Abstract
Working Memory (WM) processes help keep information in an active state so it can be used to guide future behavior. Although numerous studies have investigated brain activity associated with spatial WM in humans and monkeys, little research has focused on the neural mechanisms of WM for temporal order information, and how processing of temporal and spatial information might differ. Available evidence indicates that similar frontoparietal regions are recruited during temporal and spatial WM, although there are data suggesting that they are distinct processes. The mechanisms that allow for differential maintenance of these two types of information are unclear. One possibility is that neural oscillations may differentially contribute to temporal and spatial WM. In the present study, we used scalp electroencephalography (EEG) to compare patterns of oscillatory activity during maintenance of spatial and temporal information in WM. Time-frequency analysis of EEG data revealed enhanced left frontal theta (5–8 Hz), enhanced posterior alpha (9–12 Hz), and enhanced left posterior beta (14–28 Hz) power during the delay period of correct temporal order trials compared to correct spatial trials. In contrast, gamma (30–50 Hz) power at right lateral frontal sites was increased during the delay period of spatial WM trials, as compared to temporal WM trials. The present results are consistent with the idea that neural oscillatory patterns provide distinct mechanisms for the maintenance of temporal and spatial information in WM. Specifically, theta oscillations are most critical for the maintenance of temporal information in WM. Possible roles of higher frequency oscillations in temporal and spatial memory are also discussed.
Keywords: temporal, spatial, working memory, prefrontal cortex, theta oscillation
1. Introduction
Working Memory (WM) is a core cognitive function that supports the active maintenance and manipulation of various domains of information in order to complete complex and everyday tasks (Baddeley, 1992). A major breakthrough in understanding the neural mechanisms of WM originated from studies in monkeys, which demonstrated that single neurons in the prefrontal cortex (PFC; Fuster & Alexander, 1971; Kojima & Goldman-Rakic, 1982; Funahashi, Chafee, & Goldman-Rakic, 1993; Sawaguchi & Yamane, 1999) and posterior parietal cortex (Gnadt & Andersen, 1988; Chafee & Goldman-Rakic, 1998, 2000; Curtis, Rao, & D’Esposito, 2004) show persistent, location-specific activity during tasks that require the active short-term retention of spatial locations in WM. Building on this research, much of the literature on neural mechanisms for WM in humans has focused on spatial WM, and, in contrast, little is known about the neural underpinnings of memory for temporal order information.
Behavioral evidence suggests that the processes which support WM for temporal order may be different from those that support WM for spatial location information (Gmeindl, Walsh, & Courtney, 2011; Delogu, Nijboer, & Postma, 2012a,b). These findings seemingly conflict with physiological and neuroimaging studies, which indicate that the same frontal and parietal cortical regions that support spatial WM also support WM for temporal order. For instance, single neuron activity in frontal and parietal regions has been associated with the maintenance of temporal information (Funahashi, Inoue, & Kubota, 1997; Ninokura, Mushiake, & Tanju, 2003, 2004). Furthermore, human neuroimaging studies have shown that activity in prefrontal and posterior parietal is increased during tasks that require WM for temporal order (Amiez & Petrides, 2007, Marshuetz, Smith, Jonides, DeGutis, & Chenevert, 2000; Marshuetz & Smith, 2006).
One possible explanation that may resolve these inconsistencies in the literature is that, although similar brain regions might support maintenance of spatial and temporal information, it is conceivable that neural oscillations could differentially support these forms of WM. These oscillatory patterns might represent distinct mechanisms for maintaining these different types of information. Work in computational neuroscience has led to the idea that theta oscillations (5–8 Hz) might play an essential role in the retention of temporal order information (Lisman & Idiart, 1995; Wallenstein & Hasselmo, 1997; Jensen, 2006; Lisman & Buzsaki, 2008; Cutsuridis & Hasselmo, 2012). Neural oscillations in the theta band have been shown to increase at frontal sites with WM load or difficulty (Gevins, Smith, McEvoy, & Yu, 1997; McEvoy, Pellouchoud, Smith & Gevins, 2001; Jensen & Tesche, 2002; Schmiedt, Brand, Hildebrandt, & Basar-Eroglu, 2005; Meltzer et al., 2007), and with successful memory performance (Klimesch, Doppelmayr, Pachinger, & Ripper, 1997; Haenschel et al., 2009; Rutishauser, Ross, Mamelak, & Shuman, 2010; Addante, Watrous, Yonelinas, Ekstrom, & Ranganath, 2011). Furthermore, Hsieh, Ekstrom, & Ranganath (2011) compared oscillatory activity during tasks that required either active maintenance of temporal order or active maintenance of visual object details. They found that frontal theta oscillations were enhanced during maintenance of temporal order relative to visual object information.
In addition to theta, there is reason to believe that oscillatory activity in other frequency bands might also relate to temporal or spatial WM maintenance. For instance, alpha oscillations (9–12 Hz), often associated with “idling,” (Pfurtscheller, Stancak, & Neuper, 1996), have more recently been associated with cognitive control processes that support active maintenance of information in WM (Palva & Palva, 2007; Johnson, Sutterer, Acheson, Lewis-Peacock, & Postle, 2011; Hsieh et al. 2011; Nenert, Viswanathan, Dubuc, & Visscher, 2012). According to one hypothesis the primary role for alpha oscillations in WM is in the inhibition of irrelevant stimuli (Klimesch, Sauseng, & Hanslmayr, 2007; Jensen & Mazaheri, 2010; Freunberger, Werkle-Bergner, Griesmayr, Lindenberger, & Klimesch, 2011), or more generally, inhibition of brain regions that are not critical for the relevant task (Jensen & Mazaheri, 2010). This gate-keeping function may allow for more robust and efficient WM maintenance, although it is not clear whether this would differentially support spatial or temporal WM processing. Beta oscillations (14–28 Hz) have been associated with shifts in attention (for review see Wróbel, 2000), as well as filtering irrelevant visual representations (Waldhauser, Johansson, & Hanslmayr, 2012), and gamma oscillations, centered around 40 Hz, have been associated with a variety of cognitive functions, as well as the processing of both temporal (Lisman & Idiart, 1995; Lisman & Buzsaki, 2008; Lisman & Redish, 2009) and spatial (Kaiser & Lutzenberger, 2005; Van Der Werf, Buchholz, Jensen, & Medendorp, 2012) information in cognitive tasks. In light of the findings described above, we sought to determine whether alpha, beta, and gamma activity would be differentially associated with temporal or spatial WM maintenance.
To investigate the role of oscillatory activity associated with temporal and spatial WM processes, we recorded scalp EEG during two tasks that required maintenance of either the temporal order or spatial positions associated with each of four objects, which were selected from a pool of sixteen basic shapes. In this task, temporal and spatial trials were intermixed, and stimuli were presented in randomized order and location on the screen. Each trial began with an instruction that indicated the trial type. During temporal trials, subjects were instructed to maintain the order in which stimuli were presented on the screen, whereas, in spatial trials, they were instructed to maintain the quadrant of the screen in which each stimulus was presented. We anticipated that subjects would incidentally maintain both spatial and temporal information on each trial, as it is natural to do so, and many subjects reported remembering information about both domains even when they were trying to inhibit irrelevant information. However, the critical issue was that participants needed to place greater emphasis on maintenance of either spatial or temporal information in order to perform a given task accurately. Based on the findings ofHsieh et al. (2011), we predicted that theta power during the delay period of the WM task would be increased during maintenance of temporal, as compared with spatial information. In addition, based on the evidence described above, we investigated activity differences in the alpha, beta, and gamma bands as well, although we did not have strong a priori predictions about whether activity in these bands would differentiate between spatial and temporal WM maintenance.
