Abstract
Objective
To examine general dysphoric moods prospectively in women who tested positive for thyroid peroxidase autoantibodies (TPO) during pregnancy and postpartum.
Design
Longitudinal, correlational, two group, observational study.
Setting
Perinatal clinics.
Participants
Six hundred thirty one (631) pregnant women.
Methods
Participants were screened for TPO antibodies, and 63 were TPO euthyroid positive. All were asked to continue into a six month postpartum follow up and 47 agreed. A comparison group of TPO negative women (N=72) was randomly selected for follow-up. Women were visited monthly for six months and a blood sample was obtained to measure thyroid stimulating hormone (TSH), a targeted physical exam was conducted, and a thyroid symptom checklist (Perceived Stress Scale) and the Profile of Mood States (POMS) checklist were completed.
Results
Pregnant TPO positive women had significantly higher depressive symptoms and were more likely to score higher than 20 on the POMS depression scale than TPO negative women. The TPO positive women had significantly higher depression, anger, and total mood disturbance scores postpartum than TOP negative women, regardless of development of postpartum thyroiditis (N=25).
Conclusions
Our results suggest that the presence of TPO autoantibodies alone in euthyroid pregnant and postpartum women increases the possibility of negative dysphoric moods, especially depressive symptoms that cannot be explained by stress or demographic factors.
Perinatal mood disturbances are common and of concern. The most common are depression and anxiety, but these disturbances can include even psychotic manifestations. Women show a range of depressive symptoms during pregnancy, from mild dysphoria to clinical depression or anxiety. In a study of 5000 pregnant women (Koleva, Stuart, O'Hara, & Bowman-Reif, 2011), correlates of dysphoric symptoms in pregnancy included earlier week of pregnancy, less education, lower income, being unmarried, unemployment, and number of previous miscarriages. Depressive symptoms and true depression may be associated with thyroid disease during the perinatal period (Pop et al., 1991). Women who are hypothyroid often become depressed until appropriately treated. It has also been reported that postpartum depression occurs with higher frequency in women who test positive for thyroid peroxidase (TPO) immunoglobulin G (IgG) (Lazarus et al., 1996).
The presence of the TPO antibody suggests an incipient autoimmune thyroid disease. Presence of this antibody at higher than normal titers is associated with development of postpartum thyroiditis in up to 50% of the women (Lazarus et al., 1996). The predominant symptoms are related to the hypothyroidism that develops when the gland is inflamed and destroyed, and these may include symptoms of depression. However, researchers did not find an association between TPO antibodies measured 48 hours after delivery and postpartum depression occurring at 8 and 32 weeks after delivery in a study of 1053 postpartum Spanish women (Albacar et al., 2010).
In another study, TPO antibodies were not correlated with postpartum blues in the first postpartum week (Lambrinoudaki et al., 2010). On the other hand, Kuijpens et al. (2001) found that positive TPO antibody status during pregnancy increased the likelihood of future postpartum depression three-fold. Depression and anxiety did not appear to be generally associated with thyroid autoimmunity in a population based study of individuals who were neither pregnant nor postpartum (Engum, Bjoro, Mykletun, & Dahl, 2005). Therefore, it seems reasonable to suggest that unique reproductive biochemical factors might be responsible for any relationship between TPO antibodies and depressive symptoms during this time of life.
Approximately 10 percent of pregnant women are TPO positive and 50 percent of TPO positive women develop postpartum thyroiditis (PPT) (Abalovich et al., 2007). This autoimmune disease has a typical course with the majority of women developing thyroid disease during the first six postpartum months. Early symptoms of PPT are related to the initial hyperthyroid state, which usually occurs between 2-6 months after delivery and may last 1–2 months.(Stagnaro-Green, 2004). Mild symptoms of hyperthyroidism are present (heat intolerance, palpitations, weight loss, fatigue) during this initial stage. The hypothyroid phase typically develops between the 12th–24th weeks after delivery, and the most frequent symptom is depression (Muller, Drexhage, & Berghout, 2001), along with the classic symptoms of hypothyroidism. Most women return to a euthyroid stage by 12 months postpartum (Stagnaro-Green, 2004).
