Abstract
Thiazolidinedione (TZD) therapy has been associated with increased risk of bone fractures. Studies in rodents have led to a model in which decreased bone quality in response to TZDs is due to a competition of lineage commitment between osteoblasts and adipocytes for a common precursor cell resulting in decreased osteoblast numbers. Our goal was to investigate the effects of TZD exposure on osteoblast-adipocyte lineage determination from primary human bone marrow stromal cells (hBMSCs) both in vitro and in vivo from non-diabetic subjects and patients with type 2 diabetics. Our experimental design included two phases. Phase 1: An in vitro study of TZDs effects on hBMSCs differentiation into osteoblasts and adipocytes in non-diabetic subjects. Phase 2: a randomized placebo controlled trial to determine the effects of six month pioglitazone treatment in vivo on hBMSC differentiation using adipocyte/osteoblast colony forming unit assays in patients with type 2 diabetes. In vitro, TZDs (pioglitazone and rosiglitazone) enhanced adipogenesis of hBMSCs while neither altered osteoblast differentiation and/or function as measured by alkaline phosphatase activity, gene expression, and mineralization. The ability of TZDs to enhance adipogenesis occurred at a specific time/stage of the differentiation process and pre-treating with TZDs did not further enhance adipogenesis. In vivo, six month TZD treatment decreased osteoblast precursors, increased adipocyte precursors, and increased total colony number in patients with type 2 diabetes. Our results indicate that TZD exposure in vitro potently stimulates adipogenesis but does not directly alter osteoblast differentiation/mineralization or lineage commitment from hBMSCs. TZD-treatment in type 2 diabetic patients however results in decreased osteoblastogenesis from hBMSCs compared to placebo indicating an indirect negative effect on osteoblasts suggesting an alternative model by which TZDs might negatively regulate bone quality.
Keywords: osteoblast, adipocyte, human bone marrow, osteoporosis, drug-induced osteopenia
Thiazolidinediones (TZDs) are effective oral anti-diabetic agents used by approximately 20% of adult patients with type 2 diabetes in the U.S 1, 2. TZDs enhance insulin sensitivity mainly at the level of muscle and adipose tissue resulting in increased insulin-dependent glucose disposal 3, 4. TZDs function as agonists for the peroxisome proliferator-activated receptor-gamma (PPARγ) 5–7 which, when activated, regulate the transcription of multiple genes encoding proteins that modulate glucose and lipid metabolism 8. Several studies have reported that TZD therapy is also effective in preventing the progression of prediabetes to type 2 diabetes 9–11. Recent studies have linked the use of TZDs to osteoporosis and increased risk of bone fractures in patients with diabetes and prediabetes 12–15 as well as in healthy postmenopausal women 16 (reviewed in 17–20). The mechanism(s) by which TZDs negatively influence bone quality in humans and particularly in diabetics is not well understood.
A leading hypothesis to explain the negative effect of TZDs on bone quality focuses on the lineage commitment of adipocytes and bone forming osteoblasts. Adipocytes and osteoblasts originate from the same pluripotent mesenchymal stem cells and the idea that there is an inverse relationship between the two cell types has long been held 21. Commitment of pluripotent stromal cells towards the osteoblast lineage is regulated by the key osteoblastic transcription factors Runx2 and osterix (reviewed in 22) whereas adipocyte commitment is regulated by key adipocyte transcription factors including the C/EBP, ADD1 (Adipocyte Determination and Differentiation factor/sterol regulatory element binding protein (SREBP1)), and PPAR protein families (reviewed in 23). It has been proposed that a competition exists whereby increased adipogenesis from a common multipotent precursor cell would reduce the number of potential osteoblasts and this decreased bone forming potential would ultimately lead to decreased bone quality. Based on the strong activation of PPARγ by TZDs and subsequent enhanced adipogenesis the competition model has been suggested to explain the negative effect of TZDs on bone 24. Studies investigating the competition hypothesis in rodent models have produced conflicting results. Whereas two initial studies identified a direct negative effect of TZDs on osteoblast number and cell function 25, 26 another suggested TZDs only affected bone loss in estrogen deprived rats, with no change in osteoblast number 27 and yet another study identified an increase in osteoblast apoptosis 28.
The growing body of research suggests that the competition model may not be sufficient to explain the complexities of the effects of TZDs on bone quality as well as in the context of diabetes. Accordingly, to investigate the effects of TZDs (rosiglitazone (ROSI) and pioglitazone (PIO)) on bone marrow cells in diabetic patients we conducted two sets of experiments; 1) an in vitro study on hBMSC differentiation into osteoblasts and adipocytes in non-diabetic and diabetic subjects and 2) a pilot randomized placebo controlled trial to determine the effect(s) of six month PIO treatment on the differentiation of multipotent bone marrow stromal cells to either the adipocyte or osteoblast lineage in diabetic patients using colony forming unit (CFU) assays. Collectively, our findings suggest an alternative hypothesis to the current model of adipocyte-osteoblast lineage competition from BMSCs.
Materials and Methods
All studies were approved by the Emory Institutional Review Board to be performed at Grady Memorial Hospital and Emory University Hospital, Atlanta, GA and all subjects gave written informed consent. The study was registered at clinicaltrials.gov under study no. NCT 00927355.
In vitro-studies in bone marrow from non-diabetic subjects
We obtained bone marrow specimens from 9 subjects (6 males/3 females) between the ages of 27 and 54 years undergoing elective iliac crest bone graft harvest to reconstruct continuity defects of the mandible following ablative surgery for a variety of diagnoses. We excluded subjects with a history of diabetes or those who have received insulin or oral anti-diabetic therapy, relevant hepatic disease or impaired renal function (serum creatinine ≥ 2.0 mg/dl). All reconstruction was for non-cancerous patients with no history of osteoporosis or use of bisphosphonates.
Randomized controlled trial with TZD therapy in patients with type 2 diabetes
To determine the effects of PIO on BMSCs in diabetic patients, a six month randomized trial compared the effects of PIO (Takeda Pharmaceuticals Inc., Deerfield, Il), 30 mg (2×15mg) daily, with placebo (Cebocap 1: Forest Pharmaceuticals Inc., St. Louis, MO) on BMD. Ten patients with a history of type 2 diabetes were recruited from the Grady Diabetes Clinic between July 2009 and March 2010. Study drug was dispensed by the research pharmacist using block randomization for blinded treatment allocation. Exclusion and inclusion criteria and patient demographics are detailed in the Supplemental Materials.