WhileHsieh et al. (2011) reported differences in EEG oscillations related to WM for temporal order information, this study only examined the differences in activity between temporal order and visual object WM. Given that meaningful interpretations of EEG require comparison between conditions, this study could only tell us about neural oscillations during temporal WM as it relates to visual object WM, and thus leaving open the question of how oscillatory activity compares between spatial and temporal WM. This is an important question, given that recent studies in auditory WM (Gmeindl, Walsh, & Courtney, 2011; Delogu, Nijboer, & Postma, 2012a,b) indicate that spatial and temporal WM are, in fact, separable processes. Therefore, further research is necessary to provide additional information regarding the role of neural oscillations in temporal WM, and how neural oscillations uniquely contribute to temporal and spatial WM. The aim of this study was to investigate how temporal and spatial information are differentially maintained in WM. Understanding these relationships between neural oscillations is an essential step in characterizing the neural implementation of WM maintenance processes.
2. Materials and Methods
2.1 Participants
18 healthy undergraduate subjects were recruited from the University of California, Davis community. Participants ranged in age from 18–24 years of age, with an average of 20.6 years of age ± 0.381 SEM. All participants were right-handed. Seven subjects were female. Data from one subject was excluded due to poor memory performance (< 50% correct on either trial type), and data from one subject was excluded due to excessive artifacts in the recorded EEG. The study was approved by the Institutional Review Board at the University of California, Davis. Written informed consent was obtained from each subject before the experiment.
2.2 WM Task
The paradigm for the WM task is illustrated in Figure 1. Stimuli consisted of sixteen basic shapes, but the same shapes were never presented in consecutive trials. All shapes were white, and were presented on a black background. Shapes were approximately 3.5x3.5 inches in size. There were no differences in color or luminance across stimuli. Each trial began with a fixation for 1000 milliseconds, followed by an instruction slide for 1000 milliseconds, which indicated “Order” or “Location.” The instruction “Order” indicated a temporal trial, whereas “Location” indicated a spatial trial. The instruction slide served as the cue to prompt the subject to attend to and maintain either the sequence of the shapes, or the location of the shapes. Then, four stimuli were presented in randomized order and location. Each stimulus was presented for 1000 milliseconds, for a total encoding period of 4000 milliseconds. Following a 4000 milliseconds delay period, a single shape was presented in the center of the screen for 1000 milliseconds, and participants were instructed to make a response on a numbered response pad. For temporal trials, subjects were prompted to report when the shape occurred in the sequence (i.e., first, second, third or fourth). For spatial trials, each button corresponded to one quadrant of the screen, and subjects were asked to report the spatial location of the shape (button 1 corresponded to the upper left corner; button 2 was the upper right corner; 3 was the lower left corner; and 4 was the lower right corner). The task consisted of four testing blocks, with 28 trials in each block, for a total of 112 trials. 8 “catch” trials, in which a probe was presented that was not present in the trial, were included in this total. For these trials, subjects were instructed to press button 5, indicating that they did not see that shape in that trial. The purpose of these trials was to ensure that subjects maintained all four stimuli. In earlier versions of this task, subjects often used the strategy of focusing on the first three stimuli, and then responding to the fourth stimulus by process of elimination. Adding these catch trials eliminated this problem, and ensured that participants could not successfully use the strategy of maintaining only the first 3 shapes; however, these trials were not included in any of the analyses. The remaining trials included 52 temporal trials and 52 spatial trials. To ensure that subjects understood the task, participants also completed 2 practice blocks consisting of 6 trials each, with equal numbers of temporal and spatial trials, prior to beginning the testing blocks.
Figure 1.
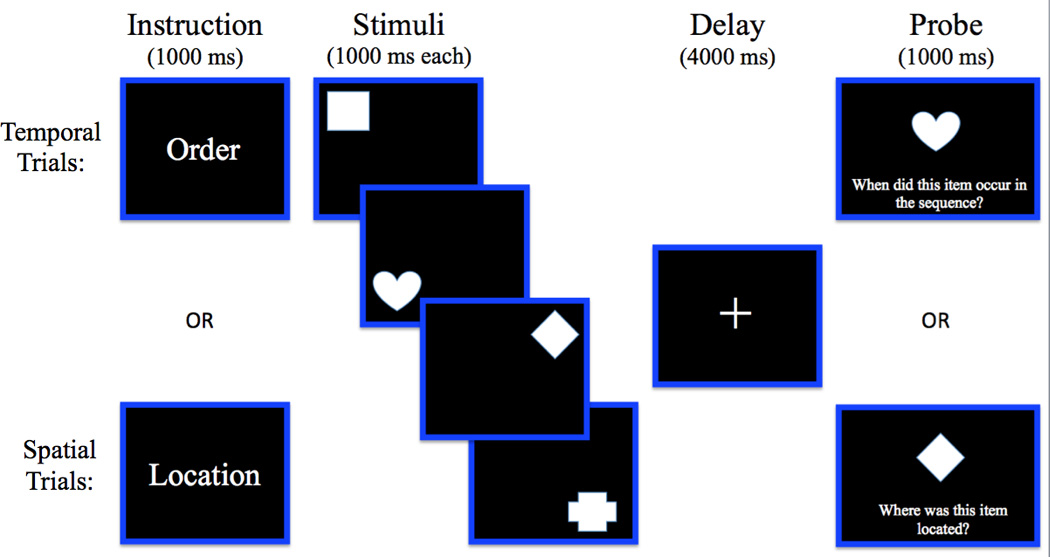
Schematic depiction of stimuli and timing of trial events in the WM task.
2.3 EEG recordings
EEG was recorded using a BioSemi (http://www.biosemi.com) Active Two system. EEG recordings were taken from 64 active Ag/AgCl electrodes embedded in an elastic cap and from active electrodes placed on the left and right mastoids, and electroculogram (EOG) recordings were taken from electrodes placed lateral to each eye and above and below the right eye. The 64 scalp electrodes were positioned in accordance with an extended version of the international 10/20 system (Klem, Luder, Jasper, & Elger, 1999). The EEG was recorded on-line with respect to a Common Mode Sense (CMS) active electrode located on the scalp near Cz. The skin under the electrodes was slightly abraded using a blunt needle, and electrodes were filled with electrolyte gel. EEG and EOG were recorded continuously at 1024 Hz. Each subject was tested individually inside an electrically shielded and sound-attenuated testing chamber. Subjects were instructed to minimize muscle tension, eye movements, and blinking, particularly during the delay period. In order to facilitate the minimization of these movements, a slide was inserted at the end of each trial that indicated it was “OK to Blink.” Participants were encouraged to time blinking, etc. to this slide, making it easier to remain still and focused during the trial.
2.4 EEG Data Analyses
The EEGLAB toolbox (Delorme & Makeig, 2004) in Matlab was used in all EEG data analyses. Analyses were performed on correct trials only. EEG data were down-sampled to 512 Hz, re-referenced to the average of the left and right mastoids, and high-pass filtered at 0.5 Hz. Data were segmented into 15 s epochs to cover the duration of the entire WM trial. Segments of EEG were visually inspected to reject any segments that appear to contain excessive noise. For the remaining trials, independent component analysis (ICA) was used to correct for artifacts (Jung et al., 2000a, b; Delorme, Sejnowski, & Makeig, 2007). Following ICA, segments of EEG were visually inspected again to remove any remaining segments that appeared to contain residual EOG, EMG, skin potential, or other artifacts. Next, data were subjected to time-frequency analysis, with different parameters used to estimate power in the low frequency bands (theta, alpha, and beta) and in the gamma band, as described below.