The purpose of this study was to analyze the relationships between TPO status, development of PPT, and dysphoric moods across pregnancy and postpartum. A group of TPO negative women was included in order to compare these relationships. The study was part of a larger parent study on trajectories of postpartum thyroiditis, so blood was tested for TSH and for a number of immune and endocrine variables that were not included in this sub-study.
Methods
Participants
Institutional Review Board approval was obtained and informed consent gathered at the start of the study. Pregnant women (n=631) were recruited at their prenatal clinics. Study participants were women first measured at mid-pregnancy and identified as either TPO positive or negative at that time. Exclusion criteria included the following: age less than 18 or greater than 45 years; known autoimmune disease; previous thyroid disease; HIV positivity; use of medications that affect immunity; chronic diseases; serious mental illness; body mass index (BMI) <20; history of hyperemesis; current multiple gestation; current pregnancy product of in vitro fertilization (IVF); fetal abnormalities; unable to understand and speak the recruiter’s language (English and Spanish); and being unable to participate in a six month postpartum follow up. These exclusion criteria helped assure the homogeneity of the sample and helped to eliminate significant confounding variables.
All TPO positive (n=63) women were invited into the postpartum phase of the study, while a convenience sample of TPO negative women were selected by a random number generator to participate in the postpartum phase (n=72). All TPO positive women had TSH measured at time of pregnancy testing and at every later postpartum visit. A subset of TPO negative women had TSH levels measured postpartum. Women recruited into this study received a monthly home visit by a research nurse for six months. Participants received a graduated honorarium of $200 for completion of six months of data collection.
Instruments
At the pregnancy visit, participants completed a demographic questionnaire (POMS) (McNair, Lorr, & Droppleman, 1992) and the Perceived Stress Scale (PSS) (Cohen, Kamarck, & Mermelstein, 1983). At the subsequent postpartum visits, these stress and mood instruments were used in addition to an investigator-developed Thyroid Symptom Checklist. Data on breastfeeding, medications, health behaviors and health status were also collected by an investigator-developed instrument.
The long form of the POMS (McNair, Lorr, & Droppleman, 1992) is a 65-item rating scale for measuring how often a mood was experienced during the past week, including the day of measurement. The POMS consists of a total mood disturbance score and six subscales: tension-anxiety, depression-dejection, anger-hostility, vigor-activity, fatigue-inertia, and confusion-bewilderment. The internal consistency reliabilities for the subscales range from .87 to .95. The validity of the scale (face validity, factorial validity, predictive validity, and construct validity) is reported to be excellent (McNair et al., 1992).
The PSS is used to measure levels of self-reported stress, and the 14-item version of the scale is used to evaluate subjects’ perception of stress with a Likert scale ranging from 0 (never) to 4 (very often). The internal consistency reliability was .84 to .86 in young adults. Congruent and criterion validity for the scale have been shown to be excellent, although predictive validity declines over time (Cohen et al., 1983).
The Thyroid Symptom Checklist was based on the literature and clinical observations and designed to inventory the symptoms of hyper- and hypo-thyroidism. It consists of 19 symptoms on Likert scales between no symptom (0) to very severe (4). The woman is asked to rate symptoms including heat intolerance, dry hair and skin, puffy face, constipation, aches and pains, etc. The validity of the scale was informally confirmed by an endocrinologist not associated with the study.
Procedures
TPO status was determined in 631 pregnant women’s plasma samples (collected between 16 and 25 weeks of pregnancy) according to kit directions by ELISA (ORGENTEC, Mainz, Germany) using standards and controls. All samples were done in duplicate and titers recorded. The coefficient of variation was always less than 5%. TPO antibody titer greater than 20 IUs/ml was used as the cutoff value for determining positivity, as a values from 0 to 20 are considered the normal range ((Prummel & Wiersinga, 2005).
