Abstract
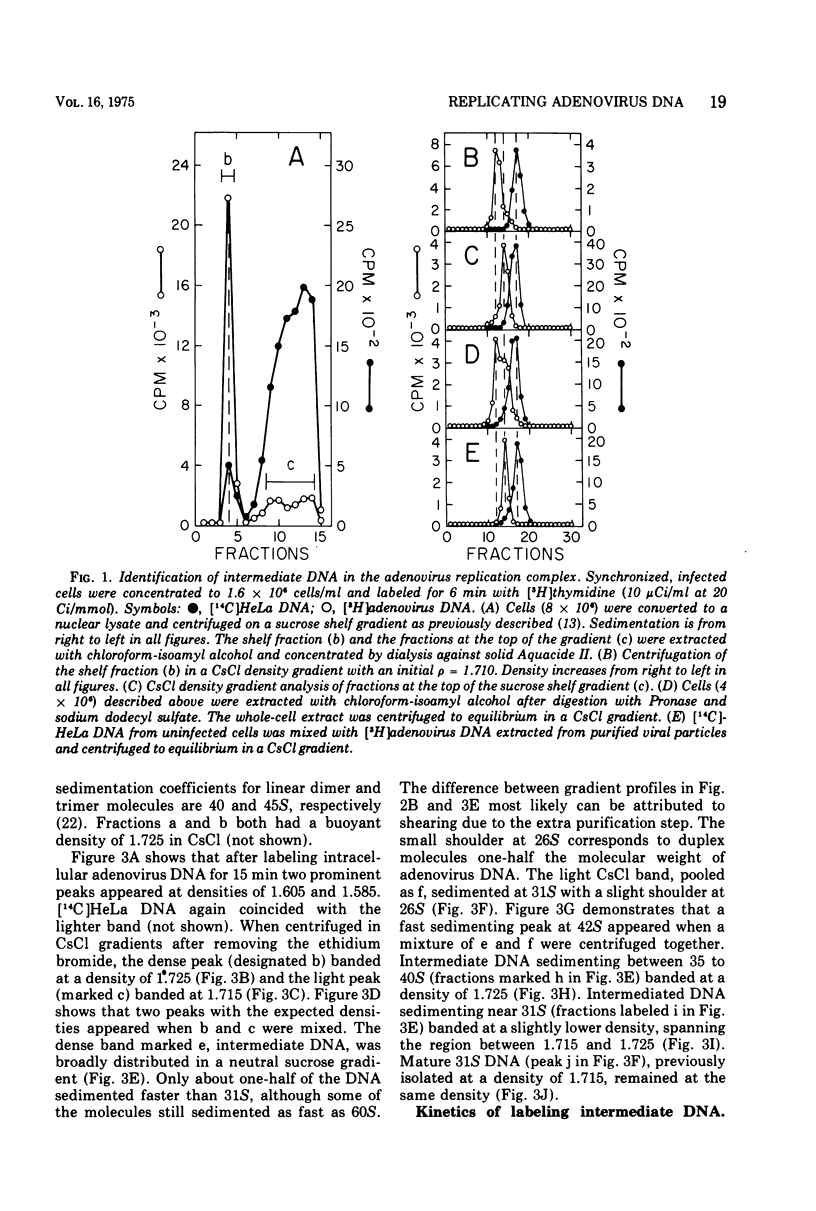
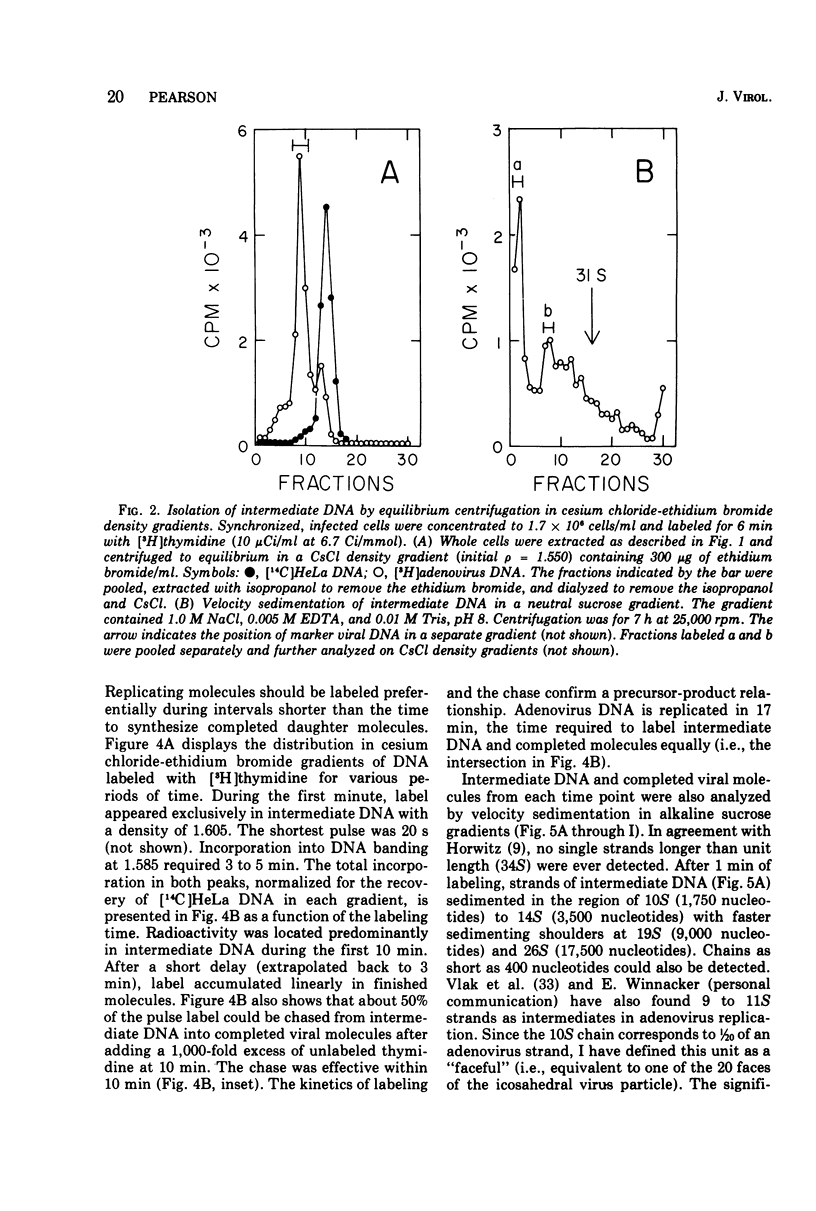
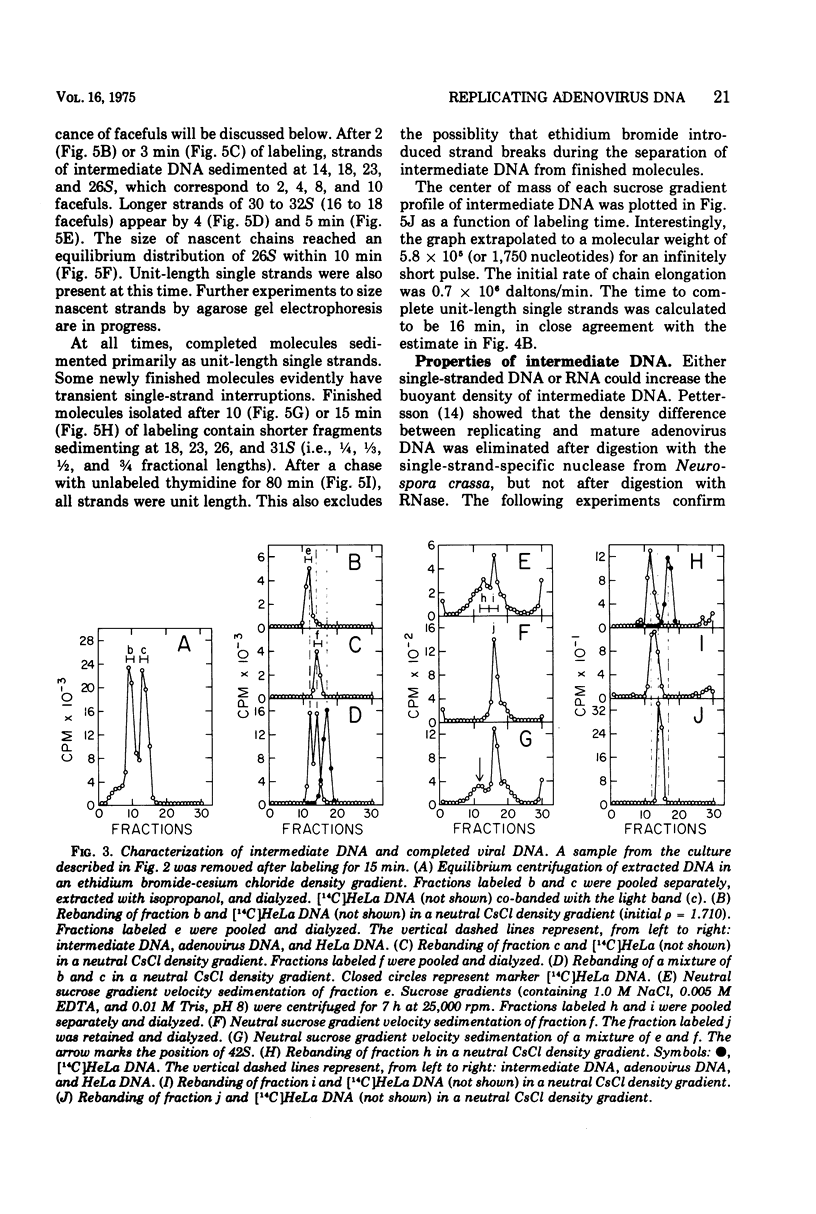
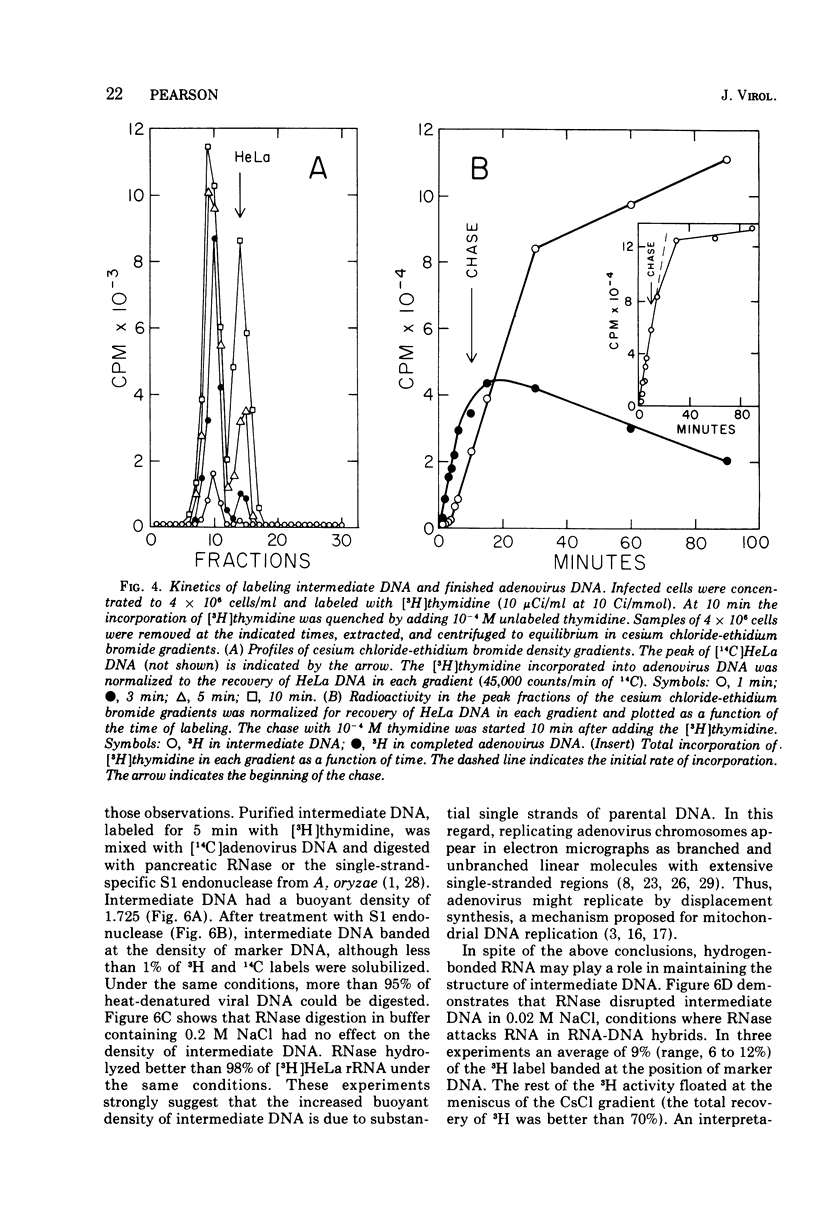
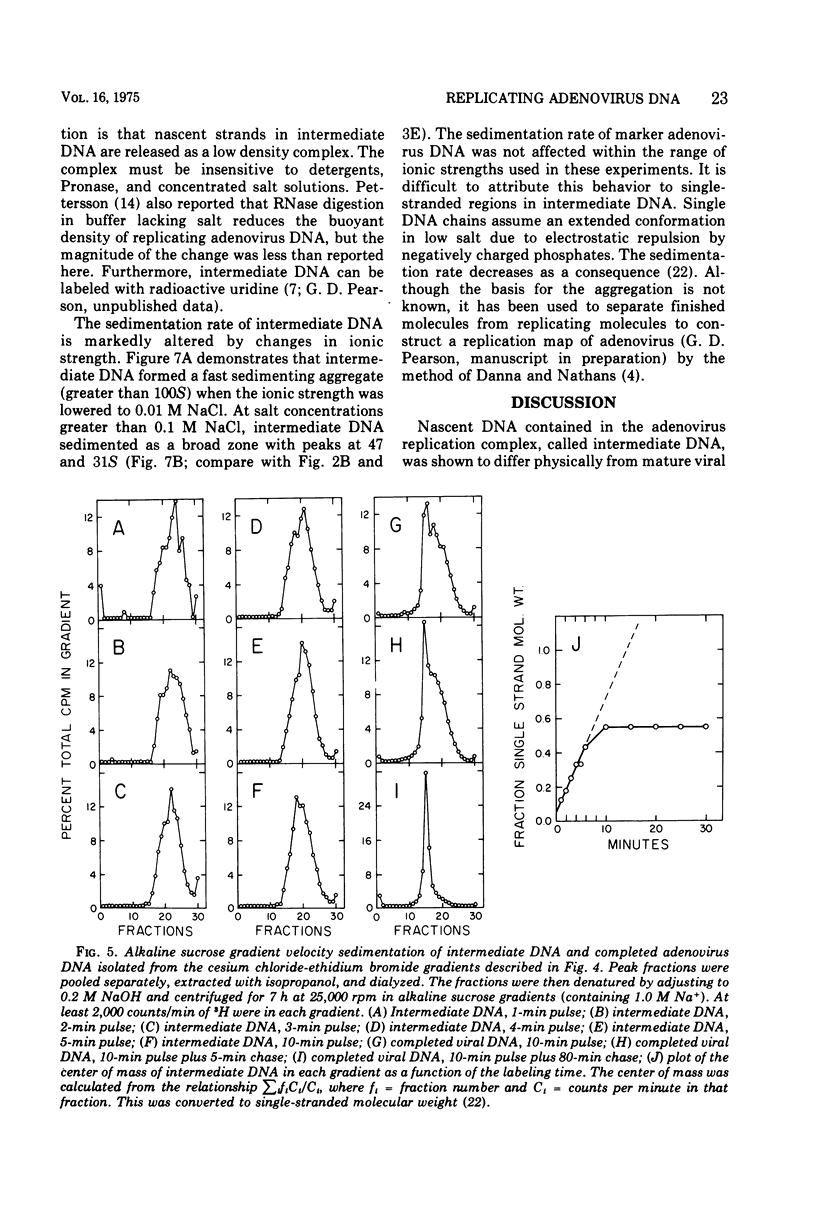
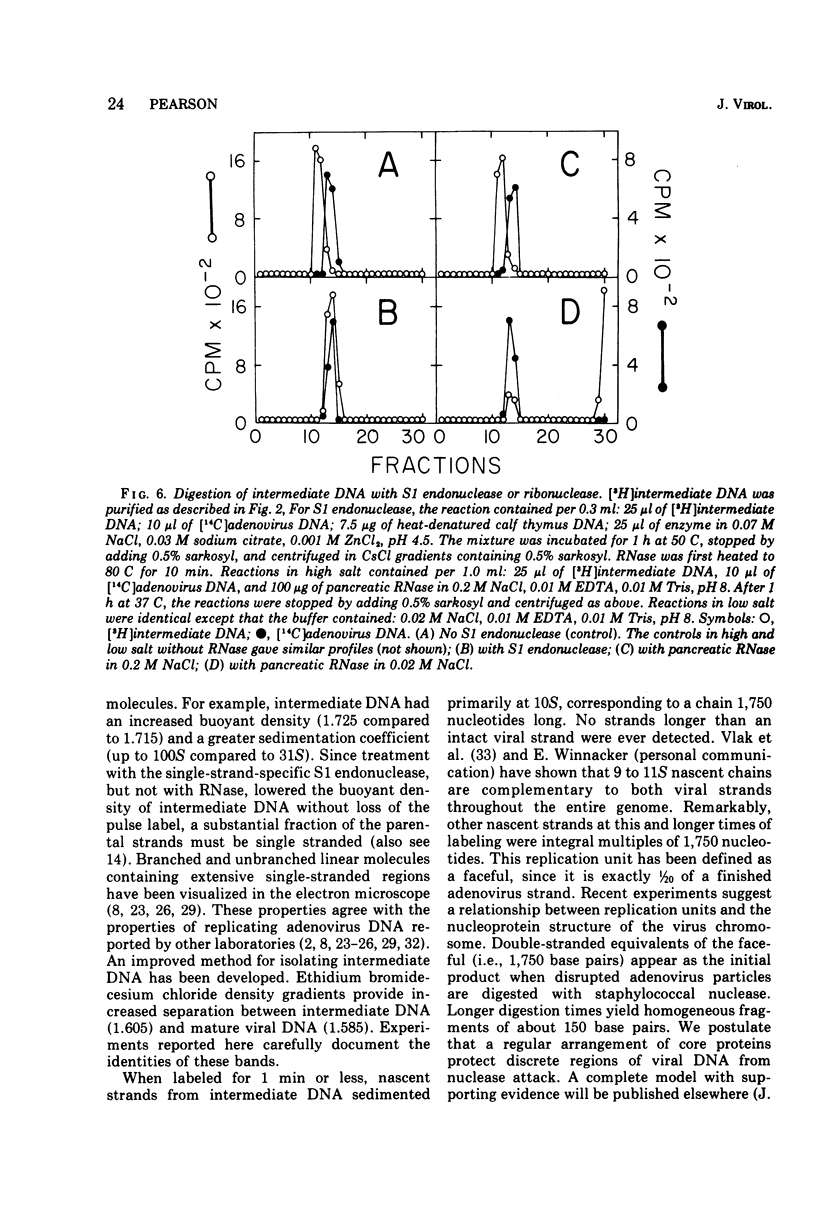
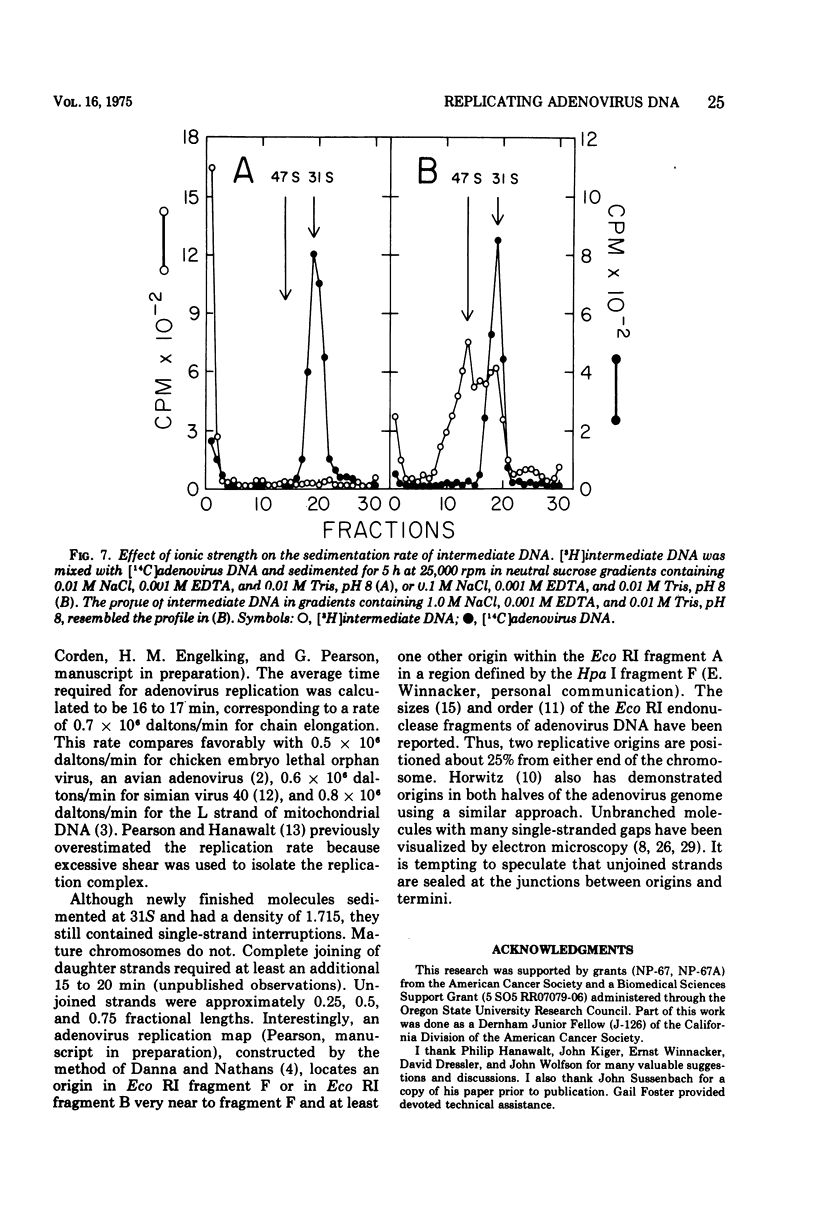
Replicating chromosomes, called intermediate DNA, have been extracted from the adenovirus replication complex. Compared to mature molecules, intermediate DNA had a greater buoyant density in CsCl gradients and ethidium bromide-cesium chloride gradients. Digestion of intermediate DNA with S1 endonuclease, but not with RNase, abolished the difference in densities. These properties suggest that replicating molecules contain extensive regions of parental single strands. Although intermediate DNA sedimented faster than marker viral DNA in neutral sucrose gradients, single strands longer than unit length could not be detected after alkaline denaturation. Integral size classes of nascent chains in intermediate DNA suggest a relationship between units of replication and the nucleoprotein structure of