2.4.1 Time-Frequency Analysis for Theta, Alpha, and Beta Oscillations
Time-frequency analysis was performed using 6-cycle Morlet wavelet decomposition (i.e., mf0σt = 6; Roach and Mathalon, 2008) ranging from 4 to 100 Hz in 1 Hz steps. The Morlet filtering was performed by convolving single-trial EEG epochs from each scalp electrode with complex Morlet wavelets. A 6-cycle Morlet wavelet was used in this analysis to provide a good tradeoff between time and frequency resolution.
The decomposition was performed on 15 s EEG epochs, which encompassed the entire duration of the WM trial. EEG epochs were extracted from −2000 ms preceding the onset of the first encoding stimulus, to 13000 ms following the onset of the first encoding stimulus in order to encompass the entire duration of a WM trial plus pre-instruction baseline period (i.e., −2000 ms to 13000 ms). Epochs began 2000 ms prior to the onset of the first encoding stimulus. This time corresponded to the baseline period in which participants focused on a fixation cross. The instruction was presented 1000 ms prior to the first encoding stimulus. At time zero, the first stimulus was presented, the second stimulus was presented at 1000 ms, the third at 2000 ms, and the fourth stimulus was presented at 3000 ms. The delay period occurred at 4000–8000 ms within the epoch. The probe was presented immediately following the offset of the delay-period fixation cross, until 9000 ms; however, the epoch was extended to 13000 ms to include the response.
After Morlet wavelet decomposition of the entire duration of the WM trial was performed, oscillatory power, defined as the square of the modulus of the resulting complex number, was then separately averaged for correct spatial trials and for correct temporal trials and power estimates for each time point were log-transformed. Analyses focused on the delay period of the task. To estimate delay-period power differences between the two types of WM trials in the theta, alpha, and beta bands, oscillatory power during the delay was extracted (4000–8000 ms) from the epoched data and normalized with respect to oscillatory power during the time window (−1120 – −1000 ms) prior to the onset of the first encoding stimulus for each trial (during the fixation period just prior to the instruction cue). Although analyses focused on delay period, oscillatory power during encoding (0–4000 ms) and probe (8000–13000 ms) periods was also extracted using the same approach.
Power estimates for each WM trial type during the delay were then binned into different frequency bands (theta, 5–8 Hz; alpha, 9–12 Hz; beta bands, 14–28 Hz) and directly compared. Because oscillatory power was computed from 4 Hz to 100 Hz in 1-Hz steps, power estimates of oscillatory activities at theta (5–8 Hz), alpha (9–12 Hz), and beta (14–28 Hz) frequency bands during WM maintenance were computed by averaging power estimates at their respective frequency ranges, and averaging across a time window spanning the WM delay (i.e., 4000 ms to 8000 ms). For instance, oscillatory power for theta frequency was calculated by averaging power estimates from 5 Hz to 8 Hz during the delay. The same approach was use to obtain power estimates at alpha and beta frequency bands. The frequency bands used here are slightly narrowed from the classically defined frequency bands. By narrowing the frequency bands, we reduced potential overlap between theta effects and alpha effects, thereby minimizing the likelihood that an alpha power enhancement would lead to a spurious increase in estimates of theta and beta power.
2.4.2 Time-Frequency Analysis for Gamma Oscillations
For analyses of power in the gamma band, time-frequency analysis was performed using 4-cycle Morlet wavelet (mf0σt = 4) decomposition from 4 – 100 Hz. Oscillatory power during the delay period was extracted (4000–8000 ms) from the epoched data using the same approach as above, averaged within a single frequency band ranging from 30–50 Hz. The same parameters were used as in 2.4.1, except that a 4-cycle wavelet length was chosen for analyses of gamma oscillations in order to facilitate visualization of the effects in this band. That is, because gamma oscillations often occur in short bursts across a relatively broad range of frequencies, these effects can be more easily visualized with a shorter wavelet. It is important to note that changing the wavelet cycle did not fundamentally change the data, nor did it affect statistical significance. All of the reported effects in the gamma band were statistically reliable when using the 6-cycle wavelet decomposition.
To circumvent the potential contamination of eye movements in scalp gamma effects (see Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008), we also conducted surface Laplacian transformation on our scalp EEG data using CSD (Current Source Density) Toolbox (Version 1.1) (Kayser & Tenke, 2006a,b), and performed time-frequency transformation on the resulting CSD estimates. Surface Laplacian transformation (second spatial derivative) of the scalp EEG data allows direct estimation of the density of the transcranial current flow and yields reference-free scalp current density estimates for the direction, location, and intensity of scalp EEG (Nunez et al., 1994; Tenke & Kayser, 2005; Nunez & Srinivasan, 2006). Single-trial EEG epochs were transformed into reference-free scalp current density estimates using the spherical spline surface Laplacian algorithm (Perrin, Pernier, Bertrand, & Echallier, 1989, 1990) with m-constant = 4 and smoothing constant =10−6. The surface Laplacian-transformed single-trial EEG epochs were then submitted to the time-frequency decomposition procedures mentioned above to estimate oscillatory power over gamma frequency band. The surface Laplacian transformation acts as a spatial filter by minimizing volume-conducted contributions from distant brain regions, thereby emphasizing the contribution of superficial neocortical sources of gamma oscillations. Therefore, gamma oscillations observed in surface Laplacian transformed EEG data should reflect the contributions of superficial neocortical brain regions instead of as the result of eye movement artifacts (Melloni, Schwiedrzik, Wibral, Rodriguez, & Singer, 2009).
2.4.3 Statistical Analyses for Contrasts in Oscillatory Power
Using the methods described above, we measured oscillatory power within each frequency band for correct spatial and temporal WM trials. Data at each site were then averaged within electrode clusters. Electrodes were grouped into 9 different clusters, with 5 electrodes in each cluster: left-frontal cluster (Fp1, AF3, AF7, F3, F5), middle-frontal cluster (Fpz, AFz, F1, Fz, F2), right-frontal cluster (Fp2, AF4, AF8, F4, F6, F8), left-central cluster (FC5, C3, C5, T7, CP5), middle-central cluster (FCz, Cz, C1, C2, CPz), right-central cluster (FC6, C4, C6, T8, CP6), left-posterior cluster (P3, P5, P7, PO3, O1), middle-posterior cluster (P1, P2, Pz, POz, Oz), and right-posterior cluster (P4, P6, P8, PO4, O2).
Next, power estimates from each cluster were averaged across a time window spanning the delay period and then subjected to statistical analysis. To minimize contamination from encoding and probe activity, all statistical analyses were performed on data averaged over the time window from 1000–3000 milliseconds after the onset of the delay. These data were then submitted to four omnibus repeated measures 3-way ANOVAs (one ANOVA per frequency band of interest). Factors in each ANOVA included Condition (spatial vs. temporal trial types), Laterality (left vs. middle vs. right) and Anterior/Posterior electrode location (frontal vs. central vs. posterior regions). Greenhouse-Geisser correction was used for violation of sphericity assumption when appropriate. Follow-up analyses to further characterize effects were performed only when significant results were found for these ANOVA’s.