Bone marrow aspiration
Bone marrow aspirations were performed on diabetic volunteers at baseline, prior to initiation of study drugs, and after 6 months of TZD treatment or placebo. As such, each volunteer acted as their own control. Local anesthesia (2% lidocaine) was applied prior to aspiration of ~10 mls of bone marrow from the posterior iliac crest using a 16 gauge bone marrow aspiration needle attached to a 20 ml heparinized syringe.
Isolation and culture of multipotent human bone marrow cells (hBMCs)
Whole bone marrow was obtained from patients described above and diluted 1:20 in DMEM (Hyclone, Thermo Fisher Scientific, Waltham, MA) containing 10% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA) supplemented with 2 mM L-glutamine (Hyclone), 100 units/ml penicillin and 100 μg/ml streptomycin (Cellgro, Mediatech, Manassas VA) (growth medium) and grown at 37°C and 5% CO2. Half of the medium was changed 5 days after initial plating. The removed medium was re-plated with an equal amount of fresh growth medium. A full medium change was performed approximately 10 days after initial plating. Ten days later the cells were subcultured onto 24 and 96 well plates as needed. Differentiation protocols were initiated approximately one week later. One potential limitation to this plating/isolation protocol is that the differentiation of these cells to other lineages such as myocytes and chondrocytes was not tested and therefore the pluripotent nature has not been fully characterized.
Osteoblast growth and differentiation studies
Osteoblast differentiation medium consisted of; alpha MEM (Irvine Scientific, Santa Ana CA) supplemented with (50μg/mL) ascorbic acid, 4mM beta-glycerophosphate and 10nM Dexamethasone (Sigma Chemical Co. (St. Louis, MO)). Differentiated osteoblasts were stained for mineralization with 0.1% Alizarin red S for 20 minutes, then rinsed in dH2O, dried and photographed as described previously 29. Differentiating osteoblasts were stained in situ for alkaline phosphatase, and alkaline phosphatase enzyme activity was measured as described previously 29. Osteoblast viability was measured using 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) assay according to the manufacturer protocol (Promega, Madison, WI). Cells were plated at approximately 5×103cells/100 μL per well in 96-well plates and after the indicated treatments the change in absorbance to XTT assay reagent was measured on a Bio-Rad Lumimark plate reader (Bio-Rad Laboratories).
Adipocyte differentiation studies
Adipogenesis was initiated 2 days after confluency with the DMEM medium containing 10%FBS in addition to insulin (10μg/mL), isomethylbutylxanthine (500μM), and dexamethasone (1μM) (Sigma). Three days after initiation cell medium was changed to DMEM+10%FBS and insulin (10μg/mL) and medium was changed every 3–5 days. TZDs (Caymen Chemical Co., Ann Arbor MI) were added as indicated. Accumulated lipid was measured on 24 well plates using Adipored according to manufacturer’s instructions (Lonza, Walkersville, MD)) and quantified on 384/96-well Fluoroskan Ascent Fluorometer/Luminometer (Thermo Labsystems, Waltham, MA).
Colony Forming Unit (CFU) assays
Bone marrow was diluted 1:20 in growth medium (DMEM+10% FBS) and plated on either 6-well or 10cm culture plates for 21 days with medium changes every 3–4 days. Osteoblast or adipocyte differentiation was performed using the protocols above. Cells on 6-well plates were stained approximately 24 days after initiation of differentiation and 10cm plates were harvested for RNA. The CFU results derived from the randomized placebo controlled trial are presented as percent change from the baseline visit for each subject.
RNA isolation and quantitative real time PCR (qPCR)
Total RNA was isolated using TRizol (Invitrogen, Carlsbad, CA). cDNA was generated using QuantiTect® Reverse Transcription kit (QIAGEN, Valencia, CA). Real-time PCR was performed using QuantiFast™ SYBR® Green PCR kit (QIAGEN) on an Applied Biosystems-7000 thermocycler. Primers were designed by the website qPrimerDepot (http://primerdepot.nci.nih.gov/) and the sequences detailed in Supplemental Table S2. Results were calculated using the ΔΔCT method.
Data Analysis
Data were analyzed by one-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparisons posttest for parametric data or Kruskal–Wallis posttest for nonparametric data and non-paired student T-test when noted, with statistical significance set at P < 0.05 using GraphPad InStat.
Results
The effects of TZDs on osteoblast differentiation in vitro
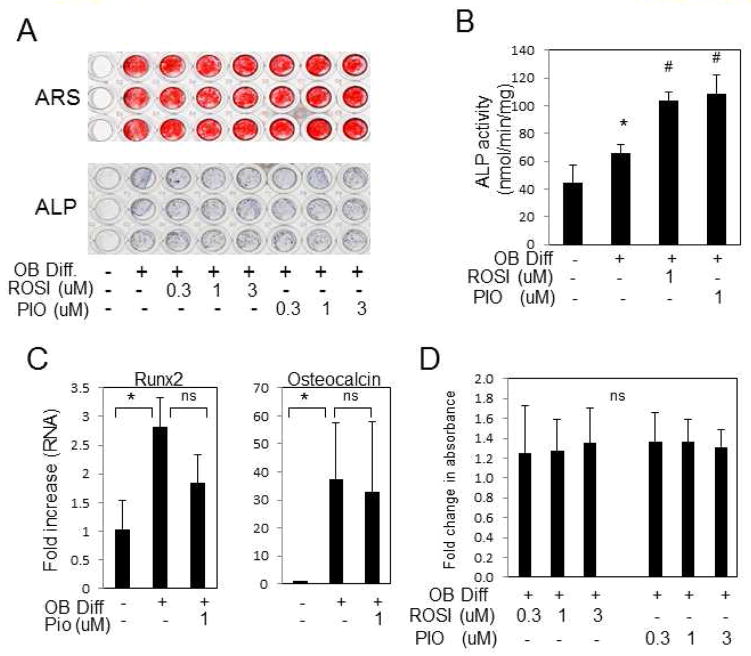
Using traditional endpoints of mineralization, lipid storage, and gene expression we confirmed the ability of our hBMSC culture methods to differentiate to multiple lineages including osteoblasts (Supplemental Fig. S1,A,B,C) and adipocytes (Supplemental Fig. S2A,B). To discern the direct effects of different TZDs on osteoblast and adipocyte lineage determination from hBMSCs a series of differentiation studies were performed on subcultured cells. Osteoblast differentiation was initiated and medium supplemented with TZDs as indicated. Neither ROSI nor PIO noticeably altered osteoblast mineral deposition as measured by alizarin Red S staining (Fig. 1A). In situ staining for alkaline phosphatase also did not detect a change in response to TZDs (Fig. 1A) however a more sensitive enzyme activity assay did measure a significant increase in response to TZD treatment (Fig. 1B). Analysis of gene expression for the osteoblast marker genes Runx2 and osteocalcin revealed a small but non-significant decrease in response to PIO (Fig. 1C). Analysis of cell viability detected a small but non-significant increase in viability (Fig. 1D) using a physiological range of TZD concentrations (0.3–3.0 μM).
