Abstract
Mitogen-activated protein kinases (MAPKs) play a central role in cell signaling. Extracellular signal-regulated kinase (ERK) is a prototypic subclass of MAPKs and is densely expressed in postmitotic neurons of adult mammalian brains. Active ERK translocates into the nucleus to regulate gene expression. Additionally, ERK is visualized in neuronal peripheries, such as distal synaptic structures. While nuclear ERK is a known sensitive target of psychostimulants, little is known about the responsiveness of synaptic ERK to stimulants. In this study, we focused on ERK at synaptic versus extrasynaptic sites and investigated its responses to the psychostimulant amphetamine in the adult rat striatum and medial prefrontal cortex (mPFC) in vivo. We used a pre-validated biochemical fractionation procedure to isolate synapse- and extrasynapse-enriched membranes. We found that two common ERK isoforms (ERK1 and ERK2) were concentrated more in extrasynaptic fractions than in synaptic fractions in striatal and cortical neurons under normal conditions. At synaptic sites, ERK2 was noticeably more abundant than ERK1. Acute injection of amphetamine induced an increase in ERK2 phosphorylation in the synaptic fraction of striatal neurons, while the drug did not alter extrasynaptic ERK2 phosphorylation. Similar results were observed in the mPFC. In both synaptic and extrasynaptic compartments, total ERK1/2 proteins remained stable in response to amphetamine. Our data establish the subsynaptic distribution pattern of MAPK/ERK in striatal and cortical neurons. Moreover, the synaptic pool of ERK2 in these neurons can be selectively activated by amphetamine.
Keywords: Striatum, basal ganglia, dopamine, stimulant, postsynaptic density, extrasynaptic membrane, addiction
1. Introduction
Mitogen-activated protein kinases (MAPKs) are a family of serine/threonine protein kinases. These kinases are densely expressed in postmitotic neurons of adult mammalian brains and play a central role in cell signaling (Nozaki et al., 2001). Extracellular signal-regulated kinase (ERK) is the first subfamily of MAPKs (Pearson et al., 2001; Volmat and Pouyssegur, 2001). Like all other MAPKs, ERK is activated through a module cascade involving initial activation of small GTPases (Ras or Rac) and subsequent three-tiered protein kinase systems. As a kinase highly sensitive to diverse extracellular stimuli, ERK is vigorously involved in activity-dependent signaling transduction and synaptic plasticity in the central nervous system (reviewed in Sweatt, 2004; Thomas and Huganir, 2004; Wang et al., 2007).
As a prototype of MAPKs, ERK has been extensively investigated in its distribution and function in neurons. Traditionally, ERK, once activated, translocates into the nucleus, where it activates a discrete set of transcription factors and thus regulates gene expression (Treisman, 1996). While this is true for ERK in the cell body, a sub-pool of ERK also notably resides in neuronal peripheries, including postsynaptic dendritic spines (Boggio et al., 2007; Casar et al., 2009; Ortiz et al., 1995). In the postsynaptic density (PSD), ERK2 coexisted with all MAPK cascade components (Suzuki et al., 1995; 1999). After activation, immunostaining of phosphorylated ERK (pERK) was visualized in synaptic structures, in addition to nuclear envelopes, in hippocampal and visual cortical neurons (Boggio et al., 2007; Sindreu et al., 2007). Thus, ERK is reasoned to function at both nuclear and synaptic sites.
ERK has been well documented to be a sensitive target of addictive drugs and plays a pivotal role in neural adaptations and drug addiction (Wang et al., 2007). Among addictive drugs that readily activate ERK in the reward circuit in vivo are psychostimulants (cocaine and amphetamines). Acute injection of cocaine markedly increased phosphorylation of ERK in the striatum (Jenab et al., 2005; Valjent et al., 2000; 2005; 2006; Zhang et al., 2004). Similarly, acute amphetamine (AMPH) increased ERK phosphorylation in the striatum (Choe et al., 2002; Choe and Wang, 2002; Valjent et al., 2004; 2005; 2006). The psychostimulant-induced ERK phosphorylation requires activation of dopamine D1 receptors and group I metabotropic glutamate receptors (Choe et al., 2002; Choe and Wang, 2002; Valjent et al., 2000). Together, ERK in striatal neurons is activated in response to stimulants and is believed to participate in neuroadaptations related to the long-lasting addictive properties of drugs of abuse. However, to date, the response of ERK to stimulants was primarily detected and analyzed in the nuclear compartment. Little is known about the distribution and responsivity of nonnuclear ERK, such as the ERK at synaptic sites, to stimulants.