the virus chromosome. Adenovirus DNA was replicated at a rate of 0.7 x 10-6 daltons/min. Although newly synthesized molecules had the same sedimentation coefficient and buoyant density as mature chromosomes, they still contained single-strand interruptions. Complete joining of daughter strands required an additional 15 to 20 min.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Bellett A. J., Younghusband H. B. Replication of the DNA of chick embryo lethal orphan virus. J Mol Biol. 1972 Dec 30;72(3):691–709. doi: 10.1016/0022-2836(72)90185-4. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: asynchronous replication of strands, segregation of circular daughter molecules, aspects of topology and turnover of an initiation sequence. J Mol Biol. 1974 Jul 15;86(4):801–824. doi: 10.1016/0022-2836(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Hirsch-Kauffmann M. Intracellular forms of adenovirus deoxyribonucleic acid. I. Evidence for a deoxyribonucleic acid-protein complex in baby hamster kidney cells infected with adenovirus type 12. J Virol. 1972 Feb;9(2):297–308. doi: 10.1128/jvi.9.2.297-308.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W., Lundholm U., Rensing U., Philipson L. Intracellular forms of Adenovirus DNA. II. Isolation in dye-buoyant density gradients of a DNA-RNA complex from KB cells infected with Adenovirus type 2. J Virol. 1973 Oct;12(4):793–807. doi: 10.1128/jvi.12.4.793-807.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Ellens D. J., Sussenbach J. S., Jansz H. S. Studies on the mechanism of replication of adenovirus DNA. III. Electron microscopy of replicating DNA. Virology. 1974 Oct;61(2):427–442. doi: 10.1016/0042-6822(74)90279-7. [DOI] [PubMed] [Google Scholar]
- Horwitz M. S. Intermediates in the synthesis of type 2 adenovirus deoxyribonucleic acid. J Virol. 1971 Nov;8(5):675–683. doi: 10.1128/jvi.8.5.675-683.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S. Location of the origin of DNA replication in adenovirus Type 2. J Virol. 1974 May;13(5):1046–1054. doi: 10.1128/jvi.13.5.1046-1054.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Sharp P. A., Delius H., Pettersson U. Specific fragmentation of DNA of adenovirus serotypes 3, 5, 7, and 12, and adeno-simian virus 40 hybrid virus Ad2+ND1 by restriction endonuclease R.EcoRI. J Virol. 