Abstract
Obese patients with atrial fibrillation (AF) are frequently managed with AF ablation. We sought to examine whether there exists a body mass index (BMI) threshold beyond which odds of experiencing a complication from AF ablation increase. All patients enrolled in the Vanderbilt AF Registry who underwent catheter-based AF ablation from May 1999 to February 2012 were included. Major complications were recorded. Morbid obesity was defined as BMI >40 kg/m2, and BMI as a continuous variable was examined in multivariable analysis. Thirty-five complications (6.8%) occurred in 512 ablations. Morbidly obese patients experienced a higher rate of complications (6/42, 14.3%) than non-morbidly obese (29/470, 6.2%) (P=0.046). Using a discrete BMI cut-off, the odds of complications increased 3.1-fold in those with morbid obesity (odds ratio [OR] 3.1, 95% Confidence Interval [CI] 1.1–8.4, P=0.03) and 2.1-fold by female gender (OR 2.1, 95% CI 1.04–4.38, P=0.04). With BMI as a continuous variable, the odds of complications increased by 5% per 1 unit increase in BMI (OR 1.05, 95% CI 1.0–1.11, P=0.05) and there was a 2.2-fold increase by female gender (OR 2.2, 95% CI 1.1–4.6, P=0.03). In conclusion, morbid obesity represents a BMI threshold above which the odds of complications with AF ablation significantly increase. The increase in complications appears to be driven primarily by events in women suggesting that morbidly obese women are a special population to consider when considering AF ablation.
Keywords: Atrial Fibrillation, Ablation, Obesity, Complications, Pulmonary Vein Isolation
Introduction
Many studies have examined the predictors for procedural complications with atrial fibrillation (AF) ablation, but only a few have included obesity or body mass index (BMI) in the analysis.1–4 Previous studies have not found an association between complications from AF ablation and obesity5–7, which may be due to the non-linear effect of BMI that is recognized in a variety of other outcomes.8, 9 In studies examining the rate of complications in patients undergoing percutaneous coronary intervention (PCI), the rate of complications was found to paradoxically decrease in patients with mild/moderate obesity prior to increasing in patients with morbid obesity.8–12 Morbid obesity was considered to represent the BMI threshold where rate of complications significantly increase in patients undergoing PCI. In this study, we sought to examine the hypothesis that morbid obesity represents a threshold beyond which risk of procedural complications with AF ablation significantly increases.
Methods
We included 445 patients with symptomatic AF who were prospectively enrolled in the Vanderbilt AF Registry, a clinical and genetic database, and underwent catheter-based ablation for AF between May 1999 and March 2012.13 Ablations included de-novo and repeat procedures. Surgical and combined hybrid catheter/surgical ablation procedures were excluded. Patient characteristics and procedural details were entered into a central database.14 Paroxysmal AF was defined as episodes lasting less than 7 days and spontaneously terminating. Non-paroxsymal AF was defined as AF episodes lasting greater than 7 days and/or requiring termination with pharmacologic or electrical cardioversion. Number of lifetime direct current cardioversions (DCCV) prior to ablation was recorded based on patient report. BMI was calculated by weight in kilograms divided by height in meters squared. Based on current recommendations, obesity was defined as BMI 30–40 kg/m2 and morbid obesity as BMI >40 kg/m2.
Major complications were defined as those resulting in permanent injury, death, intervention, or requiring or prolonging hospitalization.15 Pulmonary vein (PV) stenoses were included if they required venoplasty. Acute lung injury was defined as pneumonia or fluid-overload requiring or prolonging ventilator-support.
All patients received general anesthesia during the ablation. Vascular access was obtained from the right and/or left femoral veins with or without right internal jugular veins according to operator preference for coronary sinus cannulation. Vascular access was obtained using anatomic and fluoroscopic landmarks. All ablations were performed using biplane fluoroscopy. Left atrial access was obtained using transseptal puncture under anteroposterior and left anterior oblique fluoroscopic views with assistance from intracardiac echocardiogram.
Standard peri-procedural anticoagulation strategies varied throughout the study period. In cases where pre-procedure therapeutic INR for at least one month was not documented, a transesophageal echocardiogram was performed prior to the ablation to document absence of left atrial thrombus. Prior to 2009, warfarin was discontinued 5 days before the procedure with use of a low-molecular weight heparin bridge. Warfarin was restarted the morning of the procedure, and therapeutic anticoagulation with heparin was performed upon transseptal puncture with maintenance of ACT 300–350 seconds throughout the procedure. Heparin or low molecular weight heparin was continued after the procedure until a therapeutic INR was achieved. Since 2009, catheter ablation has been performed without interruption of warfarin with a therapeutic INR goal of 2–2.5 on the day of the procedure. Since 2011, patients who received anticoagulation with dabigatran held the anticoagulant 12–48 hours prior to the procedure based on individual patient risk factors for bleeding (held for >24 hours based on patient age >75 years, creatinine clearance <30ml/min, aspirin/clopidogrel use) with resumption 4–24 hours following removal of sheath and hemostasis. Protamine was variably administered at completion of the procedure to facilitate sheath removal in patients with therapeutic INRs. Anticoagulation with warfarin or dabigatran was continued for at least 3 months following ablation.