The research nurse who made the postpartum home visits to collect data was blinded to TPO status. At each postpartum home visit, participants received a targeted physical examination. Detailed data were gathered on the investigator developed demographic tool and included feeding type (exclusive or partial breastfeeding, or bottle feeding (ounces of formula per day), number of cigarettes smoked per day,, alcohol consumption per day, and exercise (levels and minutes per day). A venipuncture was performed and 15 mls of blood were drawn into heparinized vacutainers. The blood was collected aseptically and transported to the laboratory within 2 hours. Immediately upon receipt, blood samples were centrifuged at 3800 rpm for 25 minutes at 4°C. The plasma was aliquotted into multiple Eppendorf tubes and frozen at −80°C until later analysis in batches. TSH (ALPCO, Salem, N.H.) was always measured at each visit for the TPO positive women, regardless of symptoms, and assayed according to kit directions. TPO negative women with symptom reports on the Thyroid Screening Checklist indicating occurrence of hyper- or hypo-thyroid symptoms also had TSH levels measured.
The decision to measure TSH in the lab was made by the investigator and not the research nurse, in order to preserve blinding, as blood samples were taken from all participants for other measures in the parent study. Probable PPT development was determined to occur when TSH levels of less than 0.3 or more than 3.0mIU/L were measured (Baskin et al., 2002). . Women meeting these criteria for TSH were referred to their health care provider for further diagnostic workup and treatment. They were permitted to continue in the study, and all did. Accurate follow up with health care providers was extremely difficult so we are unable to determine the natures of treatments these women received.
Plasma samples were also assayed for prolactin levels by ELISA (ALPCO, Salem, N.H.) at week 1, and then months 2, 4, and 6, to determine if breastfeeding and prolactin levels were related to TPO status, PPT development and/or depressive symptoms.
Analysis
Data were examined for normality and log transformed if necessary. T-tests were performed for analyzing group differences. Repeated measures mixed model ANOVAs were performed to analyze differences over time. ANCOVA was done to covary and determine effects on the depression symptoms/ TPO relationship. A p value of 0.05 was accepted for significance. The data were analyzed for all TPO positive women, whether or not they developed PPT, and then separately for TPO positive women who did not develop PPT in order to characterize the effect of PPT on the relationships under study. Cronbach alphas were computed for the POMS-D and POMS-anger (POMS-A) subscales and the PSS.
Results
The Cronbach alpha for the PSS was 0.839, for the POMS-depression scale, 0.91, and the for the POMS-anger scale, 0.89. During pregnancy (measured between 16 and 25 weeks) 63 of the 631 women screened were identified as TPO positive. The mean TPO IgG titer was 55.3, with a range of 20 to 220 IU/ml at the pregnancy measurement point. TSH levels in all TPO positive women were in the normal range (between 0.3 to 3.0 U/ml) at pregnancy testing. Of these 63 TPO positive women, 47 were enrolled in the postpartum phase, with 16 being lost to follow up or declining to enter into the postpartum phase. Women declined by not returning phone calls or emails to the invitation without giving a reason, and a few had moved to a different location. A comparison of demographic, anthropometric, and mood differences between the group lost to follow up of the TPO positive women showed no important statistically significant difference from the enrolled group. The TPO positive women who did not participate in the postpartum follow up were demographically not different from the corresponding group of TPO negative women (see Table 1).
Table 1.
