A injection between the groups (Table 4). However, the reduction rate of both the total and disability score of TWSTRS at 3-6 months since the initial injection was significantly higher in group 2 than those of group 1 (Table 4).
Table 4.
Comparison of Reduction Rate of TWSTRS according to Groups in Reference to Duration since the Initial BoNT-A Injection
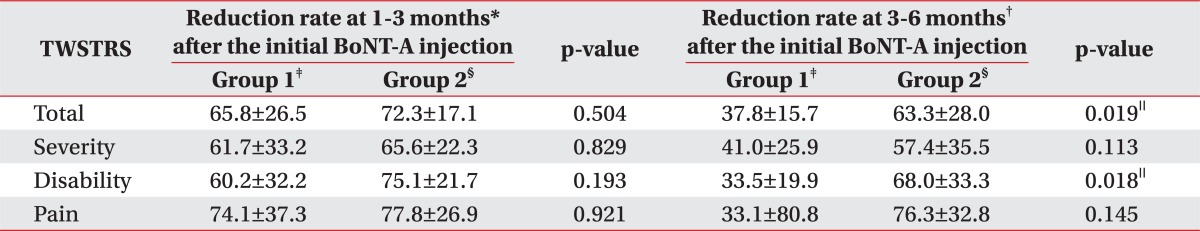
All values are expressed as means±standard deviation
TWSTRS: Toronto Western Spasmodic Torticollis Rating Scale, BoNT-A: Botulinum toxin type A
*Reduction rate at 1-3 months: Reduction rate of the TWSTRS score=(Baseline score-The follow-up score at 1-3 months)/Baseline score×100, †Reduction rate at 3-6 months: Reduction rate of the TWSTRS score=(Baseline score-The follow-up score at 3-6 months)/Baseline score×100, ‡Group 1: Clinically targeting group, §Group 2: 18F-FDG PET/CT-assisted, clinically targeting group, ∥p<0.05
The probability of reinjection-free living in both groups
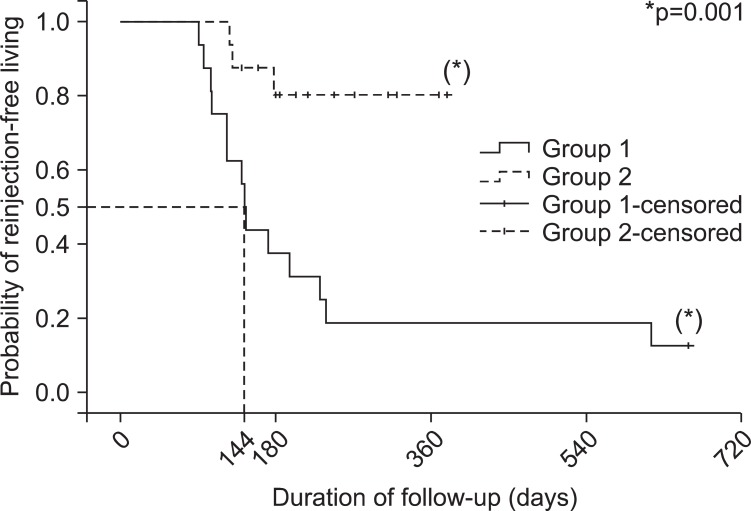
Fig. 3 shows that the probability of reinjection-free living was significantly higher in group 2 than in group 1 (p=0.001 by Log-rank test). Fifty percent of the probability of reinjection free living had come on 144 days after the initial BoNT-A injection in group 1 (Fig. 3). Meanwhile, the probability of reinjection-free living of group 2 still remained at 80%. Therefore, the probability of reinjection-free living of group 2 did not come to 50%. On 1 year of follow up, the probability of group 2 was 80%, which was 4 times higher than that of group 1, where the probability was 19% (Fig. 3).
Fig. 3.