In this study, we carried out a series of experiments in adult rats to examine the subsynaptic distribution pattern of the two common ERK isoforms (ERK1 and ERK2) in the striatum and medial prefrontal cortex (mPFC), two prime projection sites in the mesocorticolimbic dopamine system which is actively involved in mediating AMPH effects. Subsequently, we assessed the response of synaptic ERK1/2 to AMPH. Using a pre-validated subsynaptic fractionation method, synaptic and extrasynaptic ERK1/2 were isolated from the striatum and mPFC. The effect of an acute injection of AMPH on phosphorylation of ERK1/2 in those defined pools was analyzed.
2. Results
2.1. Enrichment of synaptic and extrasynatic proteins
We first evaluated the efficiency of our fractionation method in enriching synaptic and extrasynaptic proteins from the rat striatum. To this end, we separated synaptic and extrasynaptic membranes. The latter include all membranes except synaptic membranes. We then assayed the relative abundance of proteins known to be preferentially expressed in either membrane fraction. The N-methyl-D-aspartate (NMDA) glutamate receptor subunit NR2B, PSD-95, and Ca2+/calmodulin-dependent protein kinase II α/β (CaMKIIα/β) are knowingly concentrated at synaptic sites and were detected predominantly in synaptic fractions (Davies et al., 2007; Ferrario et al., 2011; Goebel-Goody et al., 2009). Consistent with this, our samples obtained from the Triton X-100-based fractionation (Fig. 1A) showed that NR2B and CaMKIIα immunoblot signals were visualized mostly in synaptic fractions, while their signals in extrasynaptic fractions were minimal (Fig. 1B). PSD-95 and CaMKIIβ were concentrated in synaptic membranes and virtually undetectable in extrasynaptic membranes (Fig. 1B). In contrast to these synaptic markers, three known extrasynaptic membrane proteins showed an opposite distribution pattern. As shown in Fig. 1C, calnexin, a Ca2+-binding protein expressed in extrasynaptic plasma membranes (Davies et al., 2007; Ferrario et al., 2011), was present in extrasynaptic fractions but was almost absent in synaptic fractions. So were the two early endosomal proteins, EEA1 (early endosome antigen 1) and Rab11, that are required for endocytosis and recycling of receptors and are enriched in extrasynaptic rather than synaptic membranes (Davies et al., 2007; Ferrario et al., 2011; Park et al., 2004; Racz et al., 2004). These results demonstrated the sufficiency of the fractionation procedure in enriching proteins from distinct subsynaptic compartments.
Figure 1. Distribution of synaptic and extrasynaptic markers in synaptic and extrasynaptic membranes.
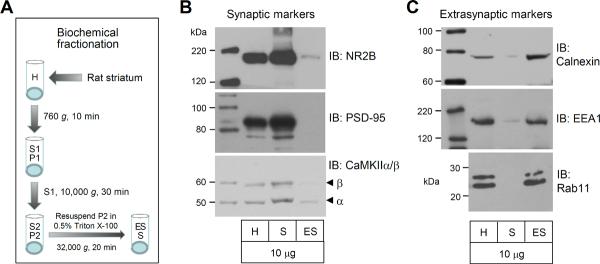
(A) Schematic illustration of Triton X-100-based fractionation procedures for enriching synaptic and extrasynaptic proteins from the rat striatum. (B) Representative immunoblots showing expression of synaptic markers (NR2B, PSD-95, and CaMKIIα/β) in synaptic and extrasynaptic membranes. (C) Representative immunoblots showing expression of extrasynaptic markers (calnexin, EEA1, and Rab11) in synaptic and extrasynaptic membranes. Note that synaptic and extrasynaptic markers are present predominantly in their respective fractions. Total proteins from homogenates (H) and enriched proteins from synaptic membranes (S) and extrasynaptic membranes (ES) were loaded at the same amount (10 μg per lane).
2.2. Synaptic and extrasynaptic distributions of ERK1/2 in the striatum
Using synaptic and extrasynaptic samples validated above, we assessed the relative abundance of pERK1/2 and ERK1/2 proteins in synaptic and extrasynaptic pools of adult rat striatal neurons. A minimal amount of pERK1 signals was seen in synaptic fractions, while pERK2 was relatively more abundant in this fraction (Fig. 2A). Both pERK1 and pERK2 were present at a higher level in extrasynaptic fractions than in synaptic fractions (Fig. 2A and 2B). Like synaptic pERK2, extrasynaptic pERK2 displayed stronger signals than pERK1, establishing a high ratio of pERK2 to pERK1 at the two sites (Fig. 2C). In immunoblot analysis of ERK1/2 expression, we found a small amount of ERK1 opposed to a higher level of ERK2 at synaptic sites (Fig. 2A and Fig. 2C). Both ERK1 and ERK2 were substantially abundant at extrasynaptic sites (Fig. 2B) and showed no obvious isoform gradient (Fig. 2C). These data demonstrate that pERK1/2 and ERK1/2 are concentrated at extrasynaptic sites. Meanwhile, a significant amount of ERK2 exists in synaptic membranes of striatal neurons.