Ablations were performed according to standard techniques contemporary for the year of ablation. The standard ablation procedure throughout the study period consisted of antral or segmental PV isolation (PVI) confirmed by entrance block, with additional linear ablation and ablation of non-PV foci based on operator discretion. From 1999 to 2003, the primary ablative approach consisted of segmental PVI with use of a multipolar circular mapping catheter. Ablation was performed with an 8 French 5mm tip temperature controlled radiofrequency (RF) catheter at the venoatrial junction. RF energy was applied with temperatures of 55–60°C, and a maximum power of 50 W for 30–45 seconds at each site. Since 2003, a wide-area circumferential antral ablation has been performed with use of a 3-D electroanatomic mapping system and placement of contiguous lesions 5 to 15 mm from the PV ostia with testing for entrance block. As of 2009, an 8 French 3.5mm tip open irrigated-tip power-controlled ablation catheter was used. For circumferential ablation, maximum power of 25 W was applied on the posterior wall and 30–35 W on the anterior wall and roof. Entrance and exit block were tested utilizing high output pacing with isoproterenol and/or adenosine administration directing additional lesions as necessary to achieve complete bidirectional electrical isolation of all PVs. Since 2011, PVI has also been performed in select cases utilizing cryoablation (Arctic Front, Medtronic, USA).
Continuous variables are expressed as mean ± SD. Categorical variables are presented as percentages and frequencies. Patients were categorized by BMI and characteristics were displayed according to BMI category. Univariate analysis was performed using a Chi-square test for nominal variables and Mann-Whitney U test for continuous variables. Multivariable analysis was performed using a binary logistic regression model to predict occurrence of complications. The covariates chosen to include in the final multivariable logistic regression analysis included age, gender, and coronary artery disease (CAD). They were pre-specified and selected based on previous studies that demonstrated them to be significant predictors of complications in multivariable analysis.1, 4, 5 Multivariable analysis was performed to examine morbid obesity defined by BMI dichotomized at 40 kg/m2 (model 1) and BMI as a continuous variable (model 2). Two-sided P-values less than 0.05 were considered statistically significant.
Results
Complete baseline patient characteristics and procedural details are shown according to BMI in Table 1. A total of 564 ablations were performed of which 512 met eligibility criteria (52 excluded for hybrid/surgical ablation). The mean BMI for the overall cohort was 31±6 kg/m2. Eight percent (n=42) were morbidly obese of which 62% (n=26) were male and 38% (n=16) were female. Morbidly obese patients were no more likely than the overall cohort to be on clopidogrel (2.4% v. 2.5%), aspirin (41% v. 45%), or to be undergoing a redo procedure (14% v. 22%).
Table 1.
Baseline Patient and Procedural Characteristics
| Variable | Body Mass Index (kg/m2)
|
||
|---|---|---|---|
| <30 (n=260) | 30–39 (n=210) | >40 (n=42) | |
| Age (years) | 61±10 | 57±9 | 56±10 |
| Male | 181(70%) | 159(76%) | 26(62%) |
| Body Mass Index (kg/m2) | 27±2.4 | 34±2.6 | 44±5 |
| Duration of AF (months) | 78±82 | 86±74 | 71±49 |
| Paroxysmal AF | 133(51%) | 88(42%) | 17(40%) |
| Number of DCCV | 1.3±1.8 | 1.4±2.0 | 1.7±3.2 |
| Coronary Artery Disease | 35(14%) | 46(22%) | 15(36%) |
| Obstructive Sleep Apnea | 36(14%) | 69(33%) | 24(57%) |
| Clopidogrel | 3(1%) | 9(4%) | 1(2%) |
| Aspirin | 117(45%) | 97(46%) | 17(40%) |
| History of amiodarone use | 84(32%) | 83(40%) | 18(43%) |
| Left Atrial Size from MRI (mm) | 37±7 | 41±7 | 46±9 |
| LVEF from MRI (%) | 63±11 | 62±12 | 60±13 |
| Redo Ablation | 54(21%) | 54(26%) | 6(14%) |
| Cavotricuspid Isthmus Ablation | 50(19%) | 46(22%) | 11(26%) |
| Roof Line | 90(35%) | 78(37%) | 23(55%) |
| Mitral Valve Line | 56(22%) | 43(20%) | 8(19%) |
| Complex Fractionated Atrial Electrogram Ablation | 18(7%) | 28(13%) | 8(19%) |
| Cryoballoon | 5(2%) | 2(1%) | 0(0%) |
| Procedure Time (min) | 280±78 | 293±78 | 310±78 |
Values are Mean ± SD when appropriate.
