Abstract
Aims and Objectives:
To compare the efficacy of sedation and time taken for extubation using dexmedetomidine and fentanyl sedation in post-operative paediatric cardiac surgical patients.
Methods:
A prospective randomized double-blind study involving 60 children undergoing open heart surgery was conducted. The patients were divided into two groups, each involving 30 patients. One group received fentanyl at 1 μg/kg/h (Group A) and the other received dexmedetomidine at 0.5 μg/kg/h (Group B) for post-operative sedation with intermittent rescue fentanyl 0.5 μg/kg bolus in either group as per requirement during suctioning. The efficacy of sedation was assessed using the Ramsay sedation score, paediatric intensive care unit sedation score and the tracheal suction score. The time taken for extubation from the stoppage of infusion was noted.
Results:
Haemodynamic parameters between the two groups were comparable. All sedation scores were comparable in the fentanyl and dexmedetomidine groups. Average time (in minutes) required for extubation was 131.0 (±51.06 SD) in the dexmedetomidine group compared with 373.0 (±121.4 SD) in the fentanyl group. The difference in mean time for extubation was statistically significant.
Conclusions:
Dexmedetomidine facilitates adequate sedation for mechanical ventilation and also early extubation as compared with fentanyl.
Keywords: Dexmedetomidine, fentanyl, mechanical ventilation, sedation
INTRODUCTION
After complex congenital heart surgery, children require prolonged mechanical ventilation. Adequate sedation and analgesia are required in the post-operative period and during procedures like endotracheal suctioning. Other considerations in the post-operative paediatric patients are maintaining synchrony with mechanical ventilation, attenuation of stress response, prevention of accidental extubation and dislodgement of intravascular devices. Benzodiazepines and opioids are commonly used for post-operative sedation.[1] These drugs are associated with significant respiratory depression and delayed recovery time after discontinuation of infusion.[1]
Dexmedetomidine provides sedation and analgesia[2,3] through its action on a2 receptor in the locus coeruleus. Patients on dexmedetomidine infusion are easily arousable, have ability to follow command and co-operate while being mechanically ventilated. The similarity between natural sleep (NREM)[4] and dexmedetomidine-induced hypnosis has been speculated to maintain cognitive and immunological function in the sleep-deprived state. Despite the adequate level of sedation with dexmedetomidine, there is limited respiratory depression,[5,6] providing a wide safety margin. Hypotension and bradycardia[7] are noticed with loading dose, which can be avoided by omitting the loading dose or limiting the loading dose to 0.4 μg/kg.
In this study, efficacy of sedation, analgesia and time required for extubation during dexmedetomidine sedation were compared with that of fentanyl.
METHODS
After the approval of the institutional ethical committee, this randomized prospective double-blind study was conducted involving 60 children; informed consent was taken from their parents. The children were randomly allocated into two groups of 30 each (randomization done by lottery method).
Inclusion criteria were paediatric patients aged between 1 and 14 years,[8] scheduled for congenital heart disease surgery and in whom overnight ventilation was anticipated (e.g., patients of Tetralogy of Fallot in whom corrective surgery with right ventricular outflow tract patch was required).
Exclusion criteria were patients less than 1 year,[9] patients undergoing re-operation, surgeries done under deep hypothermia, patients with severe liver dysfunction, second and third degree heart block and patients potentially requiring ventilation for more than 24 h.
Anaesthesia was induced with intramuscular (IM) ketamine 5 mg/kg along with atropine 0.02 mg/kg or, if intravenous (IV) access was present, then IV ketamine at a dose of 2 mg/kg was administered, followed by IV midazolam 0.1 mg/kg and fentanyl 2 μg/kg. Vecuronium 0.15 mg/kg was administered to facilitate endotracheal intubation. Anaesthesia was maintained with isoflurane in O2 and air mixture and intermittent vecuronium. During sternal closure, fentanyl 1 μg/kg and midazolam 0.05 mg/kg were repeated. Immediately after shifting to the post-operative intensive care unit, either dexmedetomidine infusion at the dose of 0.5 μgm/kg/h or fentanyl 1 μg/kg/h was started.[10]
The sedation infusion of either dexmedetomidine or fentanyl was administered by another anaesthesiologist (who was not involved in the study) in the post-operative intensive care unit randomly based on lottery method. The study observer was unaware about the identity of the drug that was being administered to the patient.