Figure 2. Distribution of pERK1/2 and ERK1/2 in synaptic and extrasynaptic fractions of the rat striatum.
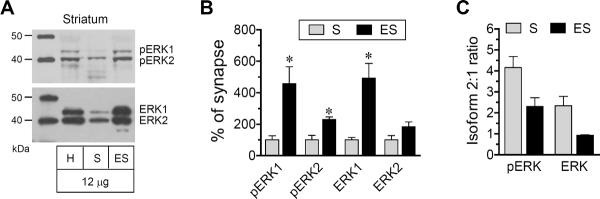
(A) Representative immunoblots showing expression of pERK1/2 and ERK1/2 proteins in homogenates (H), synaptic (S) and extrasynaptic (ES) fractions (12 μg per lane). Note that a significant amount of ERK2 was present in synaptic fractions. (B) Quantification of pERK1, pERK2, ERK1, and ERK2 expression in synaptic versus extrasynaptic fractions. (C) Ratios of pERK2 to pERK1 and ERK2 to ERK1 in the two fractions. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus synaptic fractions.
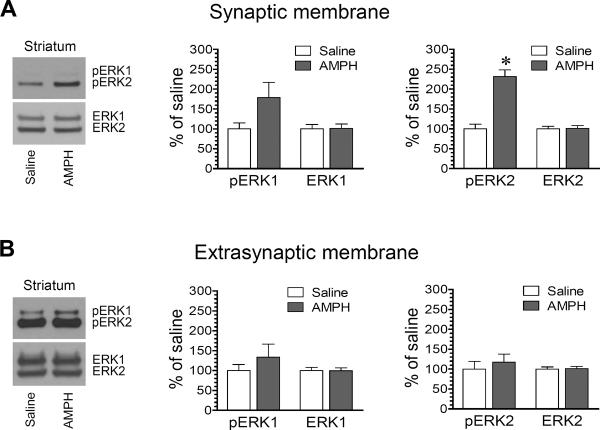
2.3. Effects of AMPH on synaptic and extrasynaptic ERK phosphorylation in the striatum
We next wanted to investigate the effect of AMPH on ERK phosphorylation and expression in the two distinct pools (synaptic versus extrasynaptic pools) of striatal neurons. We subjected rats to a single intraperitoneal (i.p.) injection of AMPH (5 mg/kg). We then sacrificed rats 15 min after drug injection. Rat brains were removed and striatal tissue was dissected for isolating synaptic and extrasynaptic proteins. Changes in phosphorylated and total ERK1/2 protein levels in the two pools were analyzed using Western blots. We found that AMPH did not significantly altered pERK1 levels in synaptic fractions, although a trend of increase was seen following AMPH administration (Fig. 3A). Noticeably, AMPH produced a greater than one-fold increase in synaptic pERK2 levels (231.6 ± 16.8% of saline, P < 0.05). The drug had a minimal impact on cellular levels of ERK1 and ERK2. At extrasynaptic sites, AMPH did not affect ERK1/2 phosphorylation and expression (Fig. 3B). Both phosphorylated and total ERK1/2 proteins showed no significant changes in AMPH-treated rats relative to saline-treated rats. These data indicate that AMPH is a stimulant that can preferentially stimulate phosphorylation of a selective synaptic pool of ERK2, while the drug has no effect on ERK1/2 phosphorylation in extrasynaptic fractions.
Figure 3. Effects of AMPH on ERK1/2 phosphorylation in the rat striatum.
(A) Effects of AMPH on ERK1/2 phosphorylation and total ERK1/2 expression in synaptic membranes. Note that AMPH markedly increased pERK2 levels in synaptic fractions. (B) Effects of AMPH on ERK1/2 phosphorylation and total ERK1/2 expression in extrasynaptic membranes. Representative immunoblots are shown left to the quantified data. Rats received a single dose of AMPH (5 mg/kg, i.p.) or saline and were sacrificed 15 min after drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 6 per group). *P < 0.05 versus saline.
