Abstract
Pulmonary tumor thrombotic microangiopathy (PTTM) is a rare, malignancy-related complication that causes marked pulmonary hypertension, right heart failure, and death. We report on a patient with locally advanced breast cancer whose course was complicated by fatal PTTM based on clinical and laboratory findings.
Keywords: Breast neoplasms, Pulmonary hypertension, Pulmonary tumor thrombotic microangiopathy
Introduction
Pulmonary tumor thrombotic microangiopathy (PTTM) is an uncommon, fatal, malignancy-related complication causing marked pulmonary hypertension, right heart failure, and death. It is diagnosed primarily during autopsy, and is rarely diagnosed antemortem.
Tumor emboli in the vasculature with consequent local activation of coagulation and by widespread fibrocellular intimal proliferation of small pulmonary arteries and arterioles, leading to increased vascular resistance, resulting in marked pulmonary hypertension [1,2]. In addition, PTTM also shows hemodynamic effects similar to those observed with microangiopathic hemolytic anemia (MAHA) and disseminated intravascular coagulation (DIC). Thus far, the majority of reported underlying malignancies have been adenocarcinomas of gastrointestinal origin [1].
We report on a 30-year-old female patient with locally advanced breast cancer who presented with acute onset of rapidly progressive dyspnea, culminating in cardiovascular collapse from right heart failure.
Case Report
In May 2009, a 30-year-old woman came to the emergency room (ER) with a five-day history of acute progressive dyspnea on exertion (NYHA class III). In 2007, ductal carcinoma in situ was diagnosed by biopsy during pregnancy. In 2008, after a full-term delivery, findings on fluorodeoxyglucose positron emission tomography computed tomography (CT) and ultrasound of the right breast revealed diffuse microcalcification and skin thickening of the right breast, and metastatic axillary lymph nodes. The patient refused further management and was lost to follow up until the ER visit.
On admission, the patient's blood pressure was 126/76 mm Hg, heart rate was 120 beats per min, respiratory rate was 18 breaths per minute, and body temperature was 36.7℃. A chest radiograph showed that the lung field was clear. Results of arterial blood gas analysis in room air indicated hypoxemia: pH 7.446, pCO2 28.2 mm Hg, pO2 44.3 mm Hg, HCO3 19.1 mmol/L, SaO2 76.8%. D-dimers were elevated to 2.59 µg/mL (normal, <0.39 µg/mL) with an elevated troponin I level to 0.24 ng/mL (normal, <0.04 ng/mL) and a brain natriuretic peptide of 774 pg/mL (normal, <100 pg/mL). Diffuse enlargement of the right breast with skin thickening and many enlarged axillary lymph nodes consistent with locally advanced breast cancer was observed on CT.
On the second day of admission, the patient was consulted with the cardiology department for possible preoperative cardiac evaluation. An electrocardiogram showed an S1Q3T3 pattern with inverted or flattened T waves in leads V1 through V4. A transthoracic echocardiogram showed normal left ventricular systolic function with right ventricular enlargement and free wall hypokinesia sparing the apex. In addition, the echocardiogram showed typical findings of acute pulmonary thromboembolism with a D-shaped left ventricle, moderate tricuspid regurgitation, and moderate pulmonary hypertension with an estimated right ventricular systolic pressure of 61 mm Hg.
Other laboratory tests showed the following results: white blood cell 7,900/mm3 with normal differential counts, hemoglobin15.4 g/dL, platelets 144,000/mm3, alanine aminotransferase 50 IU/L (normal, 0 to 40 IU/L), aspartate aminotransferase 218 IU/L (normal, 0 to 40 IU/L), total bilirubin 1.4 mg/dL (normal, 0.2 to 1.2 mg/dL), C-reactive protein 0.81 mg/dL (normal, 0 to 0.30 mg/dL), prothrombin time (PT) international normalized ratio (INR) of 1.36 (normal, 0.8 to 1.2), activated partial thromboplastin time (aPTT) of 45.7 seconds (normal, 27.0 to 45.0 seconds), fibrinogen of 196 mg/dL (normal, 200 to 400 mg/dL), and fibrin degradation products (FDP) of 1 : 2 positive (normal, negative). A peripheral blood smear showed increased numbers of schistocytes and reticulocytes consistent with MAHA (Fig. 1).
Fig. 1.