Fig. 1. TZDs do not negatively alter osteoblastogenesis from hBMSCs.
hBMSCs were cultured in growth or osteoblast differentiation medium (OB Diff) in the presence of varying concentrations of PIO- or ROSI-glitazone for 21–24 days. (A) hBMSCs were stained for mineralization with Alizarin Red S (ARS) top panel or alkaline phosphatase (ALP) bottom panel. (B) Alkaline phosphatase enzyme activity was quantified using PNPP as a substrate. (Rep. of 3 patients) (C) Gene expression was measured by real-time qRT-PCR (mean of 5 patients). (D) A cell viability assay was performed and data presented as fold change from control from 3–6 patients. Results are presented as mean +/−SEM. * p < 0.05. ns:not significant
To determine the effect of TZDs on precursor cells, hBMSCs were treated with PIO five days prior to addition of differentiation medium. No change was detected in either mineralization or ALP activity (Fig. 2A,B). Treatment of cells after the initiation of differentiation likewise had no discernible effect on mineralization (Fig. 2A, bottom panel). The phosphate transport inhibitor, Foscarnet, known to inhibit mineralization was used as a positive control and in fact inhibited mineralization (Fig. 2C). Taken together, the results suggest no detectable direct negative effect of TZDs on the differentiation of hBMSCs to osteoblasts.
Fig 2. TZDs do not negatively alter osteoblast differentiation from hBMSCs.
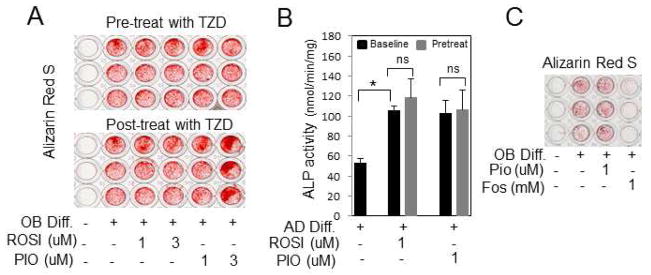
(A) hBMSCs were pretreated with TZDs for 5 days or post-treated with TZDs for 5 days relative to initiation of OB differentiation as indicated and stained with alizarin Red S. (Rep. of 3 patients). Similar results were obtained with 14 days of pretreatment. (B) Cells were pretreated with TZDs for 5 days followed by 5 days in OB differentiation medium as indicated and ALP enzyme activity measured. (Rep. of 3 patients). (C) hBMSCs were treated with OB differentiation medium for 21 days and treated with Pio (1μM) or the phosphate transport inhibitor Foscarnet (Fos-1mM) as indicated. The resulting cells were stained for alizarin Red S. Foscarnet was used as a positive control for inhibition (Rep. of 3 patients). * p < 0.05. ns:not significant.
The effects of TZDs on adipocyte differentiation in vitro
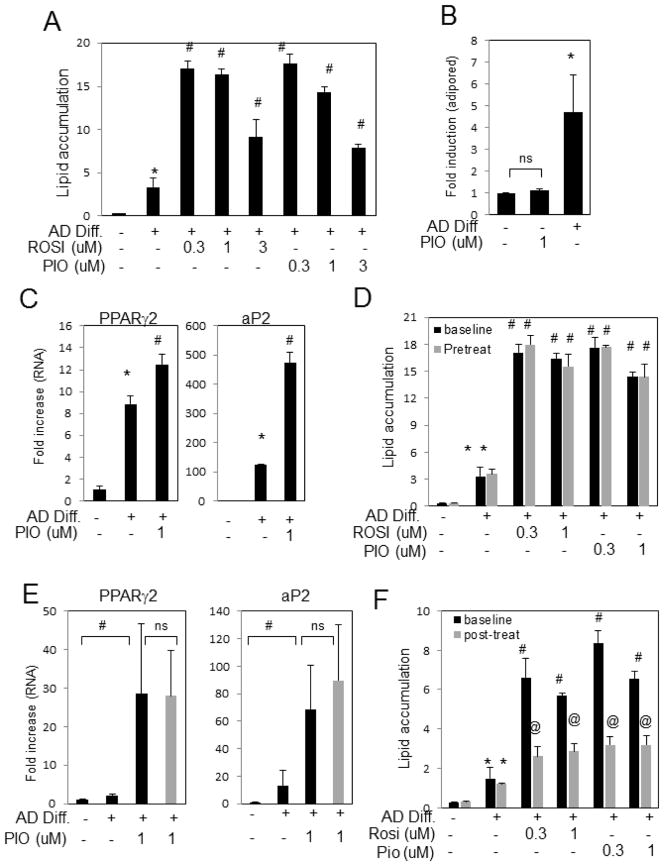
To determine the effect of TZDs on adipogenesis, hBMSCs were analyzed at various stages of adipocyte differentiation. TZDs added at the initiation of differentiation significantly enhanced lipid accumulation (Fig. 3A) which was also detectable by light microscopy and Oil-red-O staining (not shown), similar to previous results from rodent models 24. Results are similar with samples from both non-diabetic and diabetic patients. TZDs alone, in the absence of differentiation medium, did not increase adipogenesis as measured by lipid accumulation (Fig. 3B). As expected from the increase in lipid storage, addition of TZDs increased the expression of adipocyte differentiation genes such as PPARγ and aP2 (Fig. 3C). To determine the effects of TZDs on precursor cells, hBMSCs were treated with TZDs five days prior to addition of differentiation medium and lipid accumulation was measured after twenty days. Results revealed no additional increase in lipid accumulation relative to cells treated with TZDs at the time of differentiation initiation (Fig. 3D). Gene expression studies correlated these findings (Fig. 3E). Contrary to the pretreatment results, addition of TZDs five days after the switch to differentiation medium resulted in significantly less accumulated lipid relative to treatment with TZDs at initiation (Fig. 3F). The results suggest that the ability of TZDs to enhance adipogenesis only occurs at a specific time of the differentiation process and that at least in vitro, TZDs in the absence of the differentiation program do not alter adipocyte precursor number.