Demographics of participants
| TPO negative | TPO positive | |
|---|---|---|
| Ethnicity | ||
| White | 46.5% | 45.7% |
| African American | 12.9% | 14.3% |
| Asian/Pacific | 3.2% | 2.9% |
| Native American | 1.6% | 0 |
| Hispanic | 29% | 28.6% |
| Other | 6.5% | 9.5% |
| Income | ||
| $0–$14999 | 16.5% | 18.2% |
| $15000–$24999 | 10.2% | 13.7% |
| $25000–$39999 | 8.7% | 12.9% |
| $40,000+ | 64.6% | 55.3% |
| Education | ||
| Middle school | 7.8% | 5.2% |
| High school | 38.8 | 42.2% |
| College graduate | 39.8% | 41.8% |
| Post-graduate | 13.6% | 10.8% |
| Marital status | ||
| Divorced | 23.2% | 10% |
| Married | 72.5% | 63.15 |
| Single | 4.4% | 26.9% |
During pregnancy
The 63 TPO positive pregnant women had statistically significantly higher scores on the POMS depression-dejection (POMS-D) subscale (8.5) compared to TPO negative women (5.9) at the time of pregnancy measurement (t=2.2 (df=590), p=.028 The other dysphoric mood scores were higher in the TPO positive pregnant women but not at statistically significant levels. There were 48 cases of women in the total pregnancy sample (6.8%) with scores of 20 or greater on the POMS-depression-subscale. Twice as many TPO positive women had scores of 20 or greater compared to TPO negative women. The difference was significant (t (df=590)= 2.2, p=.03).The only demographic difference between TPO positive and negative groups was that the pregnant TPO positive women were slightly older than the TPO negative pregnant women (29.5 years compared to 28 years, p=.047).
During the postpartum period
At the initial postpartum testing, there were no differences in BMI, and reproductive history (number of pregnancies, preterm births, miscarriages, ectopic pregnancies) between the TPO positive and negative women. There were no differences in socioeconomic status, exercise, breastfeeding, smoking, or family histories of thyroid disease between TPO positive and negative women. With regard to ethnicity in the TPO negative group (N=72), 41.7 % were White, 13.9% were African American, 31.9% were Hispanic, and the remainders were of other ethnicities. The TPO positive group was comprised of 39.1 % White, 10.9 % African American, 19.6% Hispanic, and 30.4% from other ethnicities.
To examine if any differences postpartum were related to TSH levels rather than TPO status, levels of TSH in TPO positive compared to TPO negative women were measured. The TSH levels were actually slightly higher (1.8+/−1.4µIU/m) in TPO negative women compared to TPO positive women who did not develop PPT (1.5+/−.75µIU/m).
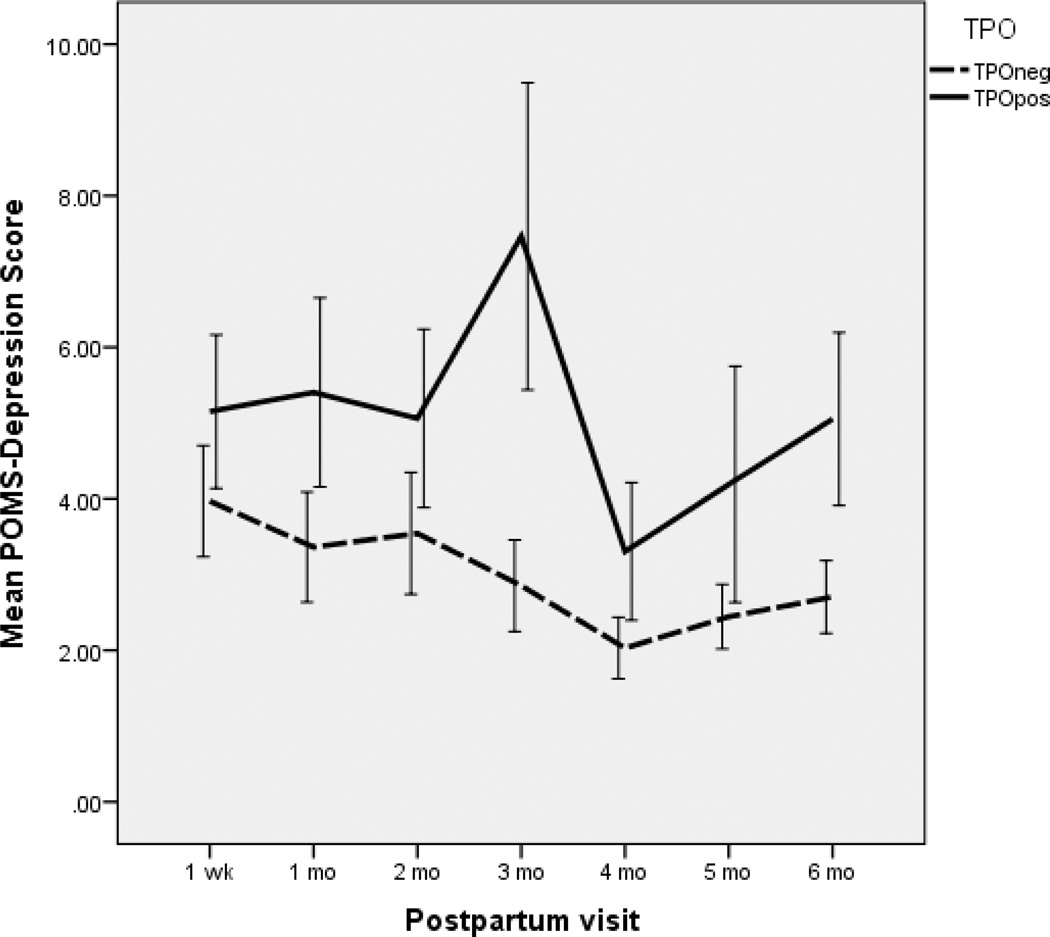