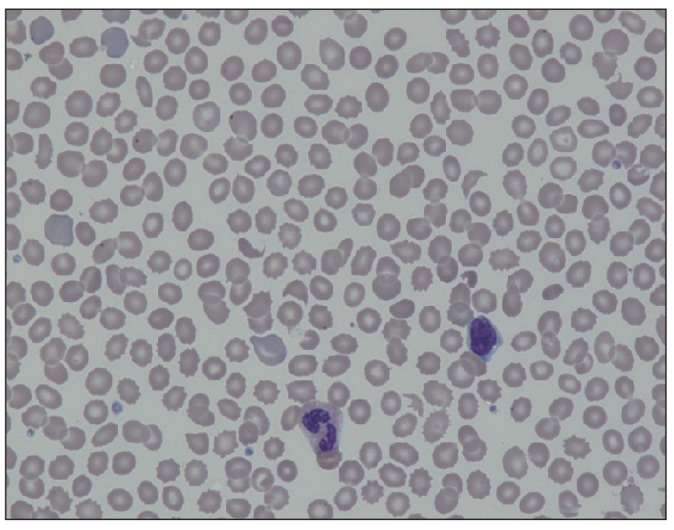
Peripheral blood smear showing schistocytes and reticulocytes (×400).
We measured serum vascular endothelial growth factor (VEGF) and interleukin 6 (IL-6) in view of preexisting information on VEGF, a critical angiogenic molecule and IL-6, a multifunctional cytokine promoting tumor growth. Serum VEGF levels were 26.9 pg/mL on the second day in the hospital and 9.5 pg/mL on the third day in the hospital (normal, 88.7 to 1,048.7 pg/mL); IL-6 levels were 50.3 pg/mL and 25.6 pg/mL on the second and third days, respectively (normal range, 0.4 to 8.6 pg/mL).
Anticoagulation therapy with enoxaparin was startedunder the clinical diagnosis of submassive acute pulmonary thromboembolism. While no evidence of pulmonary thromboembolism was observed on pulmonary CT angiography (Fig. 2), an echocardiogram showed acute right ventricular pressure overload (Fig. 3), and a perfusion lung scan showed multiple small wedge-shaped perfusion defects throughout both lungs (Fig. 4). Therefore, with a diagnosis of PTTM based on clinical and laboratory findings, the patient was transferred to the intensive care unit and received 1 mg/kg enoxaparin subcutaneously, 0.05 mg/kg dexamethasone intravenously, and 2 mg warfarin sodium orally.
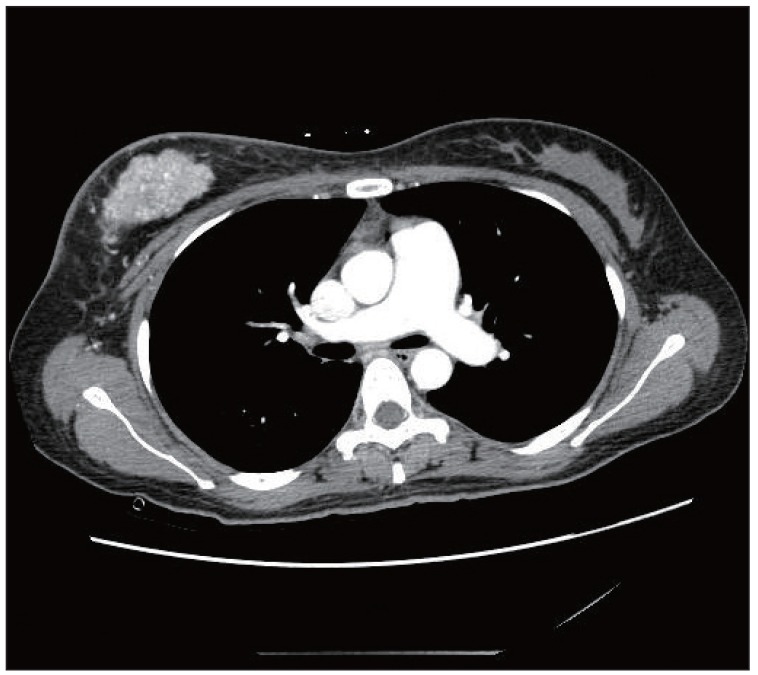
Fig. 2.
Chest computed tomography image shows an enhanced mass in the right breast and dilatation of the pulmonary artery. There was no evidence of thromboembolism in either the pulmonary artery or segmental artery.