1974 Jul;14(1):68–77. doi: 10.1128/jvi.14.1.68-77.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Specific origin in SV40 DNA replication. Nat New Biol. 1972 Apr 19;236(68):200–202. doi: 10.1038/newbio236200a0. [DOI] [PubMed] [Google Scholar]
- Pearson G. D., Hanawalt P. C. Isolation of DNA replication complexes from uninfected and adenovirus-infected HeLa cells. J Mol Biol. 1971 Nov 28;62(1):65–80. doi: 10.1016/0022-2836(71)90131-8. [DOI] [PubMed] [Google Scholar]
- Pettersson U. Letter: Some unusual properties of replicating adenovirus type 2 DNA. J Mol Biol. 1973 Dec 25;81(4):521–527. doi: 10.1016/0022-2836(73)90521-4. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Pulse-labeled components in the replication of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Jun 25;248(12):4512–4514. [PubMed] [Google Scholar]
- Robberson D. L., Clayton D. A. Replication of mitochondrial DNA in mouse L cells and their thymidine kinase - derivatives: displacement replication on a covalently-closed circular template. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3810–3814. doi: 10.1073/pnas.69.12.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Analysis of adenovirus 12 temperature-sensitive mutants defective in viral DNA replication. Virology. 1974 Oct;61(2):474–485. doi: 10.1016/0042-6822(74)90283-9. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Yamaguchi K. The viral DNA replication complex of adenovirus 12. Virology. 1974 Jul;60(1):192–199. doi: 10.1016/0042-6822(74)90376-6. [DOI] [PubMed] [Google Scholar]
- Simmons T., Heywood P., Hodge L. D. Intranuclear site of replication of adenovirus DNA. J Mol Biol. 1974 Nov 5;89(3):423–433. doi: 10.1016/0022-2836(74)90473-2. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Ellens D. J., Jansz H. S. Studies on the mechanism of replication of adenovirus DNA. II. The nature of single-stranded DNA in replicative intermediates. J Virol. 1973 Nov;12(5):1131–1138. doi: 10.1128/jvi.12.5.1131-1138.