Of the 47 TPO positive women, 64% developed PPT at some point during the first six postpartum months. The Thyroid Symptom Checklist had a hyperthyroid subscale and a hypothyroid subscale. These women with PPT had higher scores on the hypothyroid subscale (F (df=1,739)=6.7, p=.013) but not on the hyperthyroid subscale on the Thyroid Symptom Checklist compared to TPO negative women. Three TPO positive women developed visible goiters, while 9 had palpable thyroid glands. TPO positive women without PPT did not score differently on the thyroid symptom checklist compared to TPO negative women. However, there were differences by TPO status on the POMS. Depression symptom reports were higher postpartum for TPO positive but PPT negative mothers (F (1,129)=9.1, p=.003). Figure 1 shows the differences in the POMS- D by group over time. POMS-A subscale scores (F (1.131) =6.4, p=.013) and total mood disturbance scores (F(1,130)=5.3, p=.023) were also higher in the TPO positive group than in the TPO negative group. Confusion and anxiety scores were nearly significantly higher (both were at p=.07). The mood scores remained statistically different for the TPO positive women who did not become PPT positive. During the postpartum period, 13 women in the total sample had scores on the POMS-D subscale of 20 or higher, indicating potential for clinical postpartum depression. These women were all referred. Eight (61.5%) of the thirteen were TPO positive. TPO positive women did not differ from TPO negative women on their reports of perceived stress postpartum (t=.67, p=.5). Perceived stress scores are summated reports of stressful experiences during the past week, and the finding of no difference by TPO status is interesting and suggests that the dysphoric symptoms experienced may be due to biological rather than environmental causes. The PSS scores and the POMS-D scores were highly correlated (r=.53, p<.001) in both TPO positive and negative women, suggesting that highly stressed women were more likely to be experiencing dysphoric symptoms, regardless of autoantibody status.
Figure 1.
Mean Profile of Mood States (POMS) depression Scores across time (Mean +/− Standard Error of the Mean)
Age and marital status were covaried in the repeated measures mixed models and not found to contribute to the relationships (F (1,115)=.038, p=.85). Interestingly, more TPO positive (74.3%) then TPO negative women (49.3%) were breastfeeding in this study (calculated as mean frequencies over the first three postpartum months). However, prolactin was not significantly different in TPO positive compared to negative women or in women who developed postpartum thyroiditis. Prolactin was not correlated with moods at any time point.