Haemodynamic parameters and the sedation score [Table 1: Ramsay sedation score (RSS), paediatric intensive care unit (PICU) sedation score and tracheal suction score (TSS)][11,12] were monitored hourly. Rescue sedation of 0.5 μg/kg of fentanyl was given in both groups for agitated and distressed patients, and the frequency of rescue sedation requirement was noted. Sedation was stopped at 6 am on the following day to allow an early extubation trial. The total duration of sedation was the time from start of dexmedetomidine or fentanyl infusion to its cessation on the next day at 6 am. The time till extubation after discontinuation of the sedative drug was also noted.
Table 1.
Clinical sedation scales used for the study

Patients were considered ready to be weaned from the ventilation when they met our extubation criteria, which included the following: (a) awake state (b) stable haemodynamics (c) normothermic (d) with PaO2/FiO2 of >200 torr after corrective surgery (e) normocapnic with less than 10 cm H2O pressure support and (f) breathing spontaneously at the rate appropriate for age.
Extubation was performed and supplemental oxygen was administered via nasal cannula.
The severity of haemodynamic compromise was classified as (a) mild: when requiring no intervention, (b) moderate: when treated by reducing the dose of sedation and (c) severe: when sedation had to be discontinued or required pharmacological intervention or temporary pacing. The inotrope score was used to compare the amount of inotrope consumed in each group.[13]
Data was analysed using SPSS V14 software. Unpaired t test was used to compare demographic data (age, weight) and haemodynamic parameters (pulse, systolic blood pressure (SBP) and diastolic blood pressure (DBP). A two-tailed Fischer test was used to compare demographic data (gender distribution). Numerical variables were presented as the mean±standard deviation (SD). Sedation score being non-parametric, data was analyzed by the Mann Whitney test and presented as median with interquartile range. A P-value of<0.05 was considered significant.
Based on the pilot study results, with 10 patients in each group, at a level of significance (α) of 5% and power (1-β) of 85%, the sample size required to detect the difference of 180 min in time for extubation was 11 in each group and the sample size to detect the difference of 0.6 in RSS was 26 in each group.
Hence, sample sizes of 30 patients in each group were included.
RESULTS
The demographic data of the two groups (fentanyl group and dexmedetomidine group) were comparable. There was no statistically significant difference in type and duration of surgery.
The average duration of sedation was around 13 h in both groups. There was no statistically significant difference in the requirement of rescue sedation between the two groups as shown in Table 2.
Table 2.
Demographic data
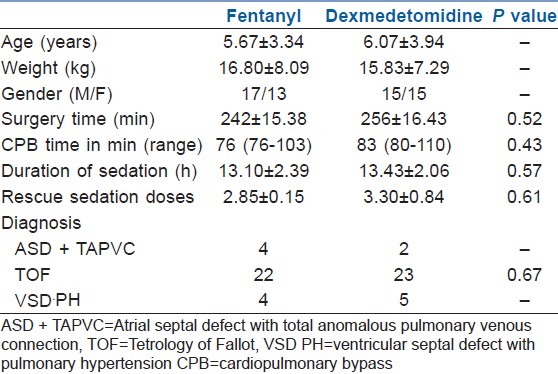
There was no statistical as well as clinically significant difference in the haemodynamic parameters, i.e. the pulse, systolic blood pressure and diastolic blood pressure, between the two groups, as seen in Figure 1. The average inotropic score in the first 24 h between the two groups was comparable with 6.37±1.72 in the fentanyl group and 7.13±2.19 in the dexmedetomidine group (P value=0.137).
Figure 1.
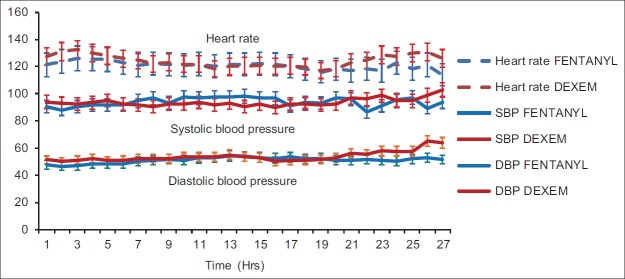
Distribution of haemodynamic parameters in the post-operative period between the two groups
The frequency of bradycardia in the fentanyl group was significantly less. In the dexmedetomidine group, even though heart rate decreased in the first few hours, it was<10 to 15% of baseline and did not require any intervention. There was no significant hypotension in either group.