3. Results
3.1 WM Performance
Overall accuracy on the WM task was high for both temporal (average = 89.5% correct ± 1.55 SEM) and spatial (average = 86.6% correct ± 1.60 SEM) trials, and there were no significant differences between the two trial types (ANOVA: F [1, 30] = 1.630; p=0.211). Reaction times (RT) were also similar between temporal (average = 1.38s ± 0.088 SEM) and spatial (average = 1.55s ± 0.051 SEM) trials, with no significant differences between trial types (ANOVA: F [1, 30] = 3.178; p = 0.085). Furthermore, accuracy was not significantly correlated between temporal and spatial trial types (r [15] = 0.253, p=0.344), suggesting that performance on these two tasks was at least partially driven by different factors.
3.2 EEG Analyses
Given our previous findings of enhanced frontal theta power during maintenance of temporal order information (Hsieh, Ekstrom, & Ranganath, 2011), we hypothesized that frontal theta would be enhanced during temporal trials relative to spatial trials. Statistical analysis confirmed a significant Condition (temporal vs. spatial trial types)×Laterality (left vs. middle vs. right) interaction (ANOVA: F [1.2, 18.5] = 5.712; p=0.022), confirming that the theta (5–8 Hz) power enhancement during temporal order maintenance was reliable. As shown in Fig. 2, theta power was enhanced during temporal, as compared to spatial WM maintenance, maximal at the left frontal cluster (t [15] = 8.014; p = 0.013).
Figure 2.
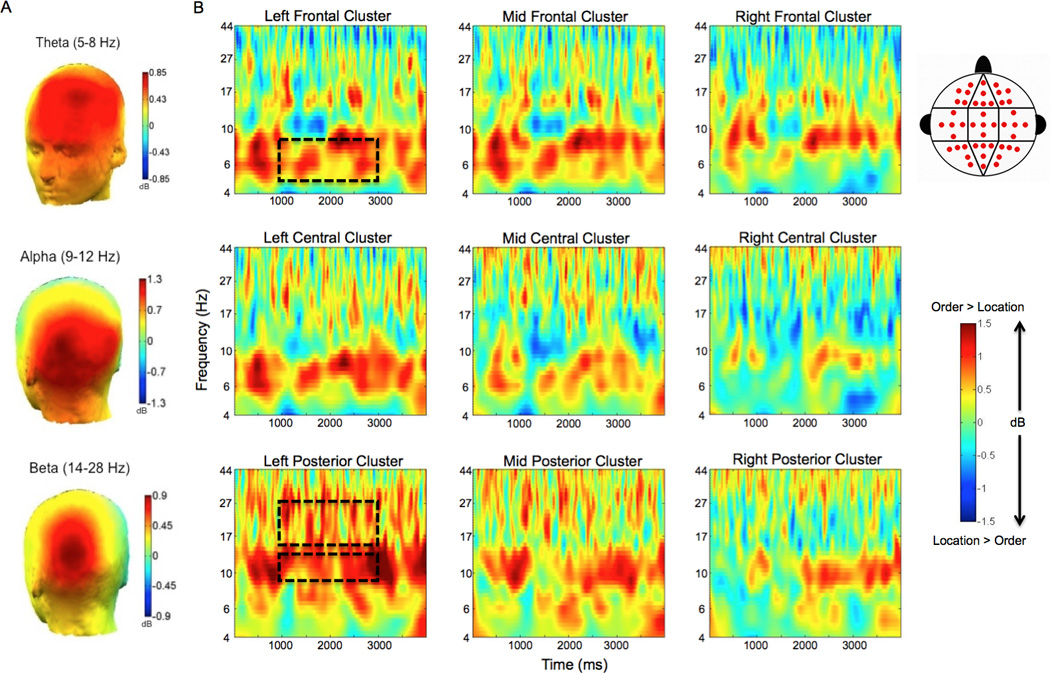
Theta, alpha, and beta oscillations are enhanced during maintenance of temporal order relative to maintenance of spatial location. A. Topographic maps of the difference in oscillatory power between correct order and correct spatial location trials are shown in theta (5–8 Hz; top), alpha (9–12 Hz; middle), and beta (14–28 Hz; bottom) frequency bands during the delay period. B. Averaged time-frequency spectrograms of the temporal – spatial power difference are shown across all electrode clusters. The distribution of electrode sites is depicted in the cluster map within the legend. Time (ms) during the delay period is plotted on the x-axis and frequency (log scale) is plotted on the y-axis. These plots display activity throughout the entire duration of the delay period. Time zero indicates the offset of the final encoding stimulus, and the last time point (4000 ms) represents the offset of the delay period fixation cross. Warm/hot colors represent enhanced power in temporal order trials as compared to spatial location trials, whereas cool/cold colors represent enhanced power in spatial location trials as compared to temporal order trials. Dashed boxes highlight the effects observed in the theta band at left frontal electrode sites, and the alpha and beta effects at left posterior electrode sites. Dashed boxes also reflect the time window used in statistical analyses (from 1000 – 3000 ms).
Our next analysis focused on the alpha band (9–12 Hz), as alpha oscillations have previously been implicated in visual WM maintenance (Gevins, Smith, McEvoy, & Yu, 1997; McEvoy, Pellouchoud, Smith & Gevins, 2001; Klimesch, Sauseng, & Hanslmayr, 2007; Jensen & Mazaheri, 2010; Freunberger, Werkle-Bergner, Griesmayr, Lindenberger, & Klimesch, 2011). Consistent with these observations, a significant Condition (temporal vs. spatial trial types)×Anterior/Posterior electrode location (frontal vs. central vs. posterior regions) interaction (ANOVA: F [1.9, 29.2] = 4.181; p=0.026) was observed. As shown in Fig. 2, the enhancement of alpha power during temporal, relative to spatial trials, was maximal at the left posterior cluster (t [15] = 8.511; p = 0.011).
Within the beta (14–28 Hz) frequency band, statistical analysis revealed a significant Condition (temporal vs. spatial trial types)×Laterality (left vs. middle vs. right), as well as a significant condition (temporal vs. spatial trial types)×Laterality (left vs. middle vs. right)×Anterior/Posterior electrode location (frontal vs. central vs. posterior regions) three-way interaction (ANOVA: F [1.8, 27.3] = 3.555; p = 0.047). As shown in Fig. 2, these results reflected the fact that beta power was enhanced during temporal WM maintenance, and that the effect was left-lateralized and maximal over posterior electrode sites, particularly at the left posterior cluster (t [15] = 7.847; p = 0.013).
Analyses of power in the gamma band (30–50Hz) revealed that right frontal gamma power was enhanced during spatial, relative to temporal WM maintenance. We found a significant Condition (spatial vs. temporal trial types)×Anterior/Posterior electrode location (anterior vs. central vs. posterior regions) interaction (ANOVA: F [1.4, 20.7] = 5.190; p = 0.024). As shown in Figure 3 the gamma enhancement was band-limited and observed throughout the delay period at the right frontal electrode cluster (t [15] = 4.806; p = 0.045). To confirm that the gamma effect was driven by neural sources, rather than oculomotor artifact, we re-ran this analysis on CSD waveforms (see section 2.4.3 for details). The differences in gamma power between the spatial and temporal conditions remained robust in this analysis (Fig. 4). The ANOVA on delay period gamma power estimates from the CSD waveforms revealed a significant Condition (spatial vs. temporal trial types)×Anterior/Posterior electrode location (anterior vs. central vs. posterior regions) interaction (ANOVA: F [1.3, 19.1] = 45.641; p = 0.022). In addition, there was also a significant Condition (spatial vs. temporal trial types)×Laterality (left vs. middle vs. right) interaction (ANOVA: F [2.0,29.8] = 4.905; p = 0.015). As is evident in Fig. 4, these effects reflected the fact that the between condition gamma differences in the CSD-transformed data exhibited two discrete peaks—one in the left frontal (t [15] = 8,183; p = 0.012) cluster and one in the right frontal (t [15] = 6.379; p = 0.023) cluster.