2.4. Synaptic and extrasynaptic distributions of ERK1/2 in the mPFC
The terminal region of the mesocorticolimbic dopamine system, the mPFC, is another forebrain structure actively involved in stimulant action (Steketee, 2003; Van den Oever et al., 2010). We thus tested ERK responses to AMPH in this region. We first analyzed the normal distribution of the kinase in synaptic and extrasynaptic fractions of mPFC neurons. The ERK1/2 distribution in the mPFC generally resembled the pattern seen in the striatum. When the synaptic distribution was compared with the extrasynaptic distribution, all proteins (pERK1, pERK2, ERK1, and ERK2) were more abundant in extrasynaptic pools than in synaptic pools (Fig. 4A and 4B). When comparing pERK1 with pERK2, the latter was expressed at a higher level in the two pools than the former (Fig. 4A and 4C). ERK2 was also enriched at synaptic sites, while ERK1 and ERK2 were equally distributed at extrasynaptic sites (Fig. 4A and 4C).
Figure 4. Distribution of pERK1/2 and ERK1/2 in synaptic and extrasynaptic fractions of the rat mPFC.
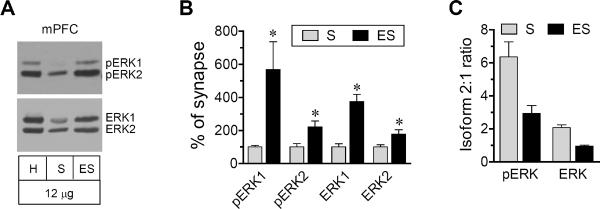
(A) Representative immunoblots showing pERK1/2 and ERK1/2 protein levels in homogenates (H), synaptic (S) and extrasynaptic (ES) fractions (12 μg per lane). (B) Quantification of pERK1/2 and ERK1/2 expression in synaptic and extrasynaptic fractions. (C) Ratios of pERK2 to pERK1 and ERK2 to ERK1 in the two fractions. Data are presented as means ± SEM (n = 4 per group). *P < 0.05 versus synaptic fractions.
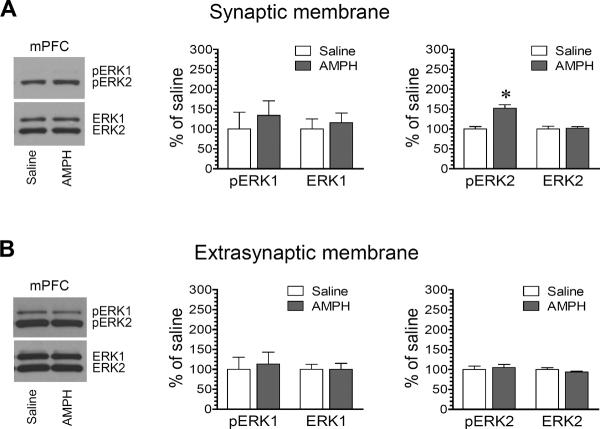
2.5. Effects of AMPH on synaptic and extrasynaptic ERK phosphorylation in the mPFC
We next examined possible changes in ERK phosphorylation in the two fractions of mPFC neurons in response to AMPH (5 mg/kg, 15 min). A single dose of AMPH induced a moderate but significant increase in ERK2 phosphorylation at synaptic sites. As shown in Fig. 5A, a significant increase in pERK2 protein levels was seen in AMPH-treated rats relative to saline-treated rats (152.1 ± 8.8% of saline, P < 0.05). AMPH did not significantly affect synaptic ERK1 phosphorylation. At extrasynaptic sites, ERK1 and ERK2 remained unchanged in their phosphorylation levels following AMPH administration (Fig. 5B). At both synaptic and extrasynaptic sites, total cellular levels of ERK1 and ERK2 remained stable in response to AMPH. These data indicate that AMPH primarily targets the synaptic species of ERK2 and stimulates its phosphorylation in mPFC neurons.
Figure 5. Effects of AMPH on ERK1/2 phosphorylation in the rat mPFC.
(A) Effects of AMPH on ERK1/2 phosphorylation and total ERK1/2 expression in synaptic membranes. (B) Effects of AMPH on ERK1/2 phosphorylation and total ERK1/2 expression in extrasynaptic membranes. Representative immunoblots are shown left to the quantified data. Rats received a single dose of AMPH (5 mg/kg, i.p.) or saline and were sacrificed 15 min after drug injection for immunoblot analysis. Data are presented as means ± SEM (n = 6 per group). *P < 0.05 versus saline.