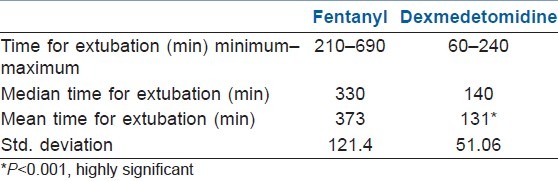
The average time for extubation from the cessation of sedative infusion was 131.0±51.06 min in the dexmedetomidine group as compared with 373.25±121.4 min in the fentanyl group, with a P value of 0.001 (statistically significant) [Table 3].
Table 3.
Descriptive analysis of extubation time between the two groups
Sedation score between the two groups were comparable with no accidental extubation because of insufficient sedation and no patient was re-intubated. The median with interquartile range of RSS, TSS and PICU sedation score in the fentanyl and dexmedetomidine groups were comparable, as shown in Figure 2.
Figure 2.
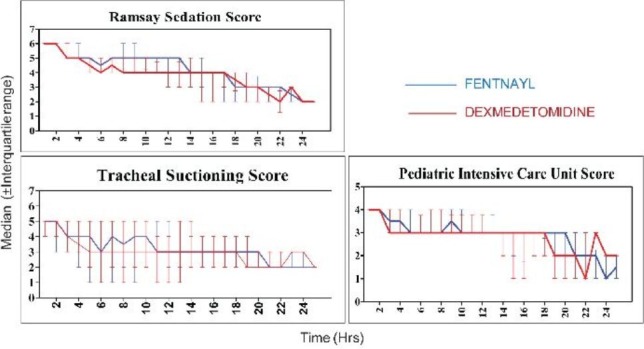
Distribution of sedation score in the post-operative period between the two groups
DISCUSSION
In post-surgical PICU, most patients require sedation and analgesia to facilitate mechanical ventilation, allay anxiety, relieve pain, encourage sleep and prevent sudden increase in systemic or pulmonary vascular resistance due to agitation and crying and to prevent inadvertent dislodgement of indwelling catheters or drainage tubes by frequent movement.
Dexmedetomidine when compared with conventional sedatives and opiates has been demonstrated to be associated with both sedative and analgesic effects, reduced delirium and agitation, minimal respiratory depression and predictable and desirable cardiovascular effects.[2,9] Central nervous system stimulation of parasympathetic outflow and inhibition of sympathetic outflow from the locus coeruleus in the brainstem plays a prominent role in sedation and anxiolysis.[4] Decreased noradrenergic output from the locus coeruleus allows increased firing of inhibitory neuron (GABA). Centrally acting α2adrenergic agonists also activate central sympatholytic effects, leading to decreased heart rate and blood pressure.[3,9] Primary analgesic effects and potentiation of opioid-induced analgesics result from the activation of the α2 adrenergic receptor in the dorsal horn of the spinal cord and inhibition of substance P release.
Chrysostosmou et al.[14] retrospectively reviewed their experience with post-operative dexmedetomidine infusion in paediatric patients undergoing cardiac surgery. Dexmedetomidine was administered in the post-operative unit at a dose of 0.1–0.5 μg/kg/h for 3–26 h, and they reported successful post-operative sedation in 93% of the patients with absent or minimal pain scores. They also reported that 87% of the patients on dexmedetomidine infusion were easily weaned and extubated.
In our study, there was a highly significant delay in extubation in the fentanyl group, the average time being 373.0±121.4 min as compared with dexmedetomidine (131.0±81.06 min). Dexmedetomidine has minimal effects on respiration and, therefore, facilitates early extubation,[9,12,14–16] whereas fentanyl, being an opioid, the respiratory depressant action is the most serious adverse effect causing delay in the extubation.[17–23]
Park et al.[24] compared hypnotic-based sedation ( propofol and/or midazolam) with analgesia-based sedation (remifentanil) in a general intensive care unit, and found that analgesia-based sedation provided more satisfactory sedation during mechanical ventilation and also allowed early extubation as compared with hypnotic-based sedation. Muellejans et al.[25] compared remifentanil versus fentanyl for analgesia-based sedation in the intensive care unit and concluded that analgesia-based sedation with fentanyl or remifentanil was comparable and helped in early extubation of the patients.