Figure 3.
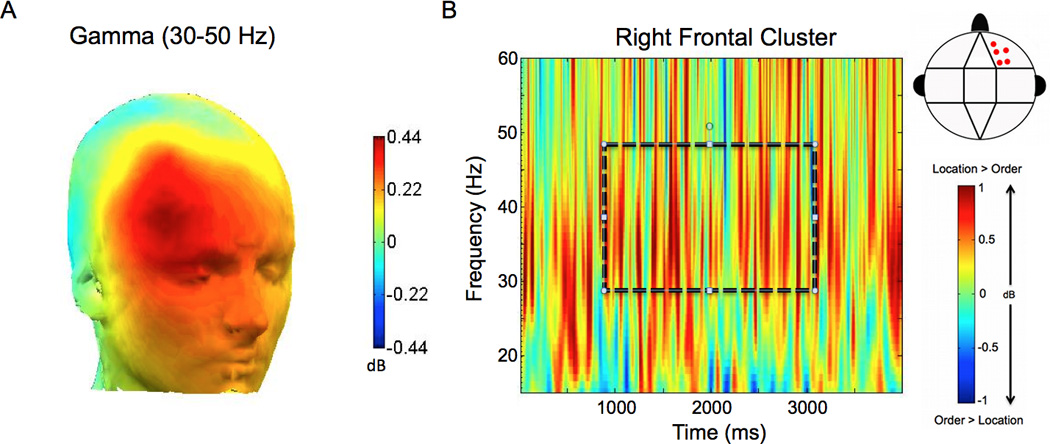
Gamma oscillations are enhanced during maintenance of spatial location relative to maintenance of temporal order. A. The topographic map of the difference in oscillatory power between correct spatial and correct temporal trials is shown in the gamma (30–50 Hz) frequency band during the delay period. B. The averaged time-frequency spectrogram of the spatial – temporal power difference is shown for the right frontal electrode cluster (see the cluster map in the legend for the locations of these electrodes). Time (ms) during the delay period is plotted on the x-axis; frequency is plotted on the y-axis. Warm/hot colors represent enhanced power in spatial location compared to temporal order trials. The dashed box highlights the frequency band and time window (1000–3000 ms) used in statistical analysis.
Figure 4.
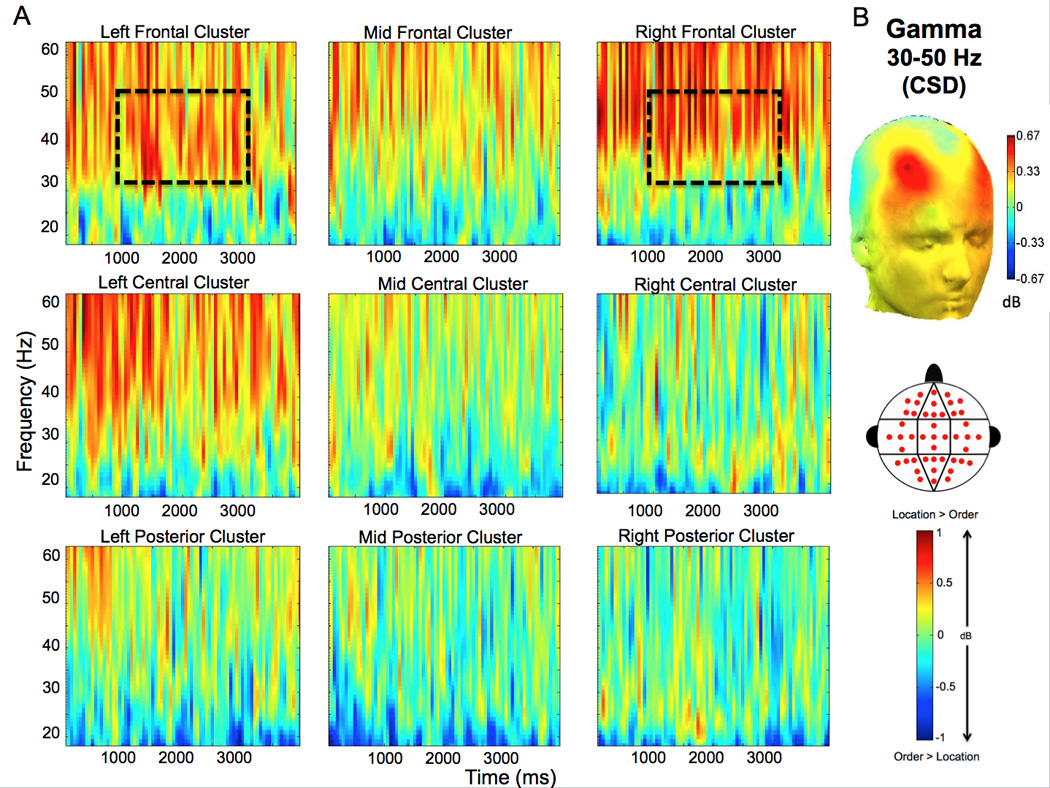
CSD demonstrates enhancement of gamma oscillations in spatial trials relative to temporal trials. A. The averaged time-frequency spectrogram (calculated from analyses of CSD waveforms) of the spatial – temporal power difference is shown across all electrode clusters (see the cluster map in the legend for the distribution of electrode sites for all clusters). Time (ms) during the delay period is plotted on the x-axis; frequency is plotted on the y-axis. Warm/hot colors represent enhanced power in spatial location compared to temporal order trials. Enhanced gamma (30–50 Hz) effects are highlighted in the dashed box, and represent the frequency band and time window (1000–3000 ms) used in statistical analysis. B. The topographic map of the difference in oscillatory power between correct spatial and correct temporal trials is shown in the gamma (30–50 Hz) frequency band during the delay period. Warm/hot colors represent enhanced power in spatial location compared to temporal order trials.
4. Discussion
In the present study, we sought to identify whether specific neural oscillations differentially support the representation of time and space in WM. We found that oscillatory activity was highly sensitive to differences between spatial and temporal WM maintenance. Whereas theta (5–8 Hz), alpha (9–12 Hz), and beta (14–28 Hz) power were enhanced during maintenance of temporal order, relative to spatial location, right frontal gamma (30–50 Hz) power was increased during spatial, relative to temporal, WM maintenance. There was no evidence to suggest that these neural activity differences could be explained solely by task difficulty, as we did not observe any significant accuracy or reaction time differences across conditions. Below, we discuss the possible roles for neural oscillations in the maintenance of temporal and spatial information in WM.