3. Discussion
In this study, we targeted a distinct pool of ERK1/2 in synaptic structures. We first defined their synaptic distribution patterns in striatal and cortical neurons. We then investigated their responses in phosphorylation to AMPH. We found that two ERK isoforms (ERK1 and ERK2) are expressed in synaptic structures. At the subsynaptic level, they are more abundantly distributed at extrasynaptic than synaptic sites. ERK2 seems to be more enriched in synaptic membranes as compared to ERK1. This synaptic pool of ERK2 was sensitive to AMPH. Acute AMPH administration increased synaptic ERK2 phosphorylation, while the drug had no impact on ERK1/2 phosphorylation in extrasynaptic fractions of striatal and cortical neurons. These results establish the subsynaptic distribution of ERK1/2 in the striatum and mPFC. More importantly, synapses represent a site where ERK2 is selectively activated by AMPH.
Several important characteristics are noted regarding the subsynaptic distribution of ERK1/2 and responsivity of these kinases to AMPH. First, the distribution of ERK1/2 in striatal and cortical neurons exhibited a preference for the extrasynaptic microdomain. Under normal conditions, ERK1/2 were concentrated at extrasynaptic sites much more than synaptic sites. Second, synaptic expression of ERK1 and ERK2 seems to show an isoform gradient. ERK2 is visualized as a major isoform positioning in synaptic fractions. Consistent with this, ERK2 rather than ERK1 was detected in the PSD of the rat brain (Suzuki et al., 1995; 1999). Moreover, AMPH induced a positive response of ERK2 but not ERK1 in phosphorylation at synaptic sites, indicating an isoform- and synapse-selective regulation of ERK2 in response to AMPH. Finally, both regions surveyed in the mesocorticolimbic dopamine system (striatum and mPFC) showed similar distributions of ERK1/2 in synaptic and extrasynaptic pools. AMPH produced no region-specific stimulation of ERK2 phosphorylation in the two areas.
Stimulants have been well documented to regulate ERK1/2 phosphorylation in the striatum. A single dose of cocaine markedly increased pERK1/2 in the striatum (Jenab et al., 2005; Sun et al., 2007; Valjent et al., 2000; 2005; 2006; Zhang et al., 2004). Similarly, AMPH increased striatal ERK1/2 phosphorylation following an acute injection (Choe et al., 2002; Choe and Wang, 2002; Valjent et al., 2004; 2005; 2006). The stimulant-induced ERK1/2 phosphorylation may be mediated through a signaling mechanism involving combined stimulation or cross-talking of multiple systems, including dopamine D1 receptors (Valjent et al., 2000; Bertran-Gonzalez et al., 2008; Shi and McGinty, 2011), glutamate NMDA receptors (Valjent et al., 2005), Src family kinases (pascoli et al., 2011), group I metabotropic glutamate receptors (Choe et al., 2002; Choe and Wang, 2002), cannabinoid type 1 receptors (Corbille et al., 2007), and Ras-guanine nucleotide-releasing factor 1 (Fasano et al., 2009). Of note, most early studies focused on detecting the nuclear ERK1/2 through immunohistochemistry or immunoblots with whole cell homogenates. One report described an increase in ERK1/2 phosphorylation in the crude synaptosomal membrane (P2) of the rat prefrontal cortex following a single injection of cocaine (Fumagalli et al., 2009). In sum, these studies provide little knowledge about synaptic versus extrasynaptic ERK1/2. In this study, we found that ERK2 is in fact expressed at synaptic sites and synaptic ERK2 is sensitive to AMPH. This information adds a new site to analyze ERK function in drug action, in addition to the nucleus where ERK1/2 are known to regulate gene expression and thereby transcriptionally contribute to enduring neuroadaptations to stimulants (Chao and Nestler, 2004; McClung and Nestler, 2008).
A large set of ERK1/2 substrates have been discovered in the nucleus (Kosako et al., 2009; Treisman, 1996). Through modifying function of these nuclear substrates in the nucleus, ERK1/2 link extracellular signals to gene expression. Like nuclear ERK1/2, synaptic ERK1/2 are believed to interact with specific local substrates to regulate synaptic transmission. At present, several synaptic proteins have been identified as efficient biochemical substrates of ERK. These substrates include protein kinase C alpha (Debata et al., 2010), Kv4.2 potassium channels (Adams et al., 2000; Schrader et al., 2006), presynaptic synapsin I (Jovanovic et al., 1996), postsynaptic PSD-93 (Guo et al., 2012), and PSD-95 (Sabio et al., 2004). However, it is unclear how ERK1/2 phosphorylation-dependently regulate their function and thereby synaptic transmission. Future studies may identify additional new ERK2 substrates at synaptic sites and elucidate how ERK2 regulates these substrates to incubate long-lasting neuroadaptations essential for enduring synaptic plasticity and stimulant seeking behavior (Chen and Xu, 2010; Gerdjikov et al., 2004; Kim and Kim, 2008).