Fig. 3. TZDs stimulate adipogenesis from hBMSCs.
hBMSCs were differentiated to adipocytes in the presence of growth (−) or adipocyte differentiation medium (AD Diff) with the indicated concentrations of PIO- or ROSI-glitazone for 14–24 days. (A) Lipid accumulation was fluorometrically quantified with Adipored and results are expressed as arbitrary units read at (485–538) (Rep. of 10 patients) (B) Lipid accumulation was measured in the presence or absence of differentiation medium and PIO and calculated as fold change (mean of 4 patients) (C) hBMSCs were treated as indicated and RNA analyzed for PPARγ2 and aP2 expression by qRT-PCR. Results are expressed as fold increase relative to untreated and representative of 7 patients. (D) hBMSCs were pretreated with TZDs for 5 days prior to addition of adipocyte differentiation medium (pretreat-gray bars) or TZDs added simultaneously (baseline-black bars) and the lipid from all samples fluorometrically quantified after 14 to 21 days. Results are expressed as fold change and representative of 7 patients. (E) RNA was harvested from cultures treated as in (D) and analyzed for the expression of PPARγ2 and aP2. (mean of 5 patients). (F) hBMCS were simultaneously treated with adipocyte differentiation medium (baseline-black bars) or treated 5 days after differentiation initiation (post-treat-gray bars). Lipid accumulation was fluorometrically quantified from all samples after 14 to 21 days and is expressed as fold change from untreated. (Rep. of 3 patients). Columns with different characters differ at p < 0.05. Results are presented as mean +/−SEM. ns:not significant
Effects of TZDs on colony forming unit assays
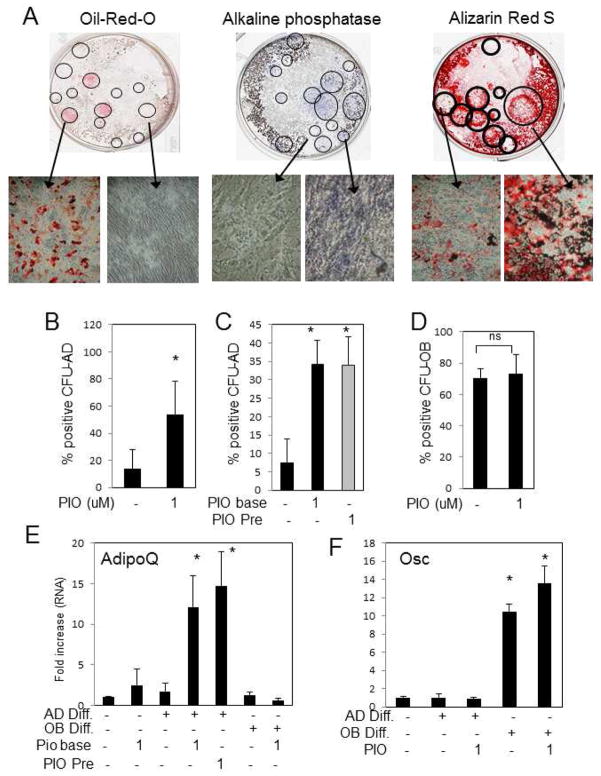
Colony forming Unit (CFU) assays were used to measure the number of “precursor” multipotent cells capable of differentiating to a particular lineage in vitro 30–32. It is possible that TZDs have a differential effect on the earliest precursor cells, thus we performed CFU-assays for adipocytes and osteoblast from individual donors in vitro to determine the direct effects of TZDs on lineage commitment. To measure the number of adipocyte positive colonies (CFU-AD) the plates were stained for lipid accumulation with Oil-Red-O and positive and negative colonies confirmed by microscopy (Fig. 4A, left panel). Colonies which stained positive and negative for Oil-Red-O were counted and the percent of positive colonies relative to the total number was calculated. The same whole bone marrow was also used analyze the ability of hBMSCs to differentiate along the osteoblast lineage (CFU-OB) staining for ALP (NBT/BCIP) or mineralization (Alizarin Red S) (Fig. 4A, middle and right panel). Similar to the sub-cultured cell populations, PIO strongly stimulated adipocyte colony formation (Fig. 4B), however pretreatment of cells with PIO for five to fourteen days did not further stimulate adipogenesis (Fig. 4C) suggesting no effect on lineage commitment. In contrast, osteoblast colony formation was not altered by addition of PIO, in vitro (Fig. 4D). The results were correlated by expression of the differentiation marker genes; adiponectin and osteocalcin (Fig. 4E&F). The results suggest that at least in vitro PIO does not alter lineage choice but promotes adipogenesis in the presence of differentiation stimuli.
Fig. 4. Pioglitazone alters adipocyte CFU but has no effect on osteoblast CFU from human bone marrow.
Whole bone marrow was diluted 1:20 in DMEM and colonies allowed to expand for twenty-one days. Cells were then treated with AD or OB differentiation medium and +/− PIO (1μM) as indicated. Colonies were treated with adipocyte (CFU-AD) or osteoblast (CFU-OB) differentiation medium and stained for adipocyte positive colonies with Oil-red-O or osteoblast positive colonies with either Alizarin Red S or NBT/BCIP (alkaline phosphatase activity). (A) Representative wells from a 6-well plate demonstrate both positive and negative colonies for the different stains. (B) The CFU-AD assay was performed in the presence or absence of 1μM PIO and positive colonies counted and expressed as a ratio relative to the total number of colonies. (mean of 7 patients). (C) Colonies were pretreated for 5–14 days with 1μM PIO prior to the initiation of adipogenesis and the percent of positive colonies relative counted and expressed as a relative ratio to the total number of colonies. (mean of 7 patients). (D) The CFU-OB assay was performed in the presence or absence of 1μM PIO and positive colonies counted and expressed as a ratio relative to the total number of colonies. (mean of 7 patients). Parallel plates were harvested for RNA analyses and analyzed for adipocyte marker genes, adiponectin shown (E), and osteoblast marker genes, osteocalcin shown (F) by qRT-PCR (Rep. of 7 patients). * p < 0.05. Results are presented as mean+/−SEM. ns:not significant
Clinical, metabolic, and Bone Mineral Density features during the 6 month TZD therapy
A total of ten diabetic patients underwent a six month pilot randomized trial comparing the effect of PIO (n=5) and placebo (n=5). Baseline patient characteristics are described in Table 1. Patients were well matched and there were no differences in clinical features, glucose and Hemoglobin A1c, between groups or between baseline and final visit, except for a larger decrease in HbA1c in the PIO treated patients from baseline, nearing significance (P=0.07) (Supplemental Fig. S3A,B,C). Patients in our study were on metformin, sulfonylurea, or a combination of both drugs resulting in stable serum glucose levels. Although the study was underpowered for the following endpoints, the data are included in the supplemental materials for the sake of completeness. The change in BMD at the femoral neck and lumbar spine was measured (Supplemental Fig. S3D,E) as well as serum markers of bone metabolism at baseline and final visit (Supplemental Table 1).