4.1 Theta Oscillatory Power in Temporal WM Maintenance
Consistent with the idea that theta oscillations support maintenance of temporal order information, we found that theta power is enhanced during the delay period of temporal, compared to spatial WM trials. When interpreting this result, it is important to note that our results only indicate a relative difference between the two tasks, which could reflect increased theta during the temporal trials or decreased theta during the spatial trials. The results from Hsieh and colleagues (2011) speak to this issue, however, as they demonstrated enhanced frontal theta power during active maintenance of temporal information in comparison to visual object information. Collectively, the results of the two studies suggest that maintenance of temporal order in WM is generally associated with increased theta activity, relative to maintenance of spatial or object information. Our results are consistent the model proposed by Lisman and colleagues (Lisman & Idiart, 1995; Lisman & Buzsaki, 2008; Lisman & Redish, 2009) that claims that temporal sequences are coded within slow theta waves. This model proposes that theta oscillations segregate the activation of the neural ensembles that represent different items, such that each ensemble will fire at a different theta phase. This idea, which receives support from recordings from rodent hippocampal cells (Lisman & Buzsaki, 2008; Lisman & Redish, 2009), implies that temporal sequence information is encoded by the relative timing of neural firing relative to the phase of theta. This model therefore suggests that theta oscillations are a crucial substrate for the temporal organization of information in WM.
In this study, it was not feasible to reliably localize the neural sources of the theta effects that we observed at the scalp, as we did not have single-subject magnetic resonance imaging data, and we lacked sufficient electrode coverage for accurate source modeling. Accordingly, we cannot make firm conclusions about the brain regions that generated theta oscillations during temporal order maintenance, but we can offer some speculations based on invasive intracranial recordings performed in previous studies. Evidence from recordings of local field potentials in monkeys suggests that the theta oscillations are generated in medial and dorsolateral prefrontal regions (Tsujimoto, Shimazu, & Isomura, et al., 2006), although the mechanism that gives rise to these oscillations is not fully understood. Electrophysiological recordings in both rodents (Buzsaki, 2002) and humans (Ekstrom et al., 2005, 2007) suggest that theta oscillations are also generated in the hippocampus, and that theta oscillations might play a role in coordinating activity between the hippocampus and PFC (Hyman, Zilli, Paley, & Hasselmo, 2005; Jones & Wilson, 2005; Siapas, Lubenov, & Wilson, 2005; Benchenane et al., 2010). Given the welldocumented role of the hippocampus in the memory for temporal sequences (Fortin, Agster, & Eichenbaum, 2002; Kesner, Gilbert, & Barua, 2002), and anatomical data from nonhuman primates showing that connections between the hippocampus and PFC are largely unidirectional (Rosene & Van Hoesen, 1977; Goldman-Rakic, Selemon, & Schwartz, 1984), it is possible that that the hippocampus might be involved in entraining prefrontal theta oscillations (Anderson, Rajagovindan, Ghacibeh, Meador, & Ding, 2010).
4.2 Alpha Oscillatory Power in Temporal WM Maintenance
Our results also revealed a clear enhancement in posterior alpha power during the delay period of temporal trials as compared to spatial location trials. This finding is compatible with a number of studies suggesting that posterior alpha activity is decreased during successful spatial WM maintenance, relative to incorrect responses. For instance, Hamidi et al. (2009) reported decreases in alpha power were associated with improved spatial WM performance, whereas this pattern of effects was not observed during object WM. Our finding also adds to results byHsieh et al. (2011), which showed an enhancement in posterior alpha during the active maintenance of visual object information compared to temporal information. Taken together, results from all of these studies suggest that alpha oscillations may be greatest during the active maintenance of visual object information, followed by temporal information, and then spatial information during WM.
The relative involvement of alpha oscillations in spatial, temporal, and object WM can potentially be explained by the “gating by inhibition” hypothesis of alpha oscillations (Jokisch & Jensen, 2007; Klimesch, Sauseng, & Hanslmayr, 2007; Jensen & Mazaheri, 2010; Freunberger, Werkle-Bergner, Griesmayr, Lindenberger, & Klimesch, 2011). According to this view, alpha oscillations result from rhythmic activity in populations of cortical interneurons (Klimesch, Sauseng, & Hanslmayr, 2007; Jensen & Mazaheri, 2010), and large-amplitude alpha oscillations may contribute to information processing by functionally inhibiting task-irrelevant cortical networks. Inhibition of task-irrelevant networks could play an important role in WM maintenance, both by allowing for increased resources in cortical regions that are necessary for task performance (Jensen & Mazaheri, 2010) and by minimizing the impact of task-irrelevant, distracting inputs during WM maintenance. Consistent with this idea, repetitive transcranial magnetic stimulation (TMS) at an alpha frequency of 10 Hz (Sauseng et al., 2009) enhances WM capacity by improving subjects’ abilities to inhibit distracting information. Collectively, these findings indicate that inhibitory control of distracting information during WM is a top-down process, which corresponds to enhancements in alpha oscillations.
In addition to inhibition of task-irrelevant networks, many reports have suggested that alpha oscillations play a direct role in the maintenance of task-relevant information (Palva & Palva, 2007; Hamidi, Slagter, Tononi, & Postle, 2009; Johnson, Sutterer, Acheson, Lewis-Peacock, & Postle, 2011; Nenert, Viswanathan, Dubuc, & Visscher, 2012). For instance, it has been proposed that alpha oscillations in extrastriate areas may contribute to the active maintenance of object or shape information in WM (Johnson, Sutterer, Acheson, Lewis-Peacock, & Postle, 2011). This idea is compatible with results fromHsieh et al. (2011), which showed enhanced alpha power during the active maintenance of visual object information as compared to temporal information. However, the idea that alpha solely reflects activation of visual object shape representations cannot explain the differences we observed between temporal and spatial conditions, as both conditions placed equal emphasis on object maintenance.
4.3 Beta Oscillatory Power in Temporal WM Maintenance
We did not predict enhancements in beta activity for temporal compared to spatial WM a priori, as there is little work that has been done on beta oscillations and WM. However, these results might be relevant to work showing that enhanced beta power is associated with changes in the focus of attention (Wróbel, 2000). For instance, local field potentials recorded from frontal and parietal cortices in monkeys have shown that activity within the beta band is implicated in top-down control of internally guided visual search and shifts of attention (Buschman & Miller, 2009; Buschman & Miller, 2010). Additionally, beta oscillations, like those in the alpha band, have also been associated with attention for and memory of irrelevant visual representations (Waldhauser, Johansson, & Hanslmayr, 2012). Furthermore, Zanto and Gazzaley (2009) have shown that beta oscillations correlate with higher WM performance due to more effective filtering of irrelevant information.
4.4 Gamma Oscillatory Power in Spatial WM Maintenance
Gamma oscillations are observed across multiple cognitive domains, including perception, attention, and WM, and have been implicated in coordinating information between brain regions in both bottom-up sensory processing and top-down goal-guided processing (Herrmann, Munk, & Engel, 2004; Buschman & Miller, 2007; Benchenane, Tiesinga, & Battaglia, 2011). To our knowledge, the enhancement in frontal gamma power during spatial WM trials as compared to temporal WM trials represents a novel finding. That said, our results are consistent with findings in spatial auditory memory (Kaiser & Lutzenberger, 2005), which have shown that memorization of the angle of a perceived sound source during short-term memory tests is characterized by gamma-band activity in frontoparietal regions. Similarly, Jokisch & Jensen (2007) reported increases in posterior gamma power during successful retention of face orientation, in comparison to face identification. Others have also demonstrated enhanced gamma-band activity during a visuo-spatial updating task in parietal cortex (Van Der Werf, Buchholz, Jensen, & Medendorp, 2012).
