Abstract
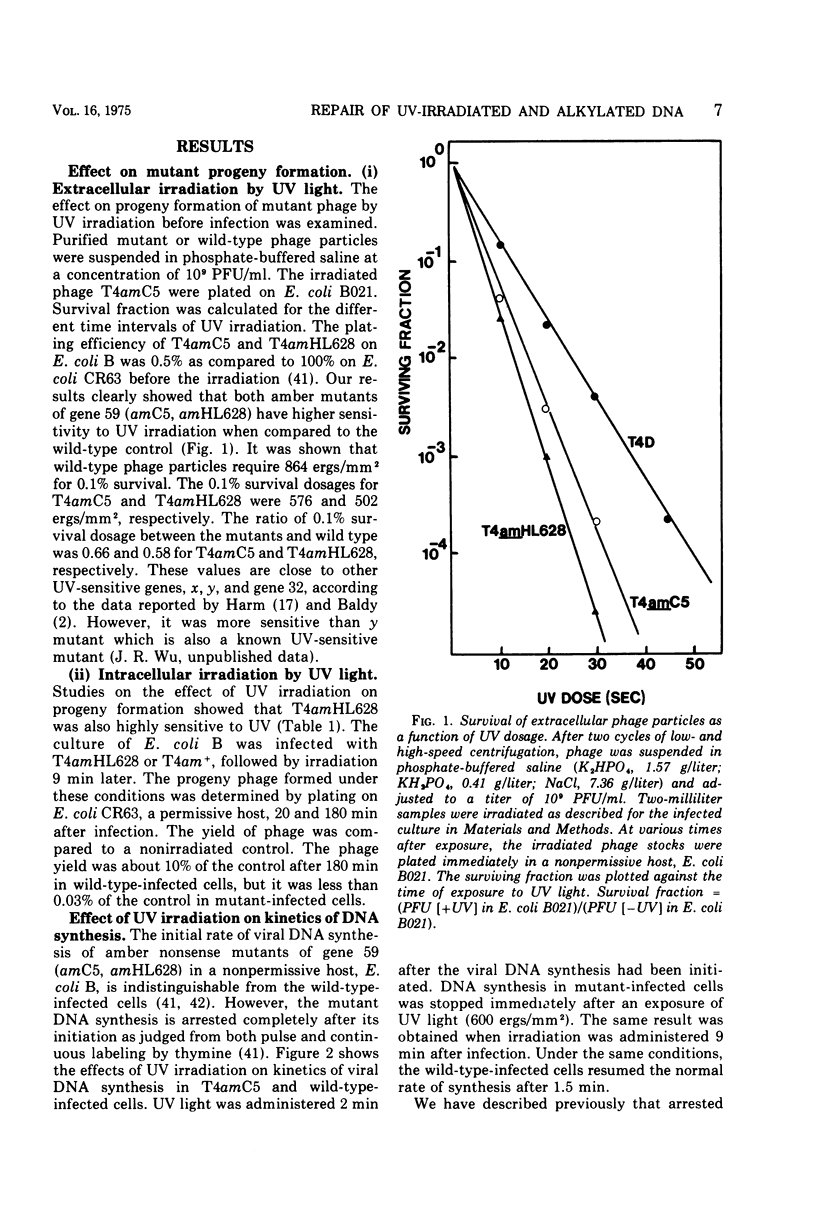
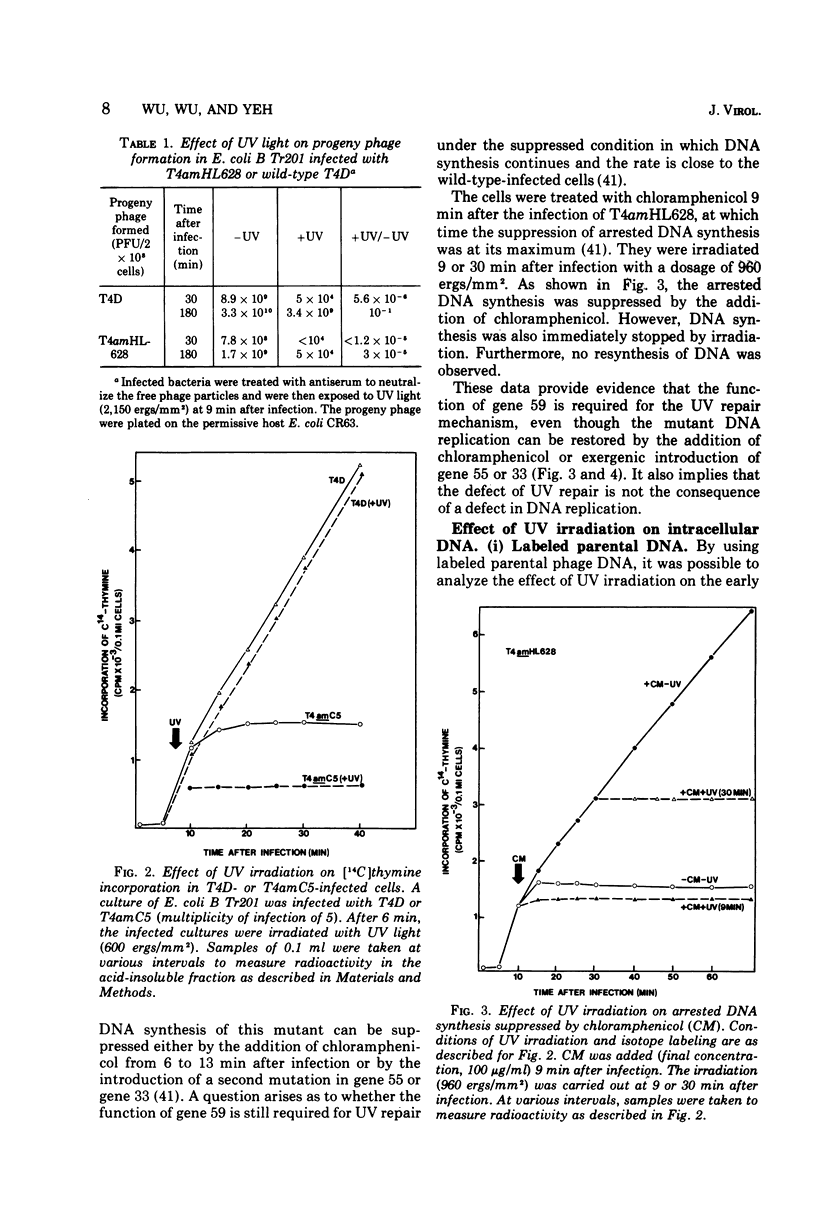
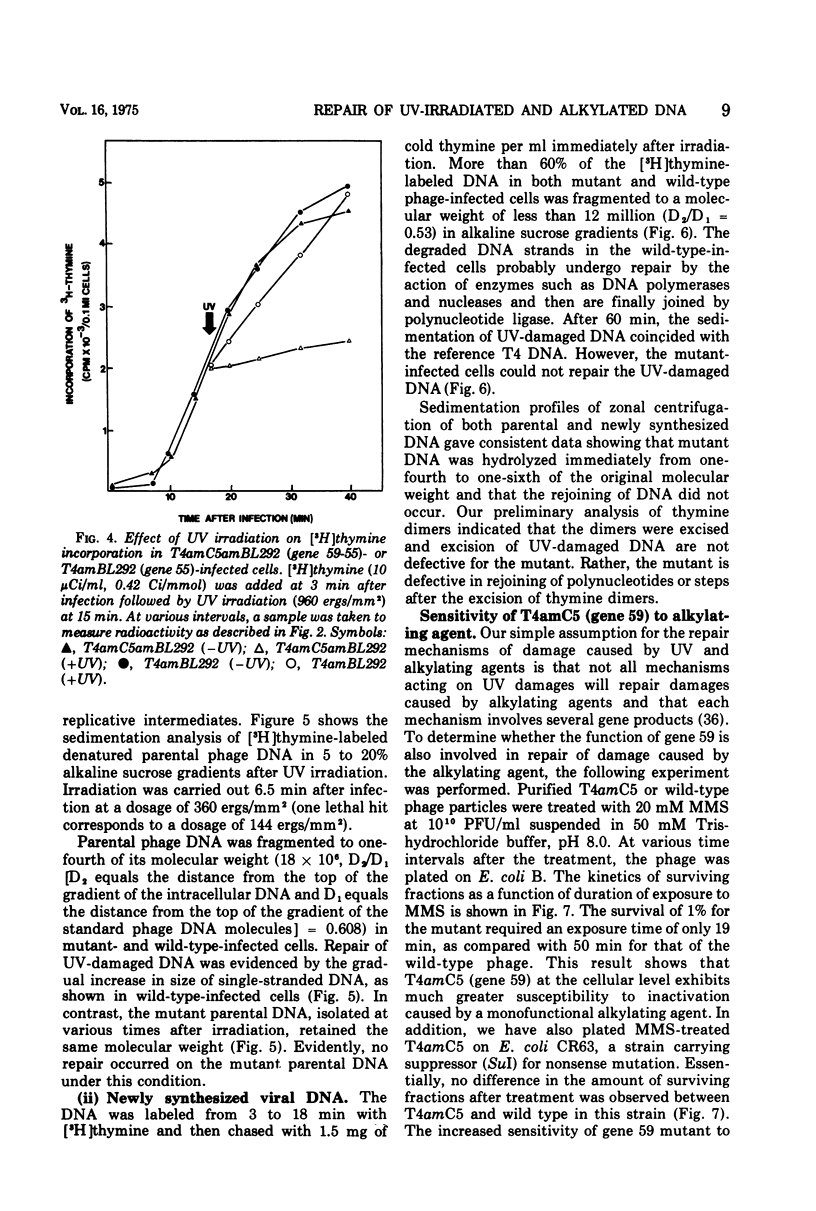
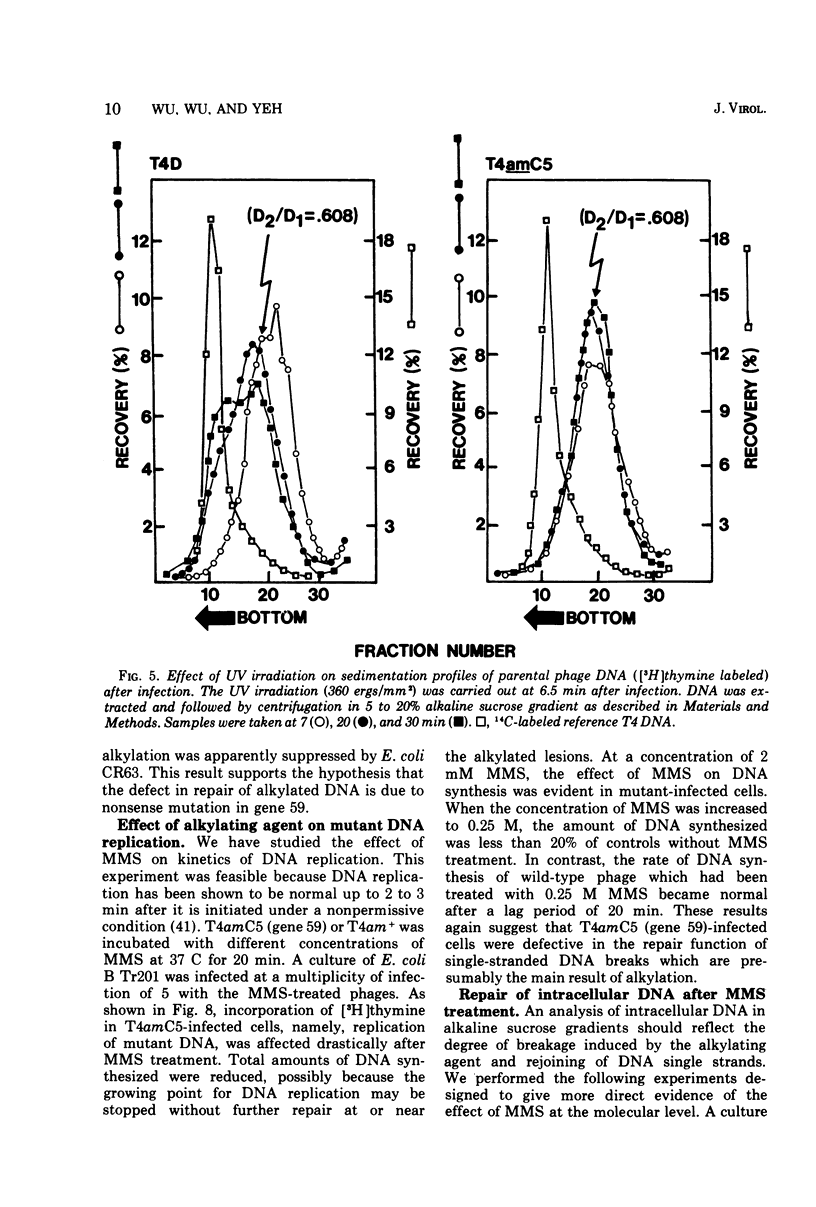
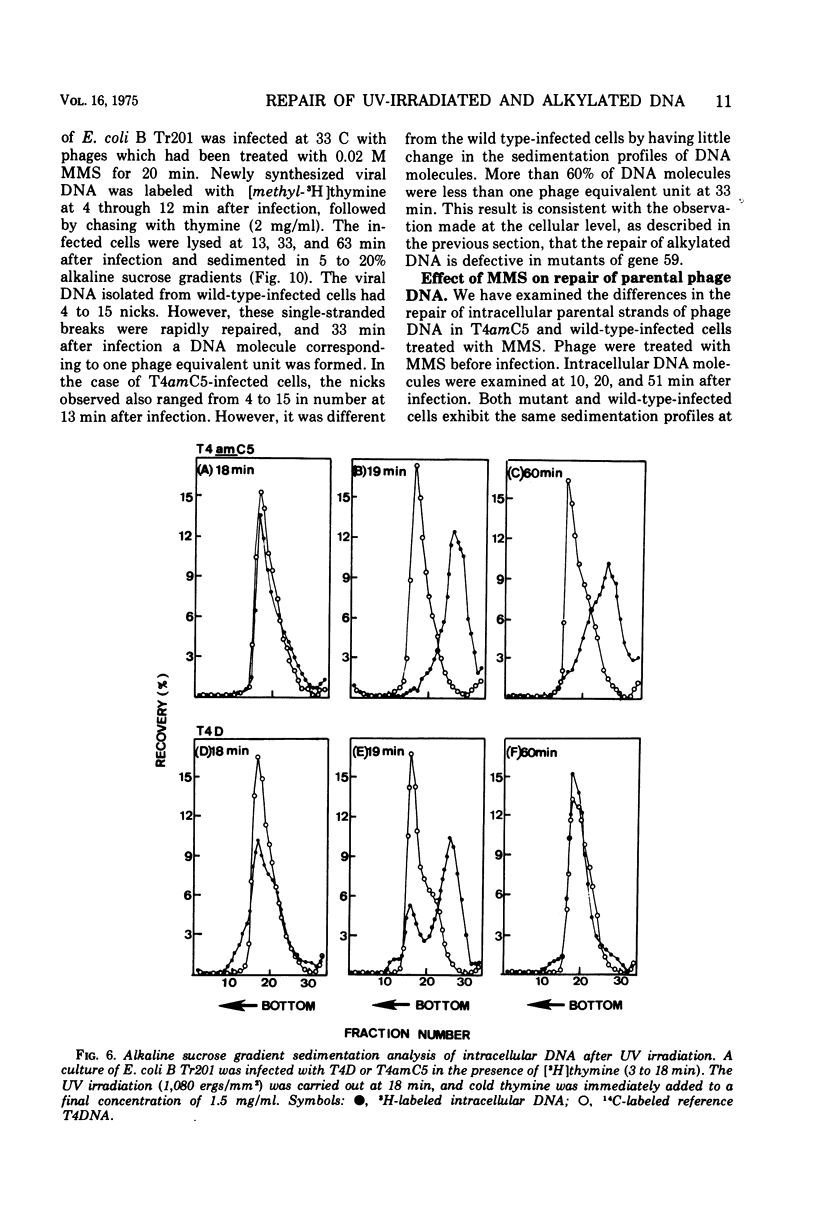
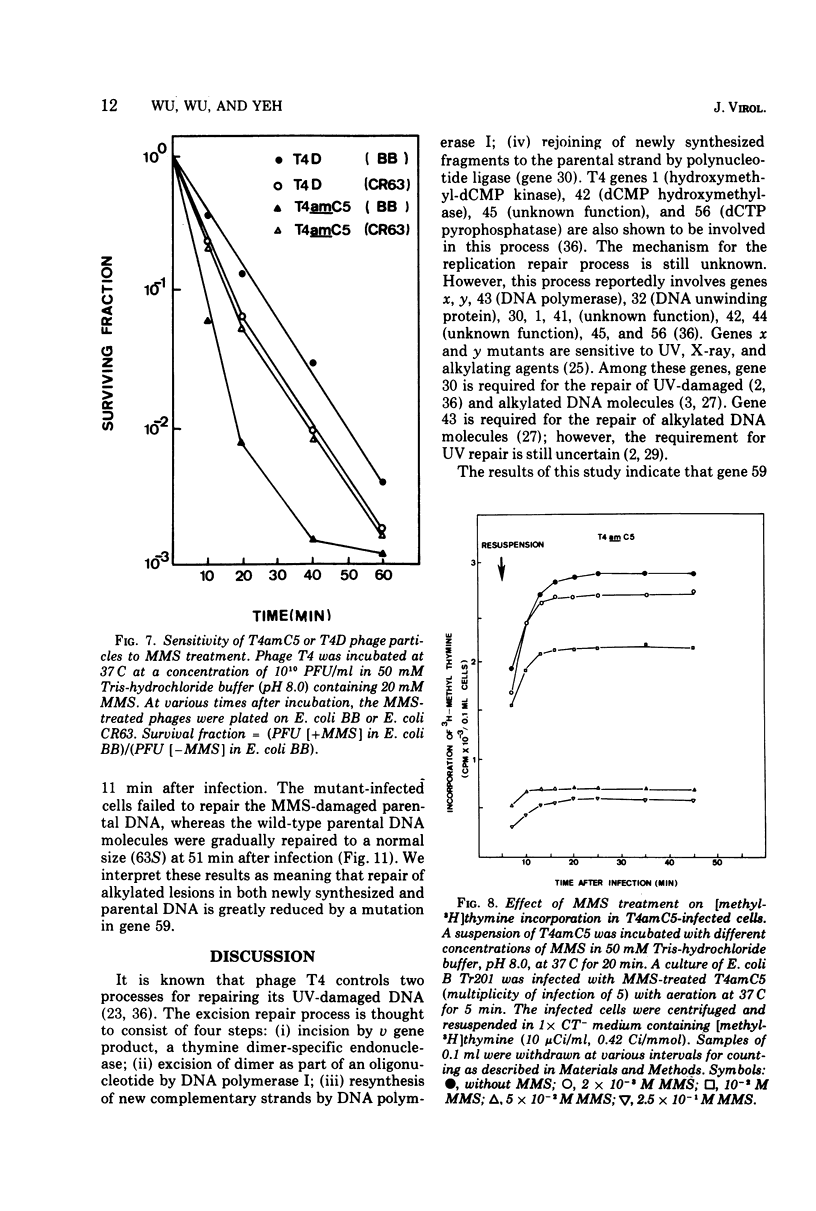
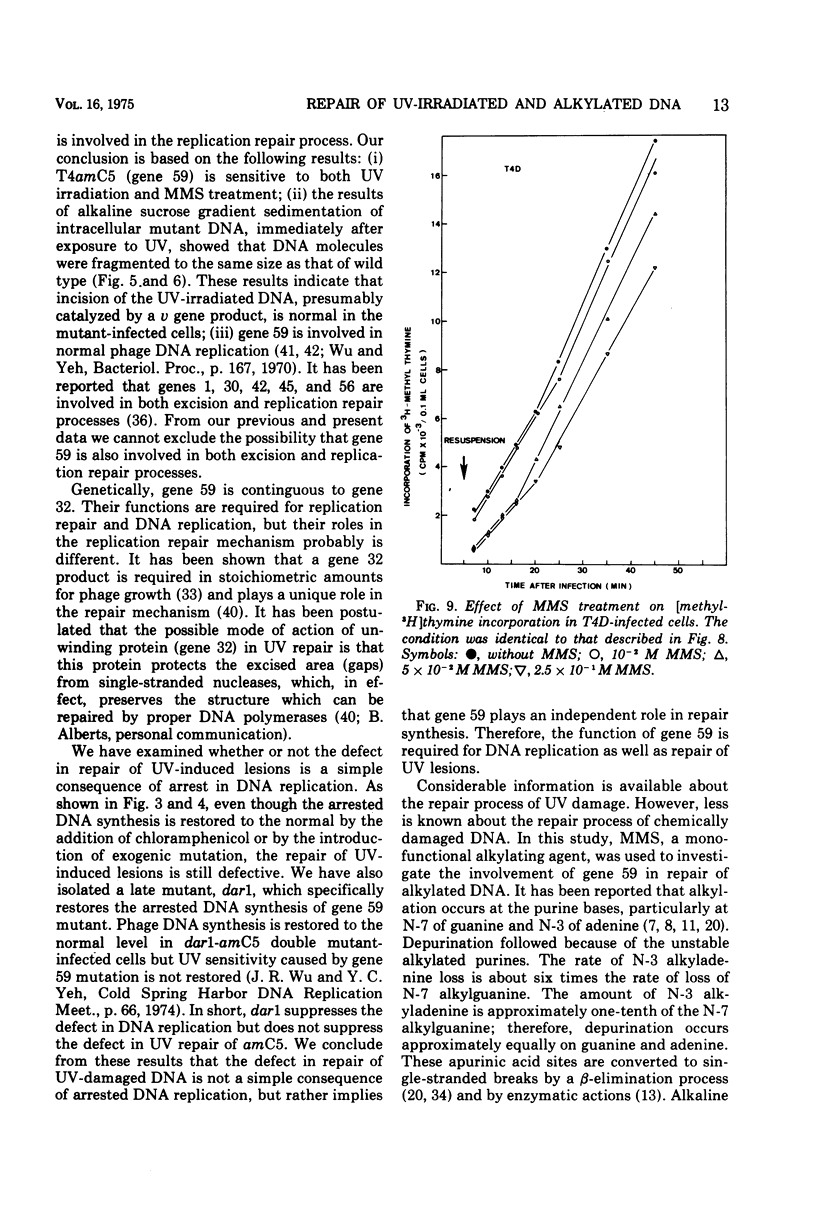
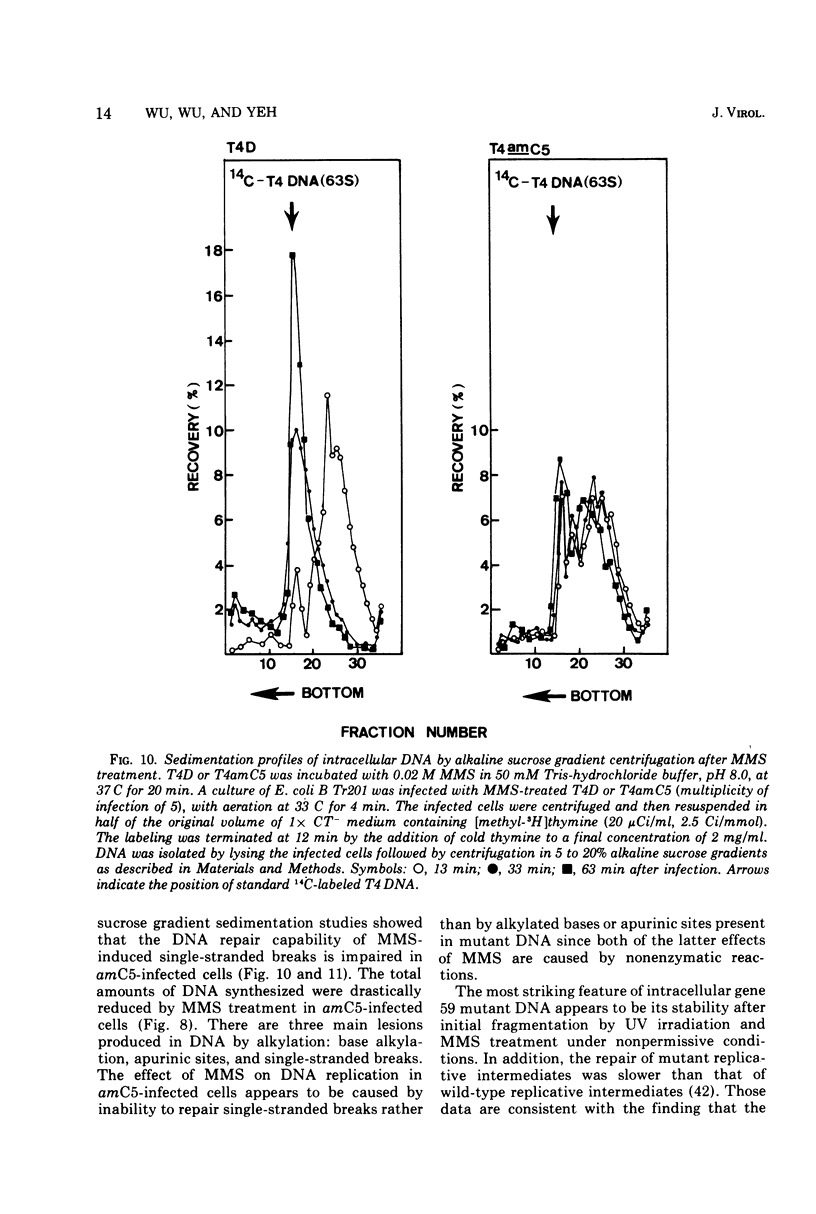
Nonsense mutants in gene 59 (amC5, amHL628) were used to study the role of this gene in the repair of UV-damaged and alkylated DNA of bacteriophage T4 in vivo. The higher sensitivity to UV irradiation and alkylation of gene 59 mutants after exposure to these agents was established by a comparison of the survival fractions with wild type. Zonal centrifugal analysis of both parental and nascent mutant intracellular DNA molecules after UV irradiation showed that immediately after exposure the size of single-stranded DNA fragments was the same as the wild-type intracellular DNA. However, the capability of rejoining fragmented