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C., Ellens D. J., Jansz H. S. Linear intermediates in the replication of adenovirus DNA. Nat New Biol. 1972 Sep 13;239(89):47–49. [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. Studies on the mechanism of replication of adenovirus DNA. I. The effect of hydroxyurea. Virology. 1973 Jul;54(1):299–303. doi: 10.1016/0042-6822(73)90142-6. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. Viral DNA synthesis in isolated nuclei from adenovirus-infected KB cells. FEBS Lett. 1972 Mar;21(1):7–10. doi: 10.1016/0014-5793(72)80149-2. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H. Temperature-sensitive formation of the DNA replication complex in adenovirus 31-infected cells. J Virol. 1974 Feb;13(2):538–540. doi: 10.1128/jvi.13.2.538-540.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Vliet P. C., Levine A. J., Ensinger M. J., Ginsberg H. S. Thermolabile DNA binding proteins from cells infected with a temperature-sensitive mutant of adenovrius defective in viral DNA synthesis. J Virol. 1975 Feb;15(2):348–354. doi: 10.1128/jvi.15.2.348-354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlak J. M., Rozijn T. H., Sussenbach J. S. Studies on the mechanism of replication of adenovirus DNA. IV. Discontinuous DNA Chain propagation. Virology. 1975 Jan;63(1):168–175. doi: 10.1016/0042-6822(75)90382-7. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Green M. Adenovirus DNA replication. I. Requirement for protein synthesis and isolation of nuclear membrane fractions containing newly synthesized viral DNA and proteins. J Virol. 1974 Sep;14(3):412–420. doi: 10.1128/jvi.14.3.412-420.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Eb A. J. Intermediates in type 5 adenovirus DNA replication. Virology. 1973 Jan;51(1):11–23. doi: 10.1016/0042-6822(73)90361-9. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Levine A. J. DNA-binding proteins specific for cells infected by adenovirus. Nat New Biol. 1973 Dec 12;246(154):170–174. doi: 10.1038/newbio246170a0. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Sussenbach J. S. The mechanism of adenovirus-DNA synthesis in isolated nuclei. Eur J Biochem. 1972 Nov 7;30(3):584–592. doi: 10.1111/j.1432-1033.1972.tb02130.x. [DOI] [PubMed] [Google Scholar]