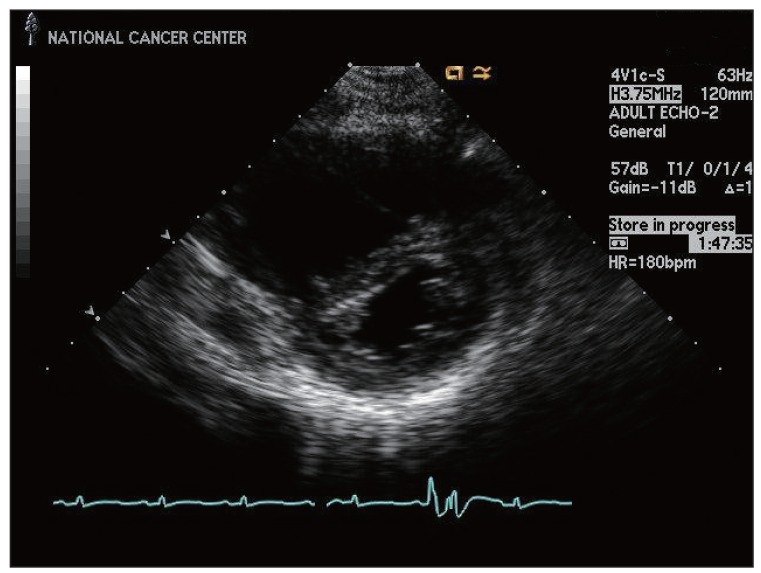
Fig. 3.
Echocardiogram showing right ventricular enlargement and a D-shaped left ventricle.
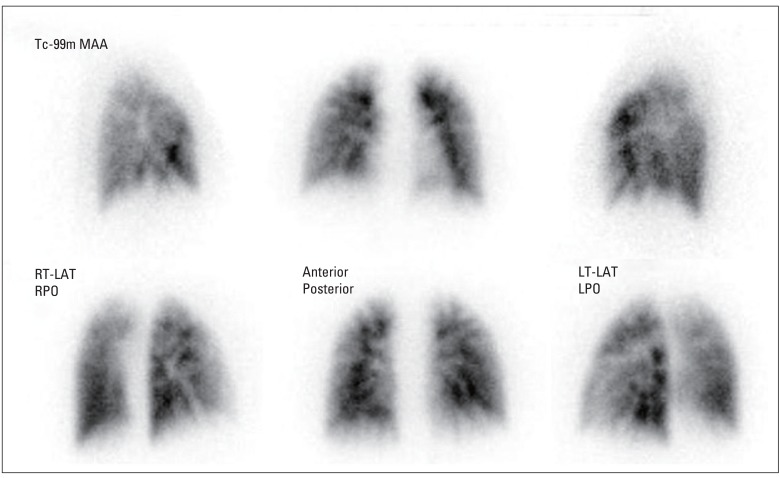
Fig. 4.
Lung perfusion scan showing multiple wedge-shaped perfusion defects in both lungs. RT-LAT, right lateral; RPO, right posterior oblique; LT-LAT, left lateral; LPO, left posterior oblique.
On the third day of admission, repeated transthoracic echocardiogram showed additional significant right ventricular pressure overload with an estimated right ventricular systolic pressure of 60 mm Hg. Abnormal liver function was the result of either passive congestion or hepatic metastasis, although the initial CT did not show definite systemic metastasis. The patient was scheduled to undergo surgical breast biopsy and appropriate chemotherapy, however, her general condition showed rapid deterioration, and she died within 48 hours of admission.
Discussion
In 1990, von Herbay et al. [1] introduced the term PTTM, because earlier terms, including carcinomatous endarteritis and embolic carcinomatosis, did not fully cover the tumor cell embolism, local thrombosis, and intimal proliferation that are the hallmark of angiopathy. PTTM differs from pulmonary tumor embolism in that the latter is not characterized by acute progressive dyspnea, significant pulmonary hypertension, MAHA, or DIC. In addition, PTTM is distinct from lymphangitic carcinomatosis, which causes less acute symptoms and is accompanied by abnormal findings on chest CT [3]. In a study of 630 carcinoma autopsies, 21 cases (3.3%) were diagnosed with PTTM. Of these, 19 cases (90.5%) had adenocarcinomas of various organs, including stomach, lung, breast, colon, pancreas, liver, bladder, and prostate. While stomach cancer was the most common, there were two cases of breast cancer [1]. The diagnosis of PTTM was based on clinical features and laboratory findings including peripheral blood smear showing MAHA, prolonged PT and aPTT, low fibrinogen levels, and positive FDP consistent with DIC.
Although the underlying molecular mechanism of PTTM remains uncertain, platelet-derived growth factor, VEGF, and osteopontin may play a role in the pathogenesis of PTTM [4-6].