In our study, we compared the analgesic fentanyl-based sedation (most common agent used in the post-operative cardiac intensive care unit because of its haemodynamic stability) with the central α2 agonist dexmedetomidine; the RSS, PICU score and TSS were comparable between the two groups as shown in Figure 2.
Tobias et al.[12] in a prospective randomized study showed that dexmedetomidine at 0.5 μg/kg/h provided more effective sedation and decreased the rescue doses of morphine. In our study, the sedation levels in the dexmedetomidine group were adequate and comparable with the fentanyl group; the rescue doses of fentanyl required were comparable in both the groups.
Venn et al.[26] in a prospective randomized study showed that dexmedetomidine at an initial loading dose of 1 μg/kg/h over 10 min followed by maintenance dose of 0.7 μg/kg/h provided optimal sedation, but 18 of 66 patients had adverse haemodynamic effects of either hypotension or bradycardia, in 11 of 18 patients the haemodynamic effects were during bolus infusion. Bloor et al.[27] and Tobias et al.[9] in their experience with dexmedetomidine concluded that the potential adverse cardiac and haemodynamic effects of dexmedetomidine, like bradycardia, sinus arrhythmia and hypotension, occur with the initial loading doses. Petroz et al.[3] in a two-centre study of pharmacokinetic and pharmacodynamics of dexmedetomidine in children at doses of 2, 4and 6 μg/kg/h showed that heart rate and systolic blood pressure decreased modestly (≤15% and ≤25%, respectively) during the first hour after dexmedetomidine infusion. The magnitude of decrease in heart rate and blood pressure increase was proportional to the dose of dexmedetomidine increased. At lower doses, the decrease were of modest clinical interest and did not warrant corrective action.
In our study, the haemodynamic effects were minimal and did not require any intervention. This was probably due to the avoidance of an initial loading dose and also a low infusion dose of 0.5 μg/kg/h.[7]
Limitations of the study
Data regarding the cardiac output, systemic vascular resistance, pulmonary vascular resistance and pulmonary artery pressure were not collected. This probably would have given a more definitive information about cardiovascular effects of both the drugs.
The efficacy, safety and the dose in infants has to be determined and its use as a sedative agent for extended periods of time in children deserves further prospective randomized controlled trials.
CONCLUSION
Dexmedetomidine provides comparable sedation, analgesic and stable haemodynamic effects as fentanyl. With minimal depression of respiratory drive and sleep-like sedation, dexmedetomidine facilitates early extubation and is a better alternative to fentanyl in paediatric post-cardiac surgical patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Greeley WJ, Berkowitz DH, Nathan AT. Anesthesia for pediatric cardiac surgery. In: Miller RD, editor. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2009. p. 2633. [Google Scholar]
- 2.Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Paediatr Drugs. 2008;10:49–69. doi: 10.2165/00148581-200810010-00006. [DOI] [PubMed] [Google Scholar]
- 3.Petroz GC, Sikich N, James M, van Dyk H, Shafer SL, Schily M, et al. A phase I, two-center study of the pharmacokinetics and pharmacodynamics of dexmedetomidine in children. Anesthesiology. 2006;105:1098–110. doi: 10.1097/00000542-200612000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–36. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32:1322–6. doi: 10.1097/01.ccm.0000128579.84228.2a. [DOI] [PubMed] [Google Scholar]
- 6.Rosen DA, Daume JT. Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation. Anesth Analg. 2006;103:68–9. doi: 10.1213/01.ane.0000216289.52261.5e. [DOI] [PubMed] [Google Scholar]
- 7.Ickeringill M, Shehabi Y, Adamson H, Ruettimann U. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: Hemodynamic effects and efficacy. Anaesth Intensive Care. 2004;32:741–5. doi: 10.1177/0310057X0403200602. [DOI] [PubMed] [Google Scholar]