intracellular DNA was greatly reduced in the mutant. In contrast, the wild-type-infected cells under the same condition resumed DNA replication and repaired its DNA to normal size. Methyl methanesulfonate induced more randomly fragmented intracellular DNA, when compared to UV irradiation. The rate of rejoining under these conditions as judged from their sedimentation profiles was also greatly reduced in mutant-infected cells. Further evidence is presented that UV repair is not a simple consequence of arrested DNA replication, which is a phenotype of the mutant when infected in a nonpermissive host, Escherichia coli B (su minus), but rather that the DNA repair function of gene 59 is independent of the replication function. These and other data presented indicate that a product(s) of gene 59 is essential for both repair of UV lesions and repair of alkylation damage of DNA in vivo. It is suggested that gene 59 may have two functions during viral development: DNA replication and replication repair of DNA molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldy M. W., Strom B., Bernstein H. Repair of alkylated bacteriophage T4 deoxyribonucleic acid by a mechanism involving polynucleotide ligase. J Virol. 1971 Mar;7(3):407–408. doi: 10.1128/jvi.7.3.407-408.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W. The UV sensitivity of some early-function temperature-sensitive mutants of phage T4. Virology. 1970 Feb;40(2):272–287. doi: 10.1016/0042-6822(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- Bridges B. A. Evidence for a further dark repair process in bacteria. Nat New Biol. 1972 Nov 8;240(97):52–53. doi: 10.1038/newbio240052a0. [DOI] [PubMed] [Google Scholar]
- Brookes P., Lawley P. D. The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J. 1961 Sep;80(3):496–503. doi: 10.1042/bj0800496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., Goldthwait D. A. Endonuclease II of E. coli. I. Isolation and purification. Proc Natl Acad Sci U S A. 1969 Mar;62(3):934–940. doi: 10.1073/pnas.62.