4. Experimental procedures
4.1. Animals
Adult male Wistar rats weighing 275–335 g (Charles River, New York, NY) were individually housed in a controlled environment at a constant temperature of 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12-h/12-h light/dark cycle. Rats were allowed 6–7 days of habituation to the animal colony. All animal use and procedures were in strict accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
4.2. Systemic drug injection
Rats were treated with an i.p. injection of saline or d-amphetamine sulfate (Sigma-Aldrich, St. Louis, MO). AMPH was injected at a dose of 5 mg/kg. The dose of the drug was calculated as the salt and was chosen based on the fact that AMPH at this dose caused typical motor stimulation and a marked increase in immediate early gene and opioid peptide gene expression in the rat striatum (Wang and McGinty, 1995). Age-matched rats received an acute injection of saline (1 ml/kg, i.p.) and served as controls. Rats were sacrificed 15 min after AMPH injection for the subsequent neurochemical analysis.
4.3. Fractionation of synaptic and extrasynaptic membranes
Subsynaptic fractionation of proteins enriched in synaptic and extrasynaptic membranes was performed according to a method developed based on the insolubility of PSD and synaptic junctions in Triton X-100 (Davies et al., 2007; 2008; Ferrario et al., 2011; Goebel-Goody et al., 2009). Briefly, rats were anesthetized with equithesin (5 ml/kg, i.p.) and decapitated. Brains were quickly removed and cut into coronal sections. The striatum was dissected and homogenized on ice in isotonic sucrose homogenization buffer containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, and a protease inhibitor cocktail (Thermo Scientific, Rochester, NY) in a glass grinding vessel with a motor driven Teflon pestle (clearance = 0.125 mm) at 700 rpm (8 strokes). The homogenate was centrifuged at 760 g for 10 min at 4°C to generate the supernatant 1 (S1) and the pellet 1 (P1). P1 was resuspended with 1 volume of the sucrose homogenization buffer, re-homogenized, and centrifuged at 760 g for 10 min at 4°C to generate the S1' and P1' fractions. The P1' fraction containing unbroken cells (including blood cells), nuclei, and large debris was discarded. The S1' fraction was added to the S1 fraction to generate the final S1 supernatant. S1 was then centrifuged at 10,000 g for 15 min at 4°C to generate the supernatant 2 (S2) containing cytosol proteins and the pellet 2 (P2) containing crude synaptosomal plasma membranes. P2 was washed once with 1 volume of the sucrose homogenization buffer and centrifuged at 10,000 g for 15 min at 4°C to produce the final P2 pellet. Washed P2 was resuspended in the sucrose homogenization buffer containing Triton X-100 (0.5%, v/v) using a motorized pestle. The suspension was incubated with gentle rotation for 20 min at 4°C, and centrifuged for 20 min (32,000 g) to yield the pellet enriched with Triton X-100-insoluable synaptic membranes and the supernatant enriched with Triton X-100-soluable extrasynaptic membranes. The extrasynaptic fraction was concentrated if needed by acetone precipitation (−20° overnight and then centrifugation at 3,000 g). The concentrated extrasynaptic pellet and the synaptic fraction were solubilized in sucrose-Triton buffer containing 1% SDS, a protease inhibitor cocktail (Thermo Scientific), and a phosphatase inhibitor cocktail (Thermo Scientific). Protein concentrations were determined and samples were stored at −80°C until use for Western blot.
4.4. Western blot analysis
Western blot was performed as described previously (Yang et al., 2006; Zhang et al., 2007). Briefly, the equal amount of protein was separated on SDS NuPAGE Novex 4–12% gels (Invitrogen, Carsbad, CA). Proteins were transferred to the polyvinylidene fluoride membrane (Millipore, Bedford, MA) and blocked in a blocking buffer (5% nonfat dry milk in phosphate-buffered saline and 0.1% Tween 20) for 1 h. The blots were washed and incubated in the blocking buffer containing a primary antibody usually at 1:1000 overnight at 4°C. This was followed by 1 h incubation in a horseradish peroxidase-linked secondary antibody against rabbit (Jackson Immunoresearch Laboratory, West Grove, PA) at 1:5000. Immunoblots were developed with the enhanced chemiluminescence reagents (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). Kaleidoscope-prestained standards (Bio-Rad, Hercules, CA) and MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. Immunoblots were measured using NIH gel analysis software.
