Abstract
Background and Aim:
Patent blue vital (PBV) dye is used for varied perioperative indications, and has a potential for causing life-threatening allergic reactions. In this retrospective case series study, at a tertiary level neurosciences centre, we analysed the nature, management and outcome of adverse drug reaction to the preoperative use of PBV for marking vertebral level prior to back surgeries.
Methods:
Patients were identified from the theatre and radiology database. Data were collected from the patients’ notes retrieved from the medical records division.
Results:
Eleven of 1247 (0.88%) patients experienced adverse reactions: 6 (0.48%) patients had minor grade I reactions (urticaria, blue hives, pruritis or generalised rash), 4 (0.32%) had grade II reactions (transient hypotension/bronchospasm/laryngospasm) and grade III reaction (hypotension requiring prolonged vasopressor support) was noted in 1 (0.08%) patient. No mortality was seen. The time of onset (range 10–45 min) frequently coincided with induction of anaesthesia or prone positioning of patient. Seven (63.6%) cases were cancelled or postponed (range 2–63 days). Treatment varied independent of the grade of reaction. Allergy workup (often incomplete) was done for 6 (54%) patients.
Conclusion:
An awareness of the time of onset and infrequency of life-threatening reactions to patent blue dye may result in better management, less postponement, more complete workup and referral of these events.
Keywords: Adverse drug reactions, anaphylaxis, microdiscectomy, patent blue V dye, urticaria
INTRODUCTION
Historically well known to be allergenic, there has been resurgence in the interest in patent blue vital (PBV) dyes and the adverse effects caused by them.[1,2] Sodium or calcium salt of diethylammonium hydroxide inner salt, it is a Food and Drug Administration approved substance having ubiquitous application in textile and paper industry, agriculture and cosmetics.
It is selectively absorbed into the lymphatics, bound to albumin, and excreted into the urine and bile. Besides its former use as an antibacterial and antifungal, it has been used for demonstrating sequential lymphatic dissemination of melanoma into sentinel nodes,[3] in the identification of sentinel nodes in breast cancer,[4] intraoperatively to identify lymphatics for the purposes of lymphaticovenular anastomosis[5] and in fistulography.[6] The isomer of patent blue V, isosulphan blue is used in the United States of America for similar indications. Blue dyes used for lymphatic mapping in sentinel lymph node biopsy cause intraoperative anaphylactic reactions in up to 2.7% of patients.[7]
We present a study of all the reactions associated with the use of PBV that have occurred in a tertiary level neurosciences centre over a period of 2 years.
Our main aim was to study the incidence and severity of adverse reactions to PBV and to compare it with the incidence seen in sentinel lymph node marking- the most common indication for its usage worldwide. We also analysed our patient management practices according to the British Society for Allergy and Clinical Immunology (BSACI) guidelines for management of drug allergy[8] and The Association of Anaesthetists of Great Britain and Ireland (AAGBI) guidelines[9] for management of patient with suspected anaphylaxis during anaesthesia. The outcome of these episodes with relation to the surgical procedure, and patient satisfaction (where applicable) were also studied.
METHODS
This was a retrospective study looking at all the patients who had a reaction of any kind to PBV after back marking from 1 April 2008 to 31 March 2010.
These patients were identified from the theatre and radiology database.
The data were collected by the audit lead from the patients’ notes retrieved from the medical records division. Data collected included patients’ preoperative demographic information, American Society of Anesthesiologists (ASA) status, current drug treatment, allergies, surgical procedure planned and consultants (surgeon and anaesthetist) involved. Data were also collected on the volume and time of dye injection at back marking, type and degree of reaction, treatment offered, serum samples for tryptase sent or not, referral to allergist, alert card in patient's case sheet and the outcome of the reaction on the procedure and patient (where applicable). The categorisation of grade of reaction was according to the grading done by Barthelmes et al., i.e. grade I (urticaria, blue hives, pruritis or generalised rash), grade II (transient hypotension/bronchospasm/laryngospasm), grade III [severe hypotension requiring vasopressor support and/or change/abandoning of planned procedure and/or high dependency unit (HDU)/intensive care unit (ICU) admission] and grade IV (cardiorespiratory arrest and/or death). Data were entered into and analysed by using Microsoft Excel.