The Kaplan-Meier plot showing the probability of reinjection-free living after BoNT-A injection in clinically targeting group (group 1) and the 18F-FDG PET/CT-assisted, clinically targeting group (group 2). The probability of reinjection-free living is 0.8 in group 2, while the rate is 0.19 in group 1 when the duration of follow up is one year (365 days). The duration of follow-up for group 1 was 144 days (95% confidence interval: 134.2-153.8) when the reinjection-free living rate had come to 50%.
A multivariate analysis for the probability of reinjection-free living was performed for two groups, using the Cox regression, which shows that the difference between the two groups significantly affected the probability of reinjection-free living (p=0.03). The hazard ratio of reinjection in group 2 was 0.231 (confidence interval: 0.062-0.864), which means that the protective effect against reinjection was 4.3 times higher in group 2 than in group 1.
DISCUSSION
The muscles with increased 18F-FDG uptake were selected as the target muscles and were given a BoNT-A injection, along with a clinically targeting method in group 2. There was a significant decrease in the number of subjects who had reinjection within 6 months of group 2 compared to that of group 1. This result could be interpreted as the result from the enhanced accuracy of muscle selection because clinically targeting and 18F-FDG PET/CT supplemented each other for the selection of target muscles.
Thirteen out of the total 16 subjects in group 2 maintained the symptom improvement even after 6 months, which had been known as the maximal duration of the BoNT response. To explain the mechanism of the effect, Giladi, in 1997, argued that BoNT blocks the α and γ motor neuron, which are connected to dystonic muscles, and this leads to changes in the afferent input, which is connected from cervical muscle spindle to the central nervous system. The afferent pathway, which has been changed by BoNT for a long time, plays a role as continuous sensory trick and finally reorganization occurs in the basal ganglia then the pathological process of CD is changed into a normal state maintaining symptom improvement.24
In group 2, in contrast to group 1, not only first considered the cervical muscles by a clinical decision but the muscles with increased 18F-FDG uptake in 18F-FDG PET/CT, were also selected as the target muscles. The muscles with increased 18F-FDG uptake were given a relatively high amount of BoNT-A injection. Therefore, the results of this research could be interpreted that the muscles, which were not selected based on clinical decision among the muscles with increased 18F-FDG uptake, could play a main role of aggravating the symptoms of CD.
The reduction rate of the follow-up TWSTRS at 1-3 months did not reveal significant differences between the two groups. However, the reduction rate in group 2 was higher than that in group 1 for the disability score by 14.9% (75.1±21.7% of group 2 vs 60.2±32.2% of group 1; Table 4). If such difference is to continue in further comparative researches with more subjects, there could be a significant difference in the reduction rate of the follow-up TWSTRS at 1-3 months. Furthermore, there were significant increases in the reduction rate of the total scores and the disability score for group 2, compared to that of group 1, after 3-6 months (Table 4). These results implies that the TWSTRS score of group 1 has increased during the period due to recurrence of CD and back up the higher number of subjects who had reinjection within 6 months in group 1.
This study has some limitations. The sample size was small. The two groups consisted of 32 subjects, of which, each group included 16 subjects. In addition, there could have been a selection bias as this research was retrospective. All the subjects who participated in this research were from one clinical facility. Further, 2 products, including Botox® or Dysport® were used as BoNT-A.
There were some cases with duration of follow-up of less than 1 year and interval censoring, which occurs after 6 months in group 2. Accordingly, a comparison which had been carried out within 180 days could have been significant, but there needs to be follow-up to ascertain the comparative results after 180 days. Furthermore, the two groups were set at different times and there had been efforts to put forth to find target muscles after December 2010, as more knowledge on CD has thereafter accumulated. Therefore, the other muscles without increased 18F-FDG uptake had been found to be dystonic muscles by the use of a physical examination and EMG in group 2, hence BoNT-A had been injected into more target muscles of group 2, compared to those of group 1. In conclusion, the time differences in the setting of the two groups could have considerably affected the results.