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Endonucleolytic cleavage of UV-irradiated DNA controlled by the V+ gene in phage T4. Biochem Biophys Res Commun. 1969 Nov 6;37(4):646–651. doi: 10.1016/0006-291x(69)90859-6. [DOI] [PubMed] [Google Scholar]
- HARM W. Mutants of phage T4 with increased sensitivity to ultraviolet. Virology. 1963 Jan;19:66–71. doi: 10.1016/0042-6822(63)90025-4. [DOI] [PubMed] [Google Scholar]
- Kim J. S., Davidson N. Electron microscope heteroduplex study of sequence relations of T2, T4, and T6 bacteriophage DNAs. Virology. 1974 Jan;57(1):93–111. doi: 10.1016/0042-6822(74)90111-1. [DOI] [PubMed] [Google Scholar]
- Lawley P. D. Effects of some chemical mutagens and carcinogens on nucleic acids. Prog Nucleic Acid Res Mol Biol. 1966;5:89–131. doi: 10.1016/s0079-6603(08)60232-9. [DOI] [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman P. H., Bootsma D. Repair mechanisms in mamalian cells. Mutat Res. 1974 Apr;23(1):147–150. doi: 10.1016/0027-5107(74)90168-7. [DOI] [PubMed] [Google Scholar]
- Maynard-Smith S., Symonds N. Involvement of bacteriophage T4 genes in radiation repair. J Mol Biol. 1973 Feb 15;74(1):33–44. doi: 10.1016/0022-2836(73)90352-5. [DOI] [PubMed] [Google Scholar]
- Miller R. C., Kozinski A. W., Litwin S. Molecular Recombination in T4 Bacteriophage Deoxyribonucleic Acid: III. Formation of Long Single Strands During Recombination. J Virol. 1970 Mar;5(3):368–380. doi: 10.1128/jvi.5.3.368-380.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortelmans K., Friedberg E. C. Deoxyribonucleic acid repair in bacteriophage T4: observations on the roles of the x and v genes and of host factors. J Virol. 1972 Oct;10(4):730–736. doi: 10.1128/jvi.10.4.730-736.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Molecular mechanisms in genetic recombination. Annu Rev Genet. 1973;7:87–111. doi: 10.1146/annurev.ge.07.120173.000511. [DOI] [PubMed] [Google Scholar]