Due to the extremely rapid progression of PTTM, almost all reported patients died within one week of onset of dyspnea [2,5]. Thus far, no predisposing factors have been identified. Therefore, in order to make an early diagnosis and administer therapeutic intervention, it is important for clinicians to be keenly aware of the disease entity. In some cases, aggressive bronchoscopic biopsy, transbronchial lung biopsy, and pulmonary microvascular cytology by a wedged pulmonary artery catheter have been suggested as tools for use in antemortem diagnosis of PTTM [3,7]. However, these procedures may not be applicable to all patients who are already experiencing severe and progressive dyspnea.
Since MAHA and DIC are part of the clinical spectrum as seen in the current patient, peripheral blood smear and DIC tests are warranted. Miyano et al. [8] reported a markedly elevated serum VEGF level in a gastric cancer with PTTM, which normalized after chemotherapy. Although serum VEGF level was not elevated in the current case, IL-6 was significantly elevated. While we do not know the exact implication in relationship with PTTM, IL-6 level was reportedly correlated with poor survival in metastatic breast cancer [9].
Since no effective management for PTTM is identified todate, chemotherapy, corticosteroids, and anticoagulation could be applied as reported in a gastric cancer patient with PTTM [9]. Type 2A serotonin receptor antagonists may also be useful by suppressing intimal proliferation [10], and new drugs for treatment of pulmonary arterial hypertension, such as endothelin antagonists, prostacyclin analogues, and phosphodiesterase type 5 inhibitors may be beneficial [11].
Footnotes
Conflicts of interest relevant to this article was not reported.
References
- 1.von Herbay A, Illes A, Waldherr R, Otto HF. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587–592. doi: 10.1002/1097-0142(19900801)66:3<587::aid-cncr2820660330>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 2.Pinckard JK, Wick MR. Tumor-related thrombotic pulmonary microangiopathy: review of pathologic findings and pathophysiologic mechanisms. Ann Diagn Pathol. 2000;4:154–157. doi: 10.1016/s1092-9134(00)90038-8. [DOI] [PubMed] [Google Scholar]
- 3.Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 19-1995. A 55 year-old woman with acute respiratory failure and radiographically clear lungs. N Engl J Med. 1995;332:1700–1707. doi: 10.1056/NEJM199506223322508. [DOI] [PubMed] [Google Scholar]
- 4.Chinen K, Fujino T, Horita A, Sakamoto A, Fujioka Y. Pulmonary tumor thrombotic microangiopathy caused by an ovarian cancer expressing tissue factor and vascular endothelial growth factor. Pathol Res Pract. 2009;205:63–68. doi: 10.1016/j.prp.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Chinen K, Kazumoto T, Ohkura Y, Matsubara O, Tsuchiya E. Pulmonary tumor thrombotic microangiopathy caused by a gastric carcinoma expressing vascular endothelial growth factor and tissue factor. Pathol Int. 2005;55:27–31. doi: 10.1111/j.1440-1827.2005.01783.x. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi F, Kumasaka T, Nagaoka T, Wakiya M, Fujii H, Shimizu K, et al. Osteopontin expression in pulmonary tumor thrombotic microangiopathy caused by gastric carcinoma. Pathol Int. 2009;59:752–756. doi: 10.1111/j.1440-1827.2009.02439.x. [DOI] [PubMed] [Google Scholar]
- 7.Babar SI, Sobonya RE, Snyder LS. Pulmonary microvascular cytology for the diagnosis of pulmonary tumor embolism. West J Med. 1998;168:47–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Miyano S, Izumi S, Takeda Y, Tokuhara M, Mochizuki M, Matsubara O, et al. Pulmonary tumor thrombotic microangiopathy. J Clin Oncol. 2007;25:597–599. doi: 10.1200/JCO.2006.09.0670. [DOI] [PubMed] [Google Scholar]
- 9.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88:1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakashita N, Yokose C, Fujii K, Matsumoto M, Ohnishi K, Takeya M. Pulmonary tumor thrombotic microangiopathy resulting from metastatic signet ring cell carcinoma of the stomach. Pathol Int. 2007;57:383–387. doi: 10.1111/j.1440-1827.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- 11.Keenan NG, Nicholson AG, Oldershaw PJ. Fatal acute pulmonary hypertension caused by pulmonary tumour thrombotic microangiopathy. Int J Cardiol. 2008;124:e11–e13. doi: 10.1016/j.ijcard.2006.11.162. [DOI] [PubMed] [Google Scholar]