4.5. Antibodies
Primary antibodies used in this study include rabbit antibodies against pERK1/2 at Thr202 and Tyr204 (Cell Signaling Technology, Beverly, MA) or ERK1/2 (Cell Signaling). Other primary antibodies include rabbit antibodies against NR2B (Millipore), PSD-95 (UC Davis/NIH NeuroMab Facility, Davis, CA), CaMKIIα/β (Santa Cruz Biotechnology, Santa Cruz, CA), calnexin (Santa Cruz), Rab11 (Invitrogen), EEA1 (Santa Cruz), or actin (Santa Cruz).
4.6. Statistics
The results are presented as means ± S.E.M., and were evaluated using a one-way analysis of variance followed by a Bonferroni (Dunn) comparison of groups using least squares-adjusted means or two-tailed unpaired Student's t-test. Probability levels of < 0.05 were considered statistically significant.
Highlights
ERK2 is present at synaptic sites in the rat striatum and prefrontal cortex.
Acute amphetamine increased ERK2 phosphorylation in the striatum.
Amphetamine also increased ERK2 phosphorylation in the medial prefrontal cortex.
Acknowledgements
This work was supported by NIH grants R01DA10355 (JQW) and R01MH61469 (JQW) and a grant from Saint Luke's Hospital Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare that there are no potential conflicts of interest.
REFERENCES
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J. Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio EM, Putignano E, Sassoe-Pognetto M, Pizzorusso T, Glustetto M. Visual stimulation activates ERK in synaptic and somatic compartments of rat cortical neurons with parallel kinetics. PLoS ONE. 2007;2(7):e604. doi: 10.1371/journal.pone.0000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casar B, Arozarena I, Sanz-Moreno V, Pinto A, Agudo-Ibanez L, Marais R, Lewis RE, Berciano MT, Crespo P. MTRas subcellular localization defines extracellular signal-regulated kinase 1 and 2 substrate specificity through distinct utilization of scaffold proteins. Mol. Cell. Biol. 2009;29:1338–1353. doi: 10.1128/MCB.01359-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu. Rev. Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- Chen L, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J. Neurochem. 2010;114:530–541. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe ES, Chung KT, Mao L, Wang JQ. Amphetamine increases phosphorylation of extracellular signal-regulated kinase and transcription factors in the rat striatum via group I metabotropic glutamate receptors. Neuropsychopharmacology. 2002;27:565–575. doi: 10.1016/S0893-133X(02)00341-X. [DOI] [PubMed] [Google Scholar]
- Choe ES, Wang JQ. CaMKII regulates amphetamine-induced ERK1/2 phosphorylation in striatal neurons. Neuroreport. 2002;13:1013–1016. doi: 10.1097/00001756-200206120-00006. [DOI] [PubMed] [Google Scholar]
- Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J. Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KD, Alvestad RM, Coultrap SJ, Browning MD. αCaMKII autophosphorylation levels differ depending on subcellular localization. Brain Res. 2007;1158:39–49. doi: 10.1016/j.brainres.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KD, Goebel-Goody SM, Coultrap SJ, Browning MD. Long-term synaptic depression that is associated with GluR1 dephosphorylation but not amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor internalization. J. Biol. Chem. 2008;283:33138–33146. doi: 10.1074/jbc.M803431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debata PR, Ranasinghe B, Berliner A, Curcio GM, Tantry SJ, Ponimaskin E, Banerjee P. Erk1/2-dependent phosphorylation of PKCalpha at threonine 638 in hippocampal 5-HT(1A) receptor-mediated signaling. Biochem. Biophys. Res. Commun. 2010;397:401–406. doi: 10.1016/j.bbrc.2010.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano S, D'Antoni A, Orban PC, Valjent E, Putignano E, Vara H, Pizzorusso T, Giustetto M, Yoon B, Soloway P, Maldonado R, Caboche J, Brambilla R. Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol. Psychiatry. 2009;66:758–768. doi: 10.1016/j.biopsych.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neurosci. Lett. 2011;490:180–184. doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Caffino L, Racagni G, Riva MA. Repeated stress prevents cocaine-induced activation of BDNF signaling in rat prefrontal cortex. Euro. Neuropsychopharmacol. 2009;19:402–408. doi: 10.1016/j.euroneuro.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav. Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. Phospho-regulation of synaptic and extrasynaptic N-methyl-D-aspartate receptors in adult hippocampal slices. Neuroscience. 2009;158:1146–1159. doi: 10.1016/j.neuroscience.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Guo ML, Xue B, Jin DZ, Mao LM, Wang JQ. Interactions and phosphorylation of postsynaptic density 93 (PSD-93) by extracellular signal-regulated kinase (ERK) Brain Res. 2012;1465:18–25. doi: 10.1016/j.brainres.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Mol. Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc. Natl. Acad. Sci. USA. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim JH. Time-dependent change of ERK phosphorylation levels in the nucleus accumbens during withdrawals from repeated cocaine. Neurosci. Lett. 2008;436:107–110. doi: 10.1016/j.neulet.2008.02.068. [DOI] [PubMed] [Google Scholar]