In addition, 6 subjects among those who had 18F-FDG PET/CT at our center were excluded from this study, as they did not have cervical muscles with increased 18F-FDG uptake. If the 6 subjects had been included in this research, the results could have been affected. Six subjects were 2 men and 4 women, who consisted of 1 subject in their 20 s, 2 in their 30 s, 1 in their 40 s and 2 in their 50 s. The duration of CD was 46.4±38.1 (range: 12-120) months and 5 out of 6 subjects appeared to have a history of previous BoNT injections before the 18F-FDG PET/CT. It was hard to check if the other one subject had experienced a previous BoNT injection. Two out of 6 subjects had taken trihexyphenidyl and clonazepam. Past medical history was not found for all 6 subjects. According to these results, it is possible that the 18F-FDG uptake could have been inhibited by the effect of the previous BoNT injections in these cases. The Initial TWSTRS was 30.7±5.3 (range: 25-39), which tended to be lower than the TWSTRS of subjects for this research. Therefore, further studies are needed on the association between TWSTRS scores and the increase in 18F-FDG uptake.
CONCLUSION
This seems to be the first report that compared the outcome of BoNT-A injection between 18F-FDG PET/CT-assisted, clinically targeting group and clinically targeting group. The number of subjects who had reinjection within 6 months was significantly lower in group 2 than group 1. The reduction rate of TWSTRS after 3-6 months and the probability of reinjection-free living were significantly higher in group 2 than in group 1. These findings suggest that 18F-FDG PET/CT study could be useful in management of CD in terms of identification of dystonic muscles if there is an increase in the 18F-FDG uptake in cervical muscle of the images. There needs to be prospective studies to compare the results of BoNT-A injection for the two groups with more subjects, and over a longer follow up.
References
- 1.Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. 1991;41:1088–1091. doi: 10.1212/wnl.41.7.1088. [DOI] [PubMed] [Google Scholar]
- 2.Dauer WT, Burke RE, Greene P, Fahn S. Current concepts on the clinical features, aetiology and management of idiopathic cervical dystonia. Brain. 1998;121:547–560. doi: 10.1093/brain/121.4.547. [DOI] [PubMed] [Google Scholar]
- 3.Jankovic J. Treatment of cervical dystonia with botulinum toxin. Mov Disord. 2004;19(Suppl 8):S109–S115. doi: 10.1002/mds.20024. [DOI] [PubMed] [Google Scholar]
- 4.Jankovic J, Tsui J, Bergeron C. Prevalence of cervical dystonia and spasmodic torticollis in the United States general population. Parkinsonism Relat Disord. 2007;13:411–416. doi: 10.1016/j.parkreldis.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Nutt JG, Muenter MD, Aronson A, Kurland LT, Melton LJ., 3rd Epidemiology of focal and generalized dystonia in Rochester, Minnesota. Mov Disord. 1988;3:188–194. doi: 10.1002/mds.870030302. [DOI] [PubMed] [Google Scholar]
- 6.Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group. A prevalence study of primary dystonia in eight European countries. J Neurol. 2000;247:787–792. doi: 10.1007/s004150070094. [DOI] [PubMed] [Google Scholar]
- 7.Jankovic J. Treatment of dystonia. Lancet Neurol. 2006;5:864–872. doi: 10.1016/S1474-4422(06)70574-9. [DOI] [PubMed] [Google Scholar]
- 8.Walker FO. Botulinum toxin therapy for cervical dystonia. Phys Med Rehabil Clin N Am. 2003;14:749–766. doi: 10.1016/s1047-9651(03)00045-7. [DOI] [PubMed] [Google Scholar]