- 8.Potts AL, Anderson BJ, Warman GR, Lerman J, Diaz SM, Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care--a pooled analysis. Paediatr Anaesth. 2009;19:1119–29. doi: 10.1111/j.1460-9592.2009.03133.x. [DOI] [PubMed] [Google Scholar]
- 9.Tobias JD. Dexmedetomidine: Applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–31. doi: 10.1097/01.PCC.0000257100.31779.41. [DOI] [PubMed] [Google Scholar]
- 10.Zwass MS, Gregory GA. Pediatric and neonatal intensive care. In: Miller RD, editor. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2009. p. 2688. [Google Scholar]
- 11.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphalaxone-alphadolone. Br Med J. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: Dexmedetomidine versus midazolam. South Med J. 2004;97:451–5. doi: 10.1097/00007611-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Skippen PW, Krahn GE. Acute renal failure in children undergoing cardiopulmonary bypass. Crit Care Resusc. 2005;7:286–91. [PubMed] [Google Scholar]
- 14.Chrysostomou C, Di Filippo S, Manrique AM, Schmitt CG, Orr RA, Casta A, et al. Use of dexmedetomidine in children after cardiac and thoracic surgery. Pediatr Crit Care Med. 2006;7:126–31. doi: 10.1097/01.PCC.0000200967.76996.07. [DOI] [PubMed] [Google Scholar]
- 15.Tobias JD, Berkenbosch JW. Initial experience with dexmedetomidine in paediatric aged patients. Paediatr Anaesth. 2002;12:171–5. doi: 10.1046/j.1460-9592.2002.00805.x. [DOI] [PubMed] [Google Scholar]
- 16.Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8. doi: 10.1186/cc712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda K. Opioids. In: Miller RD, editor. Miller's Anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2009. pp. 782–4. [Google Scholar]
- 18.Bouillon T, Bruhn J, Roepcke H, Hoeft A. Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur J Anaesthesiol. 2003;20:127–33. doi: 10.1017/s0265021503000243. [DOI] [PubMed] [Google Scholar]
- 19.Duarte LT, Fernandes Mdo C, Costa VV, Saraiva RA. The incidence of postoperative respiratory depression in patients undergoing intravenous or epidural analgesia with opioids. Rev Bras Anestesiol. 2009;59:409–20. doi: 10.1590/s0034-70942009000400003. [DOI] [PubMed] [Google Scholar]
- 20.Topacoglu H, Karcioglu O, Cimrin AH, Arnold J. Respiratory arrest after low-dose fentanyl. Ann Saudi Med. 2005;25:508–10. doi: 10.5144/0256-4947.2005.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xue FS, Huang YG, Luo LK, Deng XM, Liao X, Tong SY, et al. Observation of early postoperative hypoxaemia in children undergoing elective plastic surgery. Paediatr Anaesth. 1996;6:21–8. doi: 10.1111/j.1460-9592.1996.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 22.Khalil SN, Matuszczak ME, Maposa D, Bolos ME, Lingadevaru HS, Chuang AZ. Presurgical fentanyl vs caudal block and the incidence of adverse respiratory events in children after orchidopexy. Paediatr Anaesth. 2009;19:1220–5. doi: 10.1111/j.1460-9592.2009.03164.x. [DOI] [PubMed] [Google Scholar]
- 23.Guggenberger H, Schroeder TH, Vonthein R, Dieterich HJ, Shernan SK, Eltzschig HK. Remifentanil or sufentanil for coronary surgery: Comparison of postoperative respiratory impairment. Eur J Anaesthesiol. 2006;23:832–40. doi: 10.1017/S0265021506000251. [DOI] [PubMed] [Google Scholar]
- 24.Park G, Lane M, Rogers S, Bassett P. A comparison of hypnotic and analgesic based sedation in a general intensive care unit. Br J Anaesth. 2007;98:76–82. doi: 10.1093/bja/ael320. [DOI] [PubMed] [Google Scholar]
- 25.Muellejans B, López A, Cross MH, Bonome C, Morrison L, Kirkham AJ. Remifentanil versus fentanyl for analgesia based sedation to provide patient comfort in the intensive care unit: A randomized, double-blind controlled trial. Crit Care. 2004;8:R1–11. doi: 10.1186/cc2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 27.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]