Table 1.
Baseline Patient Characteristics
| Characteristics | Placebo | Pioglitazone |
|---|---|---|
|
| ||
| n | 5 | 5 |
|
| ||
| Age (yr) | 60 (6) | 52 (10) |
|
| ||
| Gender: Male:Female (%) | 40:60 | 20:80 |
|
| ||
| Postmenopausal Females % | 66% | 25% |
|
| ||
| Weight (kg) | 80 (17) | 92 (27) |
|
| ||
| BMI | 30 (8) | 33 (10) |
|
| ||
| Oral antidiabetic agents: | ||
| Metformin only | 40% | 40% |
| Sulfonylurea only | 20% | 0 |
| Metformin + Sulfonylurea | 40% | 60% |
| Statin use (%) | 80% | 60% |
|
| ||
| Baseline HbA1c | 7.6%(0.5) | 7.6%(0.4) |
|
| ||
| Lumbar Spine BMD (g/cm2) | 1.21 (0.12) | 1.25(0.28) |
|
| ||
| Femoral Neck BMD (g/cm2) | 1.04 (0.1) | 1.02 (0.17) |
Values are presented as the average +/− SD.
In vivo effects of TZDs on colony forming unit assays and gene expression
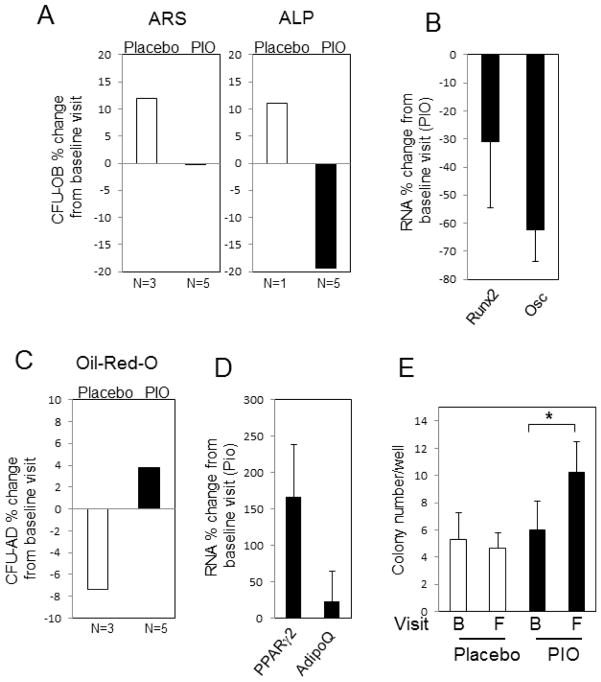
To determine the effect of PIO on BMSC lineage choice in vivo, a bone marrow aspiration was obtained from patients at baseline and after six months of treatment with PIO or placebo. The bone marrow was used for ex vivo CFU-OB and CFU-AD assays using the same protocol described for the in vitro studies above. Results revealed a decrease in the number of osteoblast positive colonies as assessed by Alizarin Red S and ALP staining in the PIO treated patients (Fig. 5A). Analysis of RNA from these colonies correlated with decreased osteoblastogenesis (Fig. 5B). Contrary to the osteoblast CFUs, adipocyte CFUs demonstrated an increase in PIO treated patients, correlated by subsequent RNA analyses (Fig. 5C&D). We additionally analyzed the number of total colonies per patient at both baseline and final visit. Treatment with PIO resulted in a significant increase in the total number of colonies formed (Fig. 5E). The data suggest that TZD treatment in vivo results in a general increase in marrow adipocytes and a decrease in osteoblasts. Taken with the lack of a direct effect of TZDs on osteoblastogenesis in vitro, these results suggest a possible indirect effect of PIO on osteoblast lineage determination. The increase in the total number of stromal cells suggests that TZDs may promote expansion as opposed to competition for a limited number of precursors.
Fig. 5. Changes in bone marrow CFU-AD and OB from study subjects treated with Pioglitazone or placebo.
Type-2 diabetic volunteers had baseline bone marrow aspirations and again following 26 weeks of treatment with placebo or PIO. (A) CFU-OB, both Alizarin Red S (ARS) and alkaline phosphatase (ALP) positive colonies were calculated as percent change from baseline for each patient and the mean calculated for each treatment group. (B) Changes in RNA levels from baseline to final visit of parallel CFU-OB assays were calculated for Runx2 and osteocalcin (Osc) from the PIO treated group. (C) The percent of CFU-AD positive colonies, based on Oil-red-O staining, was calculated as change from baseline for each patient and the mean calculated for each treatment group. (D) Changes in RNA levels from baseline to final visit of parallel CFU-AD assays were calculated for PPARγ2 and Adiponectin (AdipoQ) from the PIO treated group. (E) The total number of colonies was calculated from both the CFU-OB and CFU-AD assays, representing 12 wells per patient, for each patient group at baseline (B) and final (F) visit. Results are expressed as the mean of placebo (N=3) and 5 PIO (N=5) treated patients as indicated +/−SEM. *p<0.05 (paired student T-Test).
Discussion
Few studies have investigated the effect(s) of PPARγ activation by TZDs on primary human bone marrow stromal stem cell differentiation in vitro, and to our knowledge, this is the first study that investigated the bone effect(s) in vivo. In contrast to a number of studies in rodents, our in vitro studies did not identify a direct effect of either PIO or ROSI on osteoblast differentiation or mineralization. We utilized CFU assays to determine that exposure of multipotent hBMSCs to TZDs prior to osteoblast differentiation, likewise, had no negative effect. In agreement with our results, Bruedigam et al., found no negative effect of TZDs on osteoblast differentiation from hBMSCs. One possible explanation for the differences in human and murine cells may relate to the extent of glucocorticoid receptor expression in the different cell types previously demonstrated to influence the effects of TZDs on osteoblasts 33.