Discussion
The majority of TPO positive women are euthyroid during pregnancy, although some have subclinical hypothyroidism (Lazarus & Premawardhana, 2005). We found normal TSH levels in all of the TPO positive women during pregnancy. Nevertheless, even in the euthyroid condition, it is clear that there are pathophysiological effects associated with TPO positivity. In pregnant women, we found more clinical depression and higher depressive symptom scores in women who were TPO positive, and the same pattern continued postpartum. Autoimmune processes are somehow unmasked during the postpartum period in these women, as more than half developed PPT, often with significant thyroid gland enlargement and hypothyroid symptoms. Nevertheless, it was TPO status that was associated with the POMS scores, not PPT status.
The findings support a relationship between dysphoric moods and TPO antibody status across the perinatal period. Dysphoria is not, per se, a clinical problem, but it certainly leads to quality of life and relationship problems and nurses need to address their presence. It is also possible that dysphoria ultimately can lead to a clinical depressive or anxiety state if not addressed These scores do not indicate clinical depression and the POMS is not a diagnostic instrument. The women with highest POMS-depression scores (anyone with a score greater than 20) were referred back to their providers, and further worked up for clinical depression. Some of these women did receive antidepressants, but again the follow up with providers to accurately characterize the nature of the problem was difficult.
The relationship between TPO positivity (and autoimmune disease in general) and depression may be bidirectional. Individuals with autoimmune disease are more likely to become depressed, and depressed individuals are more likely to have altered immune status (Pitychoutis & Papadopoulou-Daifoti, 2010). Inflammatory cytokines can alter brain neurochemistry systemic and trigger depression. It has even been hypothesized that depression may have an autoimmune origin (Chen et al., 2011). Both depression and autoimmune disease are far more common in females than males, and reproductive hormones have been thought to play some role. Further research into pathophysiological mechanisms, whereby anti-thyroid antibodies, in particular, can lead to dysphoria, and even clinical depression, is needed. Dysphoric moods in the TPO positive women were not due to higher stress levels. The dysphoria cannot be explained by distressful symptoms, nor by perceptions of stress.
Currently, TPO antibody status is not routinely measured during pregnancy, but this study suggests that the risk for perinatal depressive symptoms is increased by TPO positivity. Thus, postpartum dysphoria and depression might be not only predicted but actually prevented through routine TPO antibody status determination. However this study has limitations related to the instrument used for screening dysphoria symptoms vs. clinical depression, and the relatively small number of TPO positive women.
Nurses need to be aware of this possible relationship, and particularly be concerned for women at risk for TPO positivity. This includes smokers, women with other autoimmune diseases, particularly Type 1 diabetes, and with a previous history of postpartum thyroiditis. Screening for symptoms of thyroid disease and depression is routine in many perinatal practice, but mild dysphoria itself should not be neglected. In our study TPO positive women who were euthyroid and without significant symptoms of thyroid disease were the ones at risk for dysphoria and depression.
Acknowledgement
Funded by the National Institute of Health, Grant # R01-NR05000.
Footnotes
Disclosure
The authors report no conflict of interest or relevant financial relationships.
Callouts
1. No studies have followed women who tested positive for thyroid peroxidase autoantibodies through pregnancy and postpartum.
2. Pregnant and postpartum women who tested positive for thyroid peroxidase autoantibodies, even those who remained euthyroid, had higher depression scores than controls.
3. Screening for symptoms of thyroid disease and depression is routine in many perinatal practices, but mild dysphoria itself should not be overlooked.
Contributor Information
Maureen W. Groer, Gordon Keller Professor in the College of Nursing, University of South Florida, Tampa, FL.