- 9.Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB. Double-blind study of botulinum toxin in spasmodic torticollis. Lancet. 1986;2:245–247. doi: 10.1016/s0140-6736(86)92070-2. [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Hwang YM, Kim KK, Kang JK, Jin YH. Treatment of spasmodic torticollis with botulinum toxin injection. J Korean Med Assoc. 1991;34:329–334. [Google Scholar]
- 11.Lee MS, Sohn YH, Kim JS. Botulinum toxin treatment in subjects with spasmodic torticollis. J Korean Neurol Assoc. 1997;15:790–802. [Google Scholar]
- 12.Brashear A, Watts MW, Marchetti A, Magar R, Lau H, Wang L. Duration of effect of botulinum toxin type A in adult subjects with cervical dystonia: a retrospective chart review. Clin Ther. 2000;22:1516–1524. doi: 10.1016/s0149-2918(00)83049-0. [DOI] [PubMed] [Google Scholar]
- 13.Truong D, Duane DD, Jankovic J, Singer C, Seeberger LC, Comella CL, Lew MF, Rodnitzky RL, Danisi FO, Sutton JP, et al. Efficacy and safety of botulinum type A toxin (Dysport) in cervical dystonia: results of the first US randomized, double blind, placebo controlled study. Mov Disord. 2005;20:783–791. doi: 10.1002/mds.20403. [DOI] [PubMed] [Google Scholar]
- 14.Comella CL, Buchman AS, Tanner CM, Brown-Toms NC, Goetz CG. Botulinum toxin injection for spasmodic torticollis: increased magnitude of benefit with electromyographic assistance. Neurology. 1992;42:878–882. doi: 10.1212/wnl.42.4.878. [DOI] [PubMed] [Google Scholar]
- 15.Dressler D. Electromyographic evaluation of cervical dystonia for planning of botulinum toxin therapy. Eur J Neurol. 2000;7:713–718. doi: 10.1046/j.1468-1331.2000.00161.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Gerpen JA, Matsumoto JY, Ahlskog JE, Maraganore DM, McManis PG. Utility of an EMG mapping study in treating cervical dystonia. Muscle Nerve. 2000;23:1752–1756. doi: 10.1002/1097-4598(200011)23:11<1752::aid-mus12>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Sung DH, Choi JY, Kim DH, Kim ES, Son YI, Cho YS, Lee SJ, Lee KH, Kim BT. Localization of dystonic muscles with 18F-FDG PET/CT in idiopathic cervical dystonia. J Nucl Med. 2007;48:1790–1795. doi: 10.2967/jnumed.107.044024. [DOI] [PubMed] [Google Scholar]
- 18.Lee IH, Yoon YC, Sung DH, Kwon JW, Jung JY. Initial experience with imaging-guided intramuscular botulinum toxin injection in subjects with idiopathic cervical dystonia. AJR Am J Roentgenol. 2009;192:996–1001. doi: 10.2214/AJR.08.1535. [DOI] [PubMed] [Google Scholar]
- 19.Choi KP, Chung CW, Sung DH. Verification of the dystonic muscle using 18F-fluorodeoxyglucose positron emission tomography in a subject with cervical dystonia: a case report. J Korean Acad Rehabil Med. 2010;34:91–95. [Google Scholar]
- 20.Consky E, Basinski A, Belle L, Ranawaya R, Lang A. The Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS): assessment of validity and inter-rater reliability. Neurology. 1990;40(Suppl 1):S445. [Google Scholar]
- 21.Yim SY, Lee IY, Park MC, Kim JH. Differential diagnosis and management of abnormal posture of head and neck. J Korean Med Assoc. 2009;52:705–718. [Google Scholar]
- 22.Brashear A. Botulinum toxin type A in the treatment of patients with cervical dystonia. Biologics. 2009;3:1–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Ranoux D. Cervical dystonia. In: Ranoux D, Gury C, editors. Practical handbook on botulinum toxin. Marseille: Solal; 2007. pp. 35–50. [Google Scholar]
- 24.Giladi N. The mechanism of action of botulinum toxin type A in focal dystonia is most probably through its dual effect on efferent (motor) and afferent pathways at the injected site. J Neurol Sci. 1997;152:132–135. doi: 10.1016/s0022-510x(97)00151-2. [DOI] [PubMed] [Google Scholar]