Our in vitro studies revealed a promoting effect of TZDs on adipogenesis from hBMSCs, in agreement with previous results from both rodent models 24, 34 and humans 35–37. Our study indicates that TZDs promote adipogenesis at a particular stage of differentiation from hBMSCs. Pretreatment of hBMSCs before initiation of adipogenesis did not alter the amount of lipid formed/stored and suggest that the cell must be “primed” to choose the adipocyte lineage for TZDs to effect outcome, at least in vitro. Furthermore, TZDs failed to greatly increase lipid formation five days after differentiation had been initiated but prior to terminal differentiation, suggesting the effects of TZDs are restricted to a specific stage of differentiation. Given that the early stage of adipogenesis requires the temporally coordinated regulation of a number of transcription factors 38 the result suggests that the influence of PPARγ activation on adipocyte differentiation requires activation of other factors and/or signal transduction pathways. The results together with our in vitro osteoblast findings suggest that the effect of TZDs on osteoblast differentiation and/or function might require the “context” of the bone marrow microenvironment such as the ability to interact with other cell types and/or autocrine/paracrine signals.
To determine the potential effects of TZD-treatment in the context of the marrow environment we performed, to our knowledge, the first conducted randomized placebo controlled pilot study to simultaneously examine the effects of PIO on bone at a cellular and clinical level in patients with type 2 diabetes. Our studies using CFU assays for adipocytes and osteoblasts suggest that PIO increases adipogenesis and indirectly alters osteoblastogenesis but does not alter lineage choice, a finding that is being reported in humans for the first time. Interestingly, we observed a significant increase in the number of CFUs from PIO treated patients. The increase in precursors cells is in agreement with a previous study in mice demonstrating an increase in circulating levels of mesenchymal and hematopoietic progenitor cells in response to ROSI treatment 39 and a human study demonstrating a PIO induced increase in bone marrow derived endothelial progenitor cells 40. These results provide evidence that TZDs do not necessarily limit the number of multipotent precursors but may actually expand the pool.
Our results suggest an alternative model to the competition model to explain the negative effects of TZD on bone, one that focuses on the TZD-induced effects on adipogenesis in the bone marrow as the driving influence on bone quality. The ability of adipocytes to act as endocrine cells capable of secreting biological active factors “adipokines” has gained increasing appreciation in the last decade and could represent a mechanism by which increased adipogenesis influences bone quality. A reciprocal relationship between bone quality and marrow adiposity has been observed in humans and rodents in response to TZDs 41, aging 42, 43 and glucocorticoid treatment (reviewed in 44); however, not all studies support this association 45–47. A number of adipokines are currently being investigated for potential roles in regulating bone with leptin and adiponectin being the primary focus48. Recently other potential factors have begun to gain attention such as the Wnt family of secreted signaling factors which are thought to play a key role in the regulation of osteo/adipo lineage choice 49, 50. The relationship between adipokines and bone quality is rapidly becoming an area of growing interest and future investigations are required to not only understand any regulatory relationship and adipocyte source, but also if these factors could be manipulated for human health benefits51. The roles of specific adipokines in the regulation of bone quality can be assessed using the evolving array of transgenic mouse models. A recent study in mice has identified fibroblast growth factor FGF21 as a critical regulator of adipocyte/osteoblast lineage determination in response to ROSI 52 using just such an approach and represents a novel link between adipocyte and bone quality.
The TZD class of drugs is effective in the control of diabetes; however, long-term use of TZDs may be limited by the negative effect on bone quality. It is therefore important to understand the mechanism of action of these drugs on various cell types as future design of this class might allow for the use in diabetes control with limited negative effects. An increased understanding of how this class of drugs alters the bone marrow microenvironment and the relevant target cells in humans will also shed light on the poorly understood inverse relationship between marrow adiposity and bone quality. Results presented herein provide novel insights into the deleterious effects of TZDs on bone quality in diabetics and support a model in which TZD-induced adipogenesis may be a significant influencing factor on osteoblast differentiation and function.
Supplementary Material
Supplemental Figure 1: Differentiation of hBMSCs to osteoblasts. (A) hBMSCs were treated with osteoblast differentiation medium or αMEM (non-diff.) for 14 days and stained for mineralization with Alizarin red S (10x). (B) hBMSCs were differentiated for 7 days and stained for alkaline phosphatase. (C) RNA was isolated from control and differentiated cells and analyzed by RT-PCR for Osterix (OSX), alkaline phosphatase (ALP), and Actin (loading control).
Supplemental Figure 2: Differentiation of hBMSCs to adipocytes. (A) hBMSCs at confluency were treated with differentiation medium for 7 days and photographed under light microscopy. Lipid accumulation is clearly visible. (10x and 40X). Bottom panels; Cells were then stained with Oil-red-O to confirm lipid accumulation (10x and 40X). (B) Adipocytes were generated as in (A) and gene expression analyzed by RT-PCR for PPARγ, aP2, adiponectin (AdipoQ) and actin. (Rep. of 3 patients).
Supplemental Figure 3: Baseline and final response of study subjects treated with Pioglitazone or placebo. Pre-diabetic volunteers had (B:baseline) blood, weight, and BMD measurements taken (by DeXA) and the same parameters measured (F:final) after 26 weeks of placebo (N=3) or PIO (N=5). Blood hA1C (A) and glucose (B) were measured as well as weight (C). BMD was measured by DEXA at Femoral Neck (D) and Lumbar Spine (L1–4) (E) and results are expressed as the mean +/−SEM change from baseline for each patient. Changes were not statistically different.
Supplemental Table 1: Bone metabolism markers
Supplemental Table 2: Primers used for qRT-PCR
Acknowledgments
The authors would like to thank all of the patients who contributed to the study and are grateful to Anum Ghazipura and Gonzalo Robalino, M.D. for their assistance with patient recruitment and Jane Caudell for screening the Grady Diabetes clinic database for eligible patients. Clinical Trial Registration Number: NCT 00927355.
Abbreviations
- AD
Adipocyte
- ALP
Alkaline Phosphatase
- BMD
bone mineral density
- CFU
Colony Forming Unit
- hBMSCs
human bone marrow stromal cells
- OB
Osteoblast
- PIO
Pioglitazone
- PPARγ
peroxisome proliferator-activated receptor-gamma
- ROSI
Rosiglitazone
- TZD
Thiazolidinedione
Footnotes
Disclosures Summary: The authors have nothing to disclose.
Clinical Trial Registration Number: NCT 00927355.
Disclosures: All authors have read the journal’s policy on disclosure of potential conflicts of interest. The authors confirm that there are no conflicts of interest.
Author contributions: Performed laboratory experiments: GRB. Jr, CEC, YL, LMG; Conceived project/experiments: GRB. Jr, NBK, GEU; Recruitment of patients, screening, patient data, and acquisition of bone marrow and BMD: GFB, NBK, JS, FP, DU, DS, GEU; Statistics: LP, GRB. Jr; Manuscript preparation: GRB. Jr, NBK, GEU.