Jessica H. Vaughan, Doctoral candidate in the College of Nursing, University of South Florida, Tampa, FL.
References
- Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, Levalle O. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecologic Endocrinology. 2007;23(5):279–283. doi: 10.1080/09513590701259542. doi: 779427589 [pii]10.1080/09513590701259542 [doi] [DOI] [PubMed] [Google Scholar]
- Albacar G, Sans T, Martin-Santos R, Garcia-Esteve L, Guillamat R, Sanjuan J, Vilella E. Thyroid function 48h after delivery as a marker for subsequent postpartum depression. Psychoneuroendocrinology. 2010;35(5):738–742. doi: 10.1016/j.psyneuen.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Baskin HJ, Cobin RH, Duick DS, Gharib H, Guttler RB, Kaplan MM, Segal RL. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocrinology Practice. 2002;8(6):457–469. [PubMed] [Google Scholar]
- Chen Y, Jiang T, Chen P, Ouyang J, Xu G, Zeng Z, Sun Y. Emerging tendency towards autoimmune process in major depressive patients: a novel insight from Th17 cells. Psychiatry Research. 2011;188(2):224–230. doi: 10.1016/j.psychres.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Engum A, Bjoro T, Mykletun A, Dahl AA. Thyroid autoimmunity, depression and anxiety; are there any connections? An epidemiological study of a large population. Journal of Psychosomatic Research. 2005;59(5):263–268. doi: 10.1016/j.jpsychores.2005.04.002. doi: S0022-3999(05)00087-5 [pii] 10.1016/j.jpsychores.2005.04.002 [doi] [DOI] [PubMed] [Google Scholar]
- Koleva H, Stuart S, O'Hara MW, Bowman-Reif J. Risk factors for depressive symptoms during pregnancy. Archives of Womens Mental Health. 2011;14(2):99–105. doi: 10.1007/s00737-010-0184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrinoudaki I, Rizos D, Armeni E, Pliatsika P, Leonardou A, Sygelou A, Papadias C. Thyroid function and postpartum mood disturbances in Greek women. Journal of Affective Disorders. 2010;121(3):278–282. doi: 10.1016/j.jad.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Lazarus JH, Hall R, Othman S, Parkes AB, Richards CJ, McCulloch B, Harris B. The clinical spectrum of postpartum thyroid disease. QJM. 1996;89(6):429–435. doi: 10.1093/qjmed/89.6.429. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Profile of Mood States Manual. North Tonawanda, NY: Multi-Health Systems; 1992. [Google Scholar]
- Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocrinolgy Review. 2001;22(5):605–630. doi: 10.1210/edrv.22.5.0441. [DOI] [PubMed] [Google Scholar]
- Pitychoutis PM, Papadopoulou-Daifoti Z. Of depression and immunity: does sex matter? International Journal of Neuropsychopharmacology. 2010;13(5):675–689. doi: 10.1017/S1461145710000465. [DOI] [PubMed] [Google Scholar]
- Pop VJ, de Rooy HA, Vader HL, van der Heide D, van Son M, Komproe IH, de Geus CA. Postpartum thyroid dysfunction and depression in an unselected population. New England Journal of Medicine. 1991;324(25):1815–1816. doi: 10.1056/NEJM199106203242516. doi: 10.1056/NEJM199106203242516 [doi] [DOI] [PubMed] [Google Scholar]
- Prummel MF, Wiersinga WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pracices in Research Clinical Endocrinology & Metababolism. 2005;19(1):1–15. doi: 10.1016/j.beem.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Stagnaro-Green A. Postpartum thyroiditis. Best Practices in Research Clinincal Endocrinology & Metabolism. 2004;18(2):303–316. doi: 10.1016/j.beem.2004.03.008. doi: 10.1016/j.beem.2004.03.008 [doi] S1521690X04000168 [pii] [DOI] [PubMed] [Google Scholar]