It should be noted that, although gamma oscillations are evident in invasive recordings of cortical field potentials, it is more difficult to measure gamma activity in scalp recordings. For instance, Yuval-Greenberg et al. (2008) demonstrated that artifactual gamma-band responses could result from microsaccades. We do not believe that the gamma band effects reported here can be attributed to microsaccades. Microsaccades are apparent as a temporal spike in single-trial EEG, and manifest as transient gamma power increases in trial-averaged spectrograms. In the studies by Yuval-Greenberg and colleagues, microsaccades were elicited by a transient visual (Yuval-Greenberg, Tomer, Keren, Nelken, & Deouell, 2008) or auditory (Yuval-Greenberg & Deouell, 2011) stimulus. The present study revealed sustained between-condition differences in gamma power across a 4 s memory delay, during which no stimuli were presented. Thus, in contrast to microsaccade artifacts, our results were sustained and not driven by a stimulus. To further rule out the possibility of microsaccade artifacts, we specially examined spatial WM trials with relatively high levels of delay period gamma power. Unlike the results of Yuval-Greenberg et al., we did not observe any saccade-related EEG spikes that could account for the gamma increases that we reported. In addition, time-frequency decomposition of impulse-like spike potentials elicited by saccades, should exhibit a broadband frequency profile that ranges from 30–100 Hz, which is different from the gamma effect (30–50 Hz) reported here. Also, since both conditions used a fixation cross during the delay, it is unlikely that participants would exhibit different patterns of eye movements during the delay.
Melloni et al. (2009) suggested that analyses of surface Laplacian transformed EEG data (i.e., CSD waveforms) can be used to disambiguate induced gamma responses from microsaccade artifacts. That is, CSD transformation minimizes effects at scalp electrodes that arise from distant sources (including eye movement artifacts). Results from time-frequency decomposition on the CSD waveforms generally agree with our analyses of raw EEG, but additionally revealed separate left and right frontal peaks of enhanced gamma during spatial WM maintenance (Fig. 4). The topography of these effects (i.e., peaking at site F4) is not consistent with what would be expected due to microsaccades or other oculomotor artifacts.
4.5 Conclusions
These results indicate that neural oscillations may be important mechanisms in the differential processing of temporal and spatial information during WM. This study builds upon previous research in temporal and spatial WM and indicates that, although similar frontoparietal networks may be activated during the maintenance of both temporal and spatial information, neural oscillatory patterns differ between them, and may provide a means for separate processing. Specifically, the present results provide further support for the hypothesis that theta oscillations play a critical role in the active maintenance of temporal order information in WM. Additionally, our findings revealed possible roles for higher frequency oscillations in temporal and spatial WM. These findings lay the groundwork for future studies to investigate the brain mechanisms that give rise to the oscillations observed here and the precise roles of different oscillations in memory and cognition.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01 MH068721. We thank Andrew Heusser for his technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addante RJ, Watrous AJ, Yonelinas AP, Ekstrom AD, Ranganath C. Prestimulus theta activity predicts correct source memory retrieval. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(26):10702–10707. doi: 10.1073/pnas.1014528108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiez C, Petrides M. Selective involvement of the mid-dorsolateral prefrontal cortex in the coding of the serial order of visual stimuli in working memory. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(34):13786–13791. doi: 10.1073/pnas.0706220104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Rajagovindan R, Ghacibeh GA, Meador KJ, Ding M. Theta oscillations mediate interaction between prefrontal cortex and medial temporal lobe in human memory. Cerebral Cortex. 2010;20(7):1604–1612. doi: 10.1093/cercor/bhp223. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron. 2010;66(6):921–936. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Tiesinga PH, Battaglia FP. Oscillations in the prefrontal cortex: a gateway to memory and attention. Current Opinion in Neurobiology. 2011;21(3):475–485. doi: 10.1016/j.conb.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Serial, covert shifts of attention during visual search are reflected by the frontal eye fields and correlated with population oscillations. Neuron. 2009;63(3):386–396. doi: 10.1016/j.neuron.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Shifting the spotlight of attention: evidence for discrete computations in cognition. Frontiers in Human Neuroscience. 2010;4:194. doi: 10.3389/fnhum.2010.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. Journal of Neurophysiology. 1998;79(6):2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. Journal of Neurophysiology. 2000;83(3):1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Rao VY, D'Esposito M. Maintenance of spatial and motor codes during oculomotor delayed response tasks. The Journal of Neuroscience. 2004;24(16):3944–3952. doi: 10.1523/JNEUROSCI.5640-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutsuridis V, Hasselmo M. GABAergic contributions to gating, timing, and phase precession of hippocampal neuronal activity during theta oscillations. Hippocampus. 2012 doi: 10.1002/hipo.21002. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. The Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delogu F, Nijboer TC, Postma A. Binding "When" and "Where" Impairs Temporal, but not Spatial Recall in Auditory and Visual Working Memory. Frontiers in Psychology. 2012a;3:62. doi: 10.3389/fpsyg.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delogu F, Nijboer TC, Postma A. Encoding location and serial order in auditory working memory: evidence for separable processes. Cognitive Processing. 2012b;13(3):267–276. doi: 10.1007/s10339-012-0442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A, Viskontas I, Kahana M, Jacobs J, Upchurch K, Bookheimer S, Fried I. Contrasting roles of neural firing rate and local field potentials in human memory. Hippocampus. 2007;17(8):606–617. doi: 10.1002/hipo.20300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Caplan JB, Ho E, Shattuck K, Fried I, Kahana MJ. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15(7):881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nature Neuroscience. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freunberger R, Werkle-Bergner M, Griesmayr B, Lindenberger U, Klimesch W. Brain oscillatory correlates of working memory constraints. Brain Research. 2011;1375:93–102. doi: 10.1016/j.brainres.2010.12.048. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Chafee MV, Goldman-Rakic PS. Prefrontal neuronal activity in rhesus monkeys performing a delayed anti-saccade task. Nature. 1993;365(6448):753–756. doi: 10.1038/365753a0. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Inoue M, Kubota K. Delay-period activity in the primate prefrontal cortex encoding multiple spatial positions and their order of presentation. Behavioural Brain Research. 1997;84(1–2):203–223. doi: 10.1016/s0166-4328(96)00151-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(3997):652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, McEvoy L, Y D. High-resolution EEG mapping of cortical activation related to working memory: effects of task difficulty, type of processing, and practice. Cerebral Cortex. 1997;7(4):374–385. doi: 10.1093/cercor/7.4.374. [DOI] [PubMed] [Google Scholar]