Additional Supporting Information may be found in the online version of this article
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yki-Jarvinen H. The PROactive study: some answers, many questions. Lancet. 2005;366(9493):1241–2. doi: 10.1016/S0140-6736(05)67504-6. [DOI] [PubMed] [Google Scholar]
- 2.Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168(8):820–5. doi: 10.1001/archinte.168.8.820. [DOI] [PubMed] [Google Scholar]
- 3.Nolan JJ, Ludvik B, Beerdsen P, Joyce M, Olefsky J. Improvement in glucose tolerance and insulin resistance in obese subjects treated with troglitazone. The New England journal of medicine. 1994;331(18):1188–93. doi: 10.1056/NEJM199411033311803. [DOI] [PubMed] [Google Scholar]
- 4.Bajaj M, Suraamornkul S, Kashyap S, Cusi K, Mandarino L, DeFronzo RA. Sustained reduction in plasma free fatty acid concentration improves insulin action without altering plasma adipocytokine levels in subjects with strong family history of type 2 diabetes. The Journal of clinical endocrinology and metabolism. 2004;89(9):4649–55. doi: 10.1210/jc.2004-0224. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) The Journal of biological chemistry. 1995;270(22):12953–6. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 6.Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, et al. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. Journal of medicinal chemistry. 1996;39(3):665–8. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 7.Willson TM, Lehmann JM, Kliewer SA. Discovery of ligands for the nuclear peroxisome proliferator-activated receptors. Annals of the New York Academy of Sciences. 1996;804:276–83. doi: 10.1111/j.1749-6632.1996.tb18622.x. [DOI] [PubMed] [Google Scholar]
- 8.Yki-Jarvinen H. Thiazolidinediones. The New England journal of medicine. 2004;351(11):1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 9.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk hispanic women. Diabetes. 2002;51(9):2796–803. doi: 10.2337/diabetes.51.9.2796. Epub 2002/08/28. [DOI] [PubMed] [Google Scholar]
- 10.Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet. 2006;368(9541):1096–105. doi: 10.1016/S0140-6736(06)69420-8. Epub 2006/09/26. [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. The New England journal of medicine. 2011;364(12):1104–15. doi: 10.1056/NEJMoa1010949. Epub 2011/03/25. [DOI] [PubMed] [Google Scholar]
- 12.Yaturu S, Bryant B, Jain SK. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes care. 2007;30(6):1574–6. doi: 10.2337/dc06-2606. [DOI] [PubMed] [Google Scholar]
- 13.Solomon DH, Cadarette SM, Choudhry NK, Canning C, Levin R, Sturmer T. A Cohort Study of Thiazolidinediones and Fractures in Older Adults With Diabetes. The Journal of clinical endocrinology and metabolism. 2009 doi: 10.1210/jc.2008-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancini T, Mazziotti G, Doga M, Carpinteri R, Simetovic N, Vescovi PP, et al. Vertebral fractures in males with type 2 diabetes treated with rosiglitazone. Bone. 2009 doi: 10.1016/j.bone.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, et al. Thiazolidinedione use and bone loss in older diabetic adults. The Journal of clinical endocrinology and metabolism. 2006;91(9):3349–54. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grey A, Bolland M, Gamble G, Wattie D, Horne A, Davidson J, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. The Journal of clinical endocrinology and metabolism. 2007;92(4):1305–10. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 17.Grey A. Thiazolidinedione-induced skeletal fragility--mechanisms and implications. Diabetes Obes Metab. 2009;11(4):275–84. doi: 10.1111/j.1463-1326.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 18.McDonough AK, Rosenthal RS, Cao X, Saag KG. The effect of thiazolidinediones on BMD and osteoporosis. Nat Clin Pract Endocrinol Metab. 2008;4(9):507–13. doi: 10.1038/ncpendmet0920. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz AV. Diabetes, TZDs, and Bone: A Review of the Clinical Evidence. PPAR Res. 2006;2006:24502. doi: 10.1155/PPAR/2006/24502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz AV. TZDs and Bone: A Review of the Recent Clinical Evidence. PPAR Res. 2008;2008:297893. doi: 10.1155/2008/297893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci. 1992;102 ( Pt 2):341–51. doi: 10.1242/jcs.102.2.341. [DOI] [PubMed] [Google Scholar]
- 22.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–96. doi: 10.1146/annurev.genom.9.081307.164437. Epub 2008/09/05. [DOI] [PubMed] [Google Scholar]
- 23.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–71. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 24.Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, et al. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Molecular pharmacology. 1996;50(5):1087–94. [PubMed] [Google Scholar]
- 25.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401–6. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146(3):1226–35. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- 27.Sottile V, Seuwen K, Kneissel M. Enhanced marrow adipogenesis and bone resorption in estrogen-deprived rats treated with the PPARgamma agonist BRL49653 (rosiglitazone) Calcified tissue international. 2004;75(4):329–37. doi: 10.1007/s00223-004-0224-8. [DOI] [PubMed] [Google Scholar]
- 28.Soroceanu MA, Miao D, Bai XY, Su H, Goltzman D, Karaplis AC. Rosiglitazone impacts negatively on bone by promoting osteoblast/osteocyte apoptosis. The Journal of endocrinology. 2004;183(1):203–16. doi: 10.1677/joe.1.05723. [DOI] [PubMed] [Google Scholar]
- 29.Beck GR, Jr, Sullivan EC, Moran E, Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. Journal of cellular biochemistry. 1998;68(2):269–80. doi: 10.1002/(sici)1097-4644(19980201)68:2<269::aid-jcb13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. Epub 1988/01/01. [DOI] [PubMed] [Google Scholar]
- 31.Beresford JN. Osteogenic stem cells and the stromal system of bone and marrow. Clinical orthopaedics and related research. 1989;240:270–80. Epub 1989/03/01. [PubMed] [Google Scholar]
- 32.Krebsbach PH, Kuznetsov SA, Bianco P, Robey PG. Bone marrow stromal cells: characterization and clinical application. Crit Rev Oral Biol Med. 1999;10(2):165–81. doi: 10.1177/10454411990100020401. Epub 2000/04/12. [DOI] [PubMed] [Google Scholar]