- Ray U., Bartenstein L., Drake J. W. Inactivation of bacteriophage T4 by ethyl methanesulfonate: influence of host and viral genotypes. J Virol. 1972 Mar;9(3):440–447. doi: 10.1128/jvi.9.3.440-447.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Schnitzlein C. F., Albrecht I., Drake J. W. Is bacteriophage T4 DNA polymerase involved in the repair of ultraviolet damage? Virology. 1974 Jun;59(2):580–583. doi: 10.1016/0042-6822(74)90469-3. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Setlow R. B. The photochemistry, photobiology, and repair of polynucleotides. Prog Nucleic Acid Res Mol Biol. 1968;8:257–295. doi: 10.1016/s0079-6603(08)60548-6. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Symonds N. The unexpected location of a gene conferring abnormal radiation sensitivity on phage T4. Nature. 1973 Feb 9;241(5389):395–396. doi: 10.1038/241395a0. [DOI] [PubMed] [Google Scholar]
- Snustad D. P. Dominance interactions in Escherichia coli cells mixedly infected with bacteriophage T4D wild-type and amber mutants and their possible implications as to type of gene-product function: catalytic vs. stoichiometric. Virology. 1968 Aug;35(4):550–563. doi: 10.1016/0042-6822(68)90285-7. [DOI] [PubMed] [Google Scholar]
- Strauss B., Coyle M., Robbins M. Alkylation damage and its repair. Cold Spring Harb Symp Quant Biol. 1968;33:277–287. doi: 10.1101/sqb.1968.033.01.032. [DOI] [PubMed] [Google Scholar]
- Symonds N., Heindl H., White P. Radiation sensitive mutants of phage T4. A comparative study. Mol Gen Genet. 1973 Feb 2;120(3):253–259. doi: 10.1007/BF00267156. [DOI] [PubMed] [Google Scholar]
- Taketo A., Yasuda S., Sekiguchi M. Initial step of excision repair in Escherichia coli: replacement of defective function of uvr mutants by T4 endonuclease V. J Mol Biol. 1972 Sep 14;70(1):1–14. doi: 10.1016/0022-2836(72)90160-x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wu J. R., Yeh Y. C. Requirement of a functional gene 32 product of bacteriophage T4 in UV, repair. J Virol. 1973 Oct;12(4):758–765. doi: 10.1128/jvi.12.4.758-765.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Ma F. J., Yeh Y. C. Suppression of DNA-arrested synthesis in mutants defective in gene 59 of bacteriophage T4. Virology. 1972 Jan;47(1):147–156. doi: 10.1016/0042-6822(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Wu R., Yeh Y. C. DNA arrested mutants of gene 59 of bacteriophage T4. II. Replicative intermediates. Virology. 1974 May;59(1):108–122. doi: 10.1016/0042-6822(74)90209-8. [DOI] [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. Mechanism of repair of DNA in bacteriophage. II. Inability of ultraviolet-sensitive strains of bacteriophage in inducing an enzyme activity to excise pyrimidine dimers. J Mol Biol. 1970 Jan 28;47(2):243–255. doi: 10.1016/0022-2836(70)90343-8. [DOI] [PubMed] [Google Scholar]