- Gmeindl L, Walsh M, Courtney SM. Binding serial order to representations in working memory: a spatial/verbal dissociation. Memory and Cognition. 2011;39(1):37–46. doi: 10.3758/s13421-010-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Experimental Brain Research. 1988;70(1):216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive Transcranial Magnetic Stimulation Affects behavior by Biasing Endogenous Cortical Oscillations. Frontiers in Integrative Neuroscience. 2009;3:14. doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. The Journal of Neuroscience. 2009;29(30):9481–9489. doi: 10.1523/JNEUROSCI.1428-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends in Cognitive Sciences. 2004;8(8):347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hsieh LT, Ekstrom AD, Ranganath C. Neural oscillations associated with item and temporal order maintenance in working memory. The Journal of Neuroscience. 2011;31(30):10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15(6):739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- Jensen O. Maintenance of multiple working memory items by temporal segmentation. Neuroscience. 2006;139(1):237–249. doi: 10.1016/j.neuroscience.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Frontiers in Human Neuroscience. 2010;4:186. doi: 10.3389/fnhum.2010.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. The European Journal of Neuroscience. 2002;15(8):1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- Johnson JS, Sutterer DW, Acheson DJ, Lewis-Peacock JA, Postle BR. Increased Alpha-Band Power during the Retention of Shapes and Shape-Location Associations in Visual Short-Term Memory. Frontiers in Psychology. 2011;2:128. doi: 10.3389/fpsyg.2011.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. The Journal Neuroscience. 2007;27(12):3244–3251. doi: 10.1523/JNEUROSCI.5399-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3(12):e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111(10):1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W. Human gamma-band activity: a window to cognitive processing. Neuroreport. 2005;16(3):207–211. doi: 10.1097/00001756-200502280-00001. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I Evaluation with auditory oddball tasks. Clinical Neurophysiology. 2006a;117(2):348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clinical Neurophysiology. 2006b;117(2):369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behavioral Neuroscience. 2002;116(2):286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology Suppl. 1999;52:3–6. [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Pachinger T, Ripper B. Brain oscillations and human memory: EEG correlates in the upper alpha and theta band. Neuroscience Letters. 1997;238(1–2):9–12. doi: 10.1016/s0304-3940(97)00771-4. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Research Reviews. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kojima S, Goldman-Rakic PS. Delay-related activity of prefrontal neurons in rhesus monkeys performing delayed response. Brain Research. 1982;248(1):43–49. doi: 10.1016/0006-8993(82)91145-3. [DOI] [PubMed] [Google Scholar]
- Lisman J, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophrenia Bulletin. 2008;34(5):974–980. doi: 10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Redish AD. Prediction, sequences and the hippocampus. Philosophical Transactions of the Royal Society of London. Series B Biological Sciences. 2009;364(1521):1193–1201. doi: 10.1098/rstb.2008.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267(5203):1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE. Working memory for order information: multiple cognitive and neural mechanisms. Neuroscience. 2006;139(1):195–200. doi: 10.1016/j.neuroscience.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Marshuetz C, Smith EE, Jonides J, DeGutis J, Chenevert TL. Order information in working memory: fMRI evidence for parietal and prefrontal mechanisms. Journal of Cognitive Neuroscience. 2000;12(Suppl 2):130–144. doi: 10.1162/08989290051137459. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Pellouchoud E, Smith ME, Gevins A. Neurophysiological signals of working memory in normal aging. Brain Research. Cognitive Brain Research. 2001;11(3):363–376. doi: 10.1016/s0926-6410(01)00009-x. [DOI] [PubMed] [Google Scholar]
- Melloni L, Schwiedrzik CM, Wibral M, Rodriguez E, Singer W. Response to: Yuval-Greenberg etal., Transient Induced Gamma-Band Response in EEG as a Manifestation of Miniature Saccades. Neuron. 2009;58:429–441. doi: 10.1016/j.neuron.2009.04.002. Neuron (Vol. 62, pp. 8-10; author reply 10–12). United States. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Mayes LC, Constable RT. Individual differences in EEG theta and alpha dynamics during working memory correlate with fMRI responses across subjects. Clinical Neurophysiology. 2007;118(11):2419–2436. doi: 10.1016/j.clinph.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenert R, Viswanathan S, Dubuc DM, Visscher KM. Modulations of ongoing alpha oscillations predict successful short-term visual memory encoding. Frontiers in Human Neuroscience. 2012;6:127. doi: 10.3389/fnhum.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninokura Y, Mushiake H, Tanji J. Representation of the temporal order of visual objects in the primate lateral prefrontal cortex. Journal of Neurophysiology. 2003;89(5):2868–2873. doi: 10.1152/jn.00647.2002. [DOI] [PubMed] [Google Scholar]
- Ninokura Y, Mushiake H, Tanji J. Integration of temporal order and object information in the monkey lateral prefrontal cortex. Journal of Neurophysiology. 2004;91(1):555–560. doi: 10.1152/jn.00694.2003. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Silberstein RB, Cadusch PJ, Wijesinghe RS, Westdorp AF, Srinivasan R. A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalography and Clinical Neurophysiology. 1994;90(1):40–57. doi: 10.1016/0013-4694(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electrical fields of the brain: the neurophysics of EEG. 2nd ed. New York: Oxford University Press; 2006. [Google Scholar]
- Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends in Neuroscience. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Corrigendum EEG 02274. Electroencephalography and Clinical Neurophysiology. 1990;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Jr, Neuper C. Event-related synchronization (ERS) in the alpha band--an electrophysiological correlate of cortical idling: a review. International Journal of Psychophysiology. 1996;24(1–2):39–46. doi: 10.1016/s0167-8760(96)00066-9. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia Bulletin. 2008;34(5):907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198(4314):315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Ross IB, Mamelak AN, Schuman EM. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature. 2010;464:903–907. doi: 10.1038/nature08860. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Heise KF, Gruber WR, Holz E, Karim AA, et al. Brain oscillatory substrates of visual short-term memory capacity. Current Biology. 2009;19(21):1846–1852. doi: 10.1016/j.cub.2009.08.062. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Yamane I. Properties of delay-period neuronal activity in the monkey dorsolateral prefrontal cortex during a spatial delayed matching-to-sample task. Journal of Neurophysiology. 1999;82(5):2070–2080. doi: 10.1152/jn.1999.82.5.2070. [DOI] [PubMed] [Google Scholar]
- Schmiedt C, Brand A, Hildebrandt H, Basar-Eroglu C. Event-related theta oscillations during working memory tasks in patients with schizophrenia and healthy controls. Brain Research. Cognitve Brain Research. 2005;25(3):936–947. doi: 10.1016/j.cogbrainres.2005.09.015. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron. 2005;46(1):141–151. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA) Clinical Neurophysiology. 2005;116(12):2826–2846. doi: 10.1016/j.clinph.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Shimazu H, Isomura Y. Direct recording of theta oscillations in primate prefrontal and anterior cingulate cortices. Journal of Neurophysiology. 2006;95(5):2987–3000. doi: 10.1152/jn.00730.2005. [DOI] [PubMed] [Google Scholar]
- Van Der Werf J, Buchholz VN, Jensen O, Medendorp WP. Reorganization of Oscillatory Activity in Human Parietal Cortex during Spatial Updating. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhr387. [DOI] [PubMed] [Google Scholar]
- Waldhauser GT, Johansson M, Hanslmayr S. alpha/beta oscillations indicate inhibition of interfering visual memories. The Journal of Neuroscience. 2012;32(6):1953–1961. doi: 10.1523/JNEUROSCI.4201-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Hasselmo ME. GABAergic modulation of hippocampal population activity: sequence learning, place field development, and the phase precession effect. Journal of Neurophysiology. 1997;78(1):393–408. doi: 10.1152/jn.1997.78.1.393. [DOI] [PubMed] [Google Scholar]
- Wrobel A. Beta activity: a carrier for visual attention. Acta Neurobiologiae Experimentalis (Warsaw) 2000;60(2):247–260. doi: 10.55782/ane-2000-1344. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Deouell LY. Scalp-recorded induced gamma-band responses to auditory stimulation and its correlations with saccadic muscle-activity. Brain Topography. 2011;24(1):30–39. doi: 10.1007/s10548-010-0157-7. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58(3):429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. The Journal of Neuroscience. 2009;29(10):3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