- 33.Johnson TE, Vogel R, Rutledge SJ, Rodan G, Schmidt A. Thiazolidinedione effects on glucocorticoid receptor-mediated gene transcription and differentiation in osteoblastic cells. Endocrinology. 1999;140(7):3245–54. doi: 10.1210/endo.140.7.6797. [DOI] [PubMed] [Google Scholar]
- 34.Hallakou S, Doare L, Foufelle F, Kergoat M, Guerre-Millo M, Berthault MF, et al. Pioglitazone induces in vivo adipocyte differentiation in the obese Zucker fa/fa rat. Diabetes. 1997;46(9):1393–9. doi: 10.2337/diab.46.9.1393. [DOI] [PubMed] [Google Scholar]
- 35.Mori Y, Murakawa Y, Okada K, Horikoshi H, Yokoyama J, Tajima N, et al. Effect of troglitazone on body fat distribution in type 2 diabetic patients. Diabetes care. 1999;22(6):908–12. doi: 10.2337/diacare.22.6.908. [DOI] [PubMed] [Google Scholar]
- 36.Kelly IE, Han TS, Walsh K, Lean ME. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes care. 1999;22(2):288–93. doi: 10.2337/diacare.22.2.288. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, et al. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. The Journal of clinical endocrinology and metabolism. 2002;87(6):2784–91. doi: 10.1210/jcem.87.6.8567. [DOI] [PubMed] [Google Scholar]
- 38.Siersbaek R, Nielsen R, Mandrup S. Transcriptional networks and chromatin remodeling controlling adipogenesis. Trends Endocrinol Metab. 2011 doi: 10.1016/j.tem.2011.10.001. Epub 2011/11/15. [DOI] [PubMed] [Google Scholar]
- 39.Crossno JT, Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. The Journal of clinical investigation. 2006;116(12):3220–8. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Werner C, Kamani CH, Gensch C, Bohm M, Laufs U. The peroxisome proliferator-activated receptor-gamma agonist pioglitazone increases number and function of endothelial progenitor cells in patients with coronary artery disease and normal glucose tolerance. Diabetes. 2007;56(10):2609–15. doi: 10.2337/db07-0069. [DOI] [PubMed] [Google Scholar]
- 41.Grey A, Beckley V, Doyle A, Fenwick S, Horne A, Gamble G, et al. Pioglitazone increases bone marrow fat in type 2 diabetes: results from a randomized controlled trial. European journal of endocrinology/European Federation of Endocrine Societies. 2012;166(6):1087–91. doi: 10.1530/EJE-11-1075. Epub 2012/03/13. [DOI] [PubMed] [Google Scholar]
- 42.Wren TA, Chung SA, Dorey FJ, Bluml S, Adams GB, Gilsanz V. Bone marrow fat is inversely related to cortical bone in young and old subjects. The Journal of clinical endocrinology and metabolism. 2011;96(3):782–6. doi: 10.1210/jc.2010-1922. Epub 2010/12/24. [DOI] [PubMed] [Google Scholar]
- 43.Di Iorgi N, Mittelman SD, Gilsanz V. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. International journal of obesity. 2008;32(12):1854–60. doi: 10.1038/ijo.2008.170. Epub 2008/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19(2):109–24. doi: 10.1615/critreveukargeneexpr.v19.i2.20. Epub 2009/04/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Botolin S, McCabe LR. Inhibition of PPARgamma prevents type I diabetic bone marrow adiposity but not bone loss. Journal of cellular physiology. 2006;209(3):967–76. doi: 10.1002/jcp.20804. [DOI] [PubMed] [Google Scholar]
- 46.Harslof T, Wamberg L, Moller L, Stodkilde-Jorgensen H, Ringgaard S, Pedersen SB, et al. Rosiglitazone decreases bone mass and bone marrow fat. The Journal of clinical endocrinology and metabolism. 2011;96(5):1541–8. doi: 10.1210/jc.2010-2077. Epub 2011/03/04. [DOI] [PubMed] [Google Scholar]
- 47.Justesen J, Mosekilde L, Holmes M, Stenderup K, Gasser J, Mullins JJ, et al. Mice deficient in 11beta-hydroxysteroid dehydrogenase type 1 lack bone marrow adipocytes, but maintain normal bone formation. Endocrinology. 2004;145(4):1916–25. doi: 10.1210/en.2003-1427. Epub 2004/01/13. [DOI] [PubMed] [Google Scholar]
- 48.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 49.Takada I, Kouzmenko AP, Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nature reviews Rheumatology. 2009;5(8):442–7. doi: 10.1038/nrrheum.2009.137. Epub 2009/07/08. [DOI] [PubMed] [Google Scholar]
- 50.Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone. 2012;50(2):540–5. doi: 10.1016/j.bone.2011.06.030. Epub 2011/07/13. [DOI] [PubMed] [Google Scholar]
- 51.Kawai M, Devlin MJ, Rosen CJ. Fat targets for skeletal health. Nature reviews Rheumatology. 2009;5(7):365–72. doi: 10.1038/nrrheum.2009.102. Epub 2009/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):3143–8. doi: 10.1073/pnas.1200797109. Epub 2012/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Differentiation of hBMSCs to osteoblasts. (A) hBMSCs were treated with osteoblast differentiation medium or αMEM (non-diff.) for 14 days and stained for mineralization with Alizarin red S (10x). (B) hBMSCs were differentiated for 7 days and stained for alkaline phosphatase. (C) RNA was isolated from control and differentiated cells and analyzed by RT-PCR for Osterix (OSX), alkaline phosphatase (ALP), and Actin (loading control).
Supplemental Figure 2: Differentiation of hBMSCs to adipocytes. (A) hBMSCs at confluency were treated with differentiation medium for 7 days and photographed under light microscopy. Lipid accumulation is clearly visible. (10x and 40X). Bottom panels; Cells were then stained with Oil-red-O to confirm lipid accumulation (10x and 40X). (B) Adipocytes were generated as in (A) and gene expression analyzed by RT-PCR for PPARγ, aP2, adiponectin (AdipoQ) and actin. (Rep. of 3 patients).
Supplemental Figure 3: Baseline and final response of study subjects treated with Pioglitazone or placebo. Pre-diabetic volunteers had (B:baseline) blood, weight, and BMD measurements taken (by DeXA) and the same parameters measured (F:final) after 26 weeks of placebo (N=3) or PIO (N=5). Blood hA1C (A) and glucose (B) were measured as well as weight (C). BMD was measured by DEXA at Femoral Neck (D) and Lumbar Spine (L1–4) (E) and results are expressed as the mean +/−SEM change from baseline for each patient. Changes were not statistically different.
Supplemental Table 1: Bone metabolism markers
Supplemental Table 2: Primers used for qRT-PCR