- Kosako H, Yamaguchi N, Aranami C, Ushiyama M, Kose S, Imamoto N, Taniguchi H, Nishida E, Hattori S. Phosphoproteomics reveals new ERK MAP kinase targets and links ERK to nucleoporin-mediated nuclear transport. Nat. Struct. Mol. Biol. 2009;16:1026–1035. doi: 10.1038/nsmb.1656. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- Nozaki K, Nishimura M, Hashimoto N. Mitogen-activated protein kinases and cerebral ischemia. Mol. Neurobiol. 2001;23:1–19. doi: 10.1385/MN:23:1:01. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J. Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Herve D, Pages C, Heck N, Girault JA, Caboche J, Vanhoutte P. Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol. Psychiatry. 2011;69:218–227. doi: 10.1016/j.biopsych.2010.08.031. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson BT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation of physiological functions. Endo. Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat. Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Sabio G, Reuver S, Feijoo C, Hasegawa M, Thomas GM, Centeno F, Kuhlendahl F, Leal-Ortiz S, Goedert M, Garner C, Cuenda A. Stress- and mitogen-induced phosphorylation of the synapse-associated protein SAP90/PSD-95 by activation of SAPK3/p38gamma and ERK1/ERK2. Biochem. J. 2004;380:19–30. doi: 10.1042/BJ20031628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, Sweatt JD. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am. J. Physiol. Cell. Physiol. 2006;290:C852–861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- Shi X, McGinty JF. D1 and D2 dopamine receptors differentially mediate the activation of phosphoproteins in the striatum of amphetamine-sensitized rats. Psychopharmacology. 2011;214:653–663. doi: 10.1007/s00213-010-2068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca(2+)-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD. Neurotransmitter systems of the medial prefrontal cortex: potential role in sensitization to psychostimulants. Brain Res. Rev. 2003;41:203–228. doi: 10.1016/s0165-0173(02)00233-3. [DOI] [PubMed] [Google Scholar]
- Sun WL, Zhou L, Hazim R, Quinones-Jenab V, Jenab S. Effects of acute cocaine on ERK and DARPP-32 phosphorylation pathways in the caudate-putamen of Fischer rats. Brain Res. 2007;1178:12–19. doi: 10.1016/j.brainres.2007.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Mitake S, Murata S. Presence of up-stream and downstream components of a mitogen-activated protein kinase pathway in the PSD of the rat forebrain. Brain Res. 1999;840:36–44. doi: 10.1016/s0006-8993(99)01762-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Okumura-Noji K, Nishida E. ERK2-type mitogen-activated protein kinase (MAPK) and its substrates in postsynaptic density fractions from the rat brain. Neurosci. Res. 1995;22:277–285. doi: 10.1016/0168-0102(95)00902-6. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. MAPK cascade signaling and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell. Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J. Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Trzaskos JM, Girault JA, Herve D. Role of the ERK pathway in psychostimulant-induced locomotor sensitization. BMC Neurosci. 2006;7:20. doi: 10.1186/1471-2202-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur. J. Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JG, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci. Biobehav. Rev. 2010;35:276–284. doi: 10.1016/j.neubiorev.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Volmat V, Pouyssegur J. Spatiotemporal regulation of the p42/p44 MAPK pathway. Biol. Cell. 2001;93:71–79. doi: 10.1016/s0248-4900(01)01129-7. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao LM. Regulation of mitogen-activated protein kinases by glutamate receptors. J. Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Dose-dependent alteration in zif/268 and preprodynorphin mRNA expression induced by amphetamine or methamphetamine in rat forebrain. J. Pharmacol. Exp. Ther. 1995;273:909–917. [PubMed] [Google Scholar]
- Yang L, Mao L, Chen H, Catavsan M, Kozinn J, Arora A, Liu X, Wang JQ. A signaling mechanism from Gαq-protein-coupled glutamate receptors to gene expression: role of the c-Jun N-terminal kinase pathway. J. Neurosci. 2006;26:971–980. doi: 10.1523/JNEUROSCI.4423-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Liu XY, Parelkar NK, Arora A, Yang L, Haines M, Fibuch EE, Wang JQ. In vivo regulation of Homer1a expression in the striatum by cocaine. Mol. Pharmacol. 2007;71:1148–1158. doi: 10.1124/mol.106.028399. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine-induced intracellular signaling and gene expression are oppositely regulated by the dopamine D1 and D3 receptors. J. Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]