RESULTS
A total 1247 patients underwent procedures requiring back marking in the above-mentioned time period. The procedures included primary posterior laminectomy decompression of lumbar spinal cord (179), primary microdiscectomy of lumbar intervertebral disc (758) and revisional microdiscectomy of lumbar intervertebral disc (130). Others were specified primary decompression operations on the lumbar spine (98). The rest were for similar procedures at thoracic level.
Incidence and severity of adverse events
Of the total of 1247 patients with PBV exposure, 11 adverse incidents (0.88%) were reported. None of these patients had prior history of adverse drug reactions.
Of these, 0.48% were grade I reactions (urticaria, blue hives, pruritis or generalised rash), 0.32%were grade II reactions (transient hypotension/bronchospasm/ laryngospasm including airway oedema) and 0.08% of the total were grade III reactions (severe hypotension requiring vasopressor support). None of the patients had grade IV reactions.
Time of onset and duration of adverse reactions
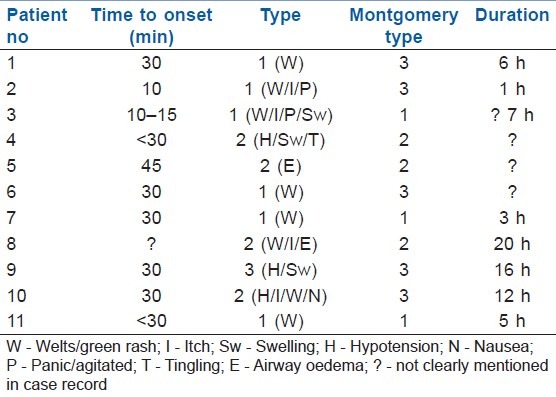
The adverse events began 10–45 min (median, 17.0 min; range 10–45 min) as first recorded after patent blue V injection. There was record of cutaneous reactions lasting from 1 h up to 20 h [Table 1]. The vasopressor support for the one patient who had a primary grade III reaction lasted for 12 h.
Table 1.
Time of onset of adverse events
Treatment of adverse events
Treatment varied in different cases: five patients were administered adrenaline; two of these had only skin reactions (grade I); one case was administered sucutaneous adrenaline. The dose of hydrocortisone varied from 100 to 400 mg with 9 (82%) patients receiving it. Inj. Chlorpheniramine was also given in all nine patients with one patient getting 10 mg orally and the rest 10 mg intravenously. Crystalloids were administered in all cases, but H2 antagonist was given for only 2 out of 11 patients (18%). Mast cell tryptase was advised and at least one serum sample for mast cell tryptase was sent in 6 (54%) cases, whereas the complete profile was sent for in only 2 (18%) cases. The reports of these tests were attached in the case files in only three cases. Allergy clinic referrals were sent for in 3 (27%) patients. Red alert card was found in records of 4 (36%) patients.
Outcome
Seven (63.6%) of the surgical procedures were postponed (2–63 days range). Of these, three were grade I reactions. Two of the cases were diagnosed in theatre. One case was cancelled post induction [Table 2].
Table 2.
Outcome of dye reaction
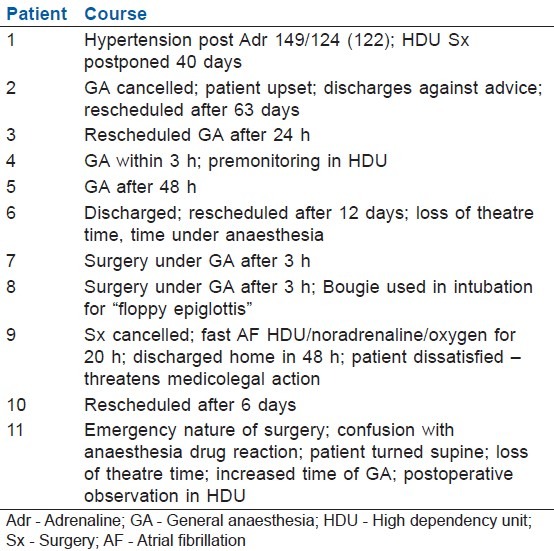
One patient recorded complaints and was discharged against advice. One patient (with grade III reaction) was unhappy enough to threaten medicolegal action.
DISCUSSION
Patent blue v is used at our centre for back marking prior to microdiscectomy and laminectomy procedures. The procedure involves injecting a small quantity (1–2 ml) of undiluted patent blue V dye into a 22-G needle passed into the interspinous ligament/ligamentum flavum for lumbar microdiscectomies at the proposed level of surgery after anteroposterior and lateral radiographs have verified the correct placement. The injection is performed by the radiologist. At operation, the presence of a clearly visible column of dye in the interspinous ligament (or ligamentum flavum), combined with prior inspection of the radiographs with the needle in situ, clearly identifies the level.[10] A dye localisation prior to anaesthesia may be expected to avoid the issues of unreliability, greater surgical exposure required and prolongation of anaesthesia time, as are the inherent problems with other techniques of vertebra level identification. This technique has been long used at this centre, with good postoperative results.[11] The practice is to send the patient for back marking prior to transfer to the theatre, sometimes even en route the preoperative area. The time taken for the patient to reach the induction room after the dye injection varies from 5 minutes to half an hour depending on factors like theatre availability. This may cause the adverse reaction to manifest just at the time of or after induction of anaesthesia, causing concern to the anaesthetist. In the incidence of an adverse reaction, the dye batch is noted and the company is informed – an attempt is made to avoid the same batch of dye if more than two such cases occur. Adverse drug reaction may occur due to various causes.[12] There have been two recent large studies involving the adverse reactions to patent blue and its isomer isosulphan blue. The paper from Britain reviews the adverse reactions of patent blue V in 7917 patients who participated in the NEW START training programme and the ALMANAC trial.[1] Among them, 72 (0.9%) patients experienced adverse reactions: 23 (0.3%) patients had minor grade I allergic skin reactions (urticaria, blue hives, pruritis, or generalised rash) and 16 (0.2%) had grade II reactions (transient hypotension/bronchospasm/laryngospasm). Severe grade III reactions (severe hypotension requiring vasopressor support and/or change of planned procedure and/or ITU admission) were noted in 5 (0.06%) patients. No mortality was recorded. The other similar large-scale retrospective analysis done in the USA for 1835 patients undergoing 1852 procedures involving isosulphan blue dye injections showed a similar incidence of adverse events in 28 patients (1.5%) of which 0.75% were classified as major or having hypotension.[2] The time of onset for adverse events was 42.2–53.9 min (median, 17.5; range 1–180 min) after isosulphan blue injection, and was significantly longer for minor reactions compared with major events (P<0.015). In yet another analysis involving 637 patients,[13] preoperative prophylaxis with chlorpheniramine, hydrocortisone, and famotidine was found to reduce the severity, but not the overall incidence, of adverse reactions to isosulphan blue dye. The incidence and severity of adverse events to patent blue V dye in our centre when used for back marking is comparable with the national and international averages. Most adverse events had appeared by 30 min and the longest reaction (cutaneous evidence of rash) lasted for 20 h. This is similar to what has been seen in other reports studying adverse reactions to patent blue V dye, and may suggest a window of safety for anaesthetising/postponing these cases without the concern of having on table adverse events or cancelling procedures. Transfer to HDU may be more frequent even for grade I because of the alarming appearance of the skin reaction to patent blue V – big blue/green hives coalescing over time to form huge welts giving rise to the name “blue urticaria.” This skin reaction is pathognomonic of PV dye reaction and was seen in all of our patients at some time in their course of hospitalisation.
Reporting of the back marking and the adverse reaction by radiologists and other doctors was poor and could be put down to not taking cutaneous manifestations “seriously.” This prevents gathering of information and data on these events which may be important for the centre given the unlicensed use of the dye for any medical procedure worldwide.
Clinical management of adverse events was generally in accordance to the AAGBI and BSACI guidelines except for the arguably unwarranted use of adrenaline in pure grade I reactions and improper dosage/route of administration of hydrocortisone in a few cases. There was a poor compliance in sending blood for mast cell tryptase and referring patient to allergy clinics; this may have resulted from a lack of awareness of the guidelines or the feeling that the reaction was "mild" and therefore did not warrant follow-up. In a few occasions, the patients were unable to attend referral centres and allergy clinics as they were not present close to their residence.
However, as these patients are mostly young, they have a lifelong risk of re-exposure to these dyes in medical or pharmaceutical products or exposure to general anaesthesia for different procedures. Repeat exposures are in general of greater severity and may even be life threatening. Our rate of postponing cases appears to be higher than the others, mainly because of the elective nature of surgery, proximity to induction and confusion with anaesthesia drug reaction.
Of the four cases which underwent surgery in spite of the reaction, one was an emergency surgery for cauda equina syndrome and two were grade II and ended uneventfully. This and data from the lymphangiography studies would show that the risks of continuing with the surgical procedure would be a reasonably safe option in most grade I and II reactions.
Finally, the two cases which were diagnosed in the theatre were both grade I reactions. One was cancelled due to concern on the part of anaesthetist about continuing the use of the anaesthetic agent and possibly increasing the severity of reaction. The second case proceeded with delay, invasive monitoring and ultimate shifting to HDU mainly due to the emergency nature of the surgery.
CONCLUSION
Patent blue v dye, which is now being used more often in medicine for varied indications, has a potential to cause adverse reactions.
It is of importance to the anaesthetist to recognise and discern this particular reaction from others. The severity is more often low. The characteristic blue/green wheals must be looked for. All patients with adverse drug reaction which may or may not confound with anaesthesia should have serial samples sent for mast call tryptase levels and referred for skin prick/intradermal tests.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Barthelmes L, Goyal A, Newcombe RG, Macneill F, Mansel RE NEW START and ALMANAC groups. Adverse reactions to patent blue dye-The NEW START and ALMANAC experience. Eur J Surg Oncol. 2010;36:399–403. doi: 10.1016/j.ejso.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Daley MD, Norman PH, Leak JA, Nguyen DT, Bui TP, Kowalski AM, et al. Adverse events associated with the intraoperative injection of isosulfan blue. J Clin Anesth. 2004;16:332–41. doi: 10.1016/j.jclinane.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Morton DL, Wen DR, Wong JH, Economou JS, Cagle LA, Storm FK, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–9. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 4.Karakousis CP, Velez AF, Spellman JE, Scarozza J. The technique of sentinel node biopsy. Eur J Surg Oncol. 1996;22:271–5. doi: 10.1016/s0748-7983(96)80017-5. [DOI] [PubMed] [Google Scholar]
- 5.Yap YL, Lim J, Shim TW, Naidu S, Ong WC, Lim TC. Patent Blue Dye in Lymphaticovenular Anastomosis. Ann Acad Med Singapore. 2009;38:704–6. [PubMed] [Google Scholar]
- 6.Ebo DG, Wets RD, Spiessens TK, Bridts CH, Stevens WJ. Flow assisted diagnosis of anaphylaxis to patent blue. Allergy. 2005;60:703–4. doi: 10.1111/j.1398-9995.2005.00730.x. [DOI] [PubMed] [Google Scholar]
- 7.Scherer K, Studer W, Figueiredo V, Bircher AJ. Anaphylaxis to isosulfan blue and cross-reactivity to patent blue V: Case report and review of the nomenclature of vital blue dyes. Ann Allergy Asthma Immunol. 2006;96:497–500. doi: 10.1016/S1081-1206(10)60921-0. [DOI] [PubMed] [Google Scholar]
- 8.Harper NJ, Dixon T, Dugué P, Edgar DM, Fay A, Gooi HC, et al. Association of Anaesthetists of Great Britain and Ireland.Suspected anaphylactic reactions associated with anaesthesia. Anaesthesia. 2009;64:199–211. doi: 10.1111/j.1365-2044.2008.05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirakian R, Ewan PW, Durham SR, Youlten LJ, Dugue P, Friedmann PS, et al. BSACI guidelines for the management of drug allergy. Clin Exp Allergy. 2009;39:43–61. doi: 10.1111/j.1365-2222.2008.03155.x. [DOI] [PubMed] [Google Scholar]
- 10.Redfern RM, Smith ET. A method of identification of vertebral level. Ann R Coll Surg Eng. 1986;68:163. [PMC free article] [PubMed] [Google Scholar]
- 11.Findlay GF, Hall BI, Musa BS, Oliveira MD, Fear SC. A 10-year follow-up of the outcome of lumbar microdiscectomy. Spine (Phila Pa 1976) 1998;23:1168–71. doi: 10.1097/00007632-199805150-00019. [DOI] [PubMed] [Google Scholar]
- 12.Riedl MA, Casillas AM. Adverse drug reactions: Types and treatment options. Am Fam Physician. 2003;68:1781–91. [PubMed] [Google Scholar]
- 13.Raut CP, Hunt KK, Akins JS, Daley MD, Hunt KK, Akins J, et al. Incidence of anaphylactoid reactions to Isosulfan Blue Dye during breast carcinoma lymphatic mapping in patients treated with preoperative prophylaxis. Cancer. 2005;104:692–9. doi: 10.1002/cncr.21226. [DOI] [PubMed] [Google Scholar]