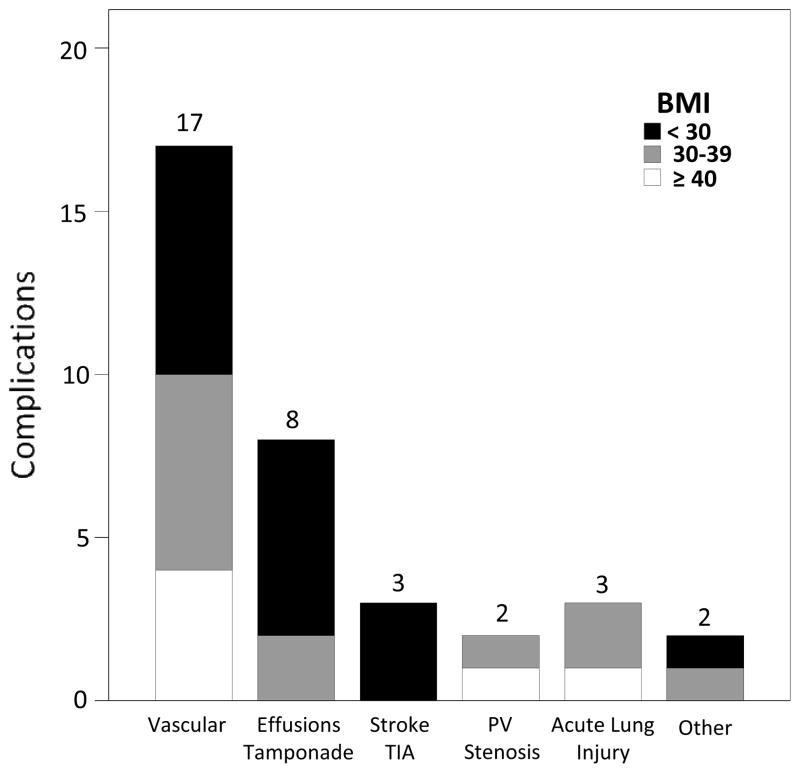
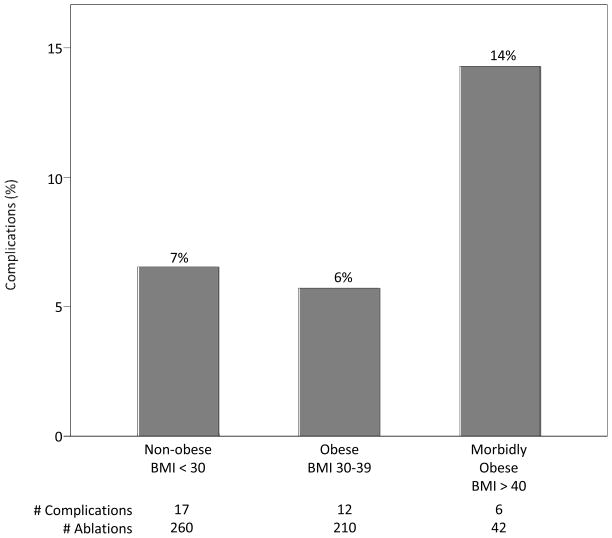
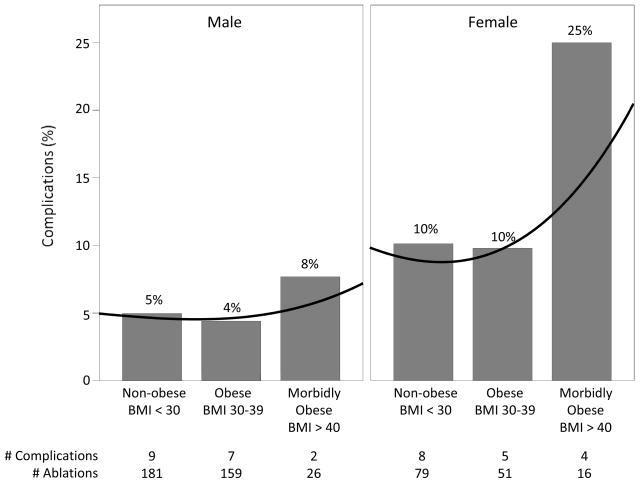
A total of 35 complications occurred for an overall rate of 6.8% (35/512, Figure 1). Six complications were observed in patients classified as morbidly obese for a complication rate of 14.3% (6/42) (Figure 2). Twenty-five percent (4/16) of morbidly obese females experienced a complication, compared to 7.7% (2/26) of males (Figure 3). Four of the six complications within the morbidly obese category were major vascular complications, 1 was an acute lung injury, and 1 was a PV stenosis requiring angioplasty.
Figure 1. Breakdown of complications by BMI category.
Other complications included an esophageal ulcer seen on upper endoscopy and an oropharyngeal bleed requiring ICU admission.
Figure 2. Complication rate according to BMI category.
The occurrence of complications marginally decreases in the BMI 30–39 kg/m2 group prior to increasing in the morbidly obese group. In this univariate analysis, P>0.05.
Figure 3. Complication rate according to BMI category stratified by gender.
The observed increase in occurrence of complications among patients with morbid obesity is driven by events in female patients. A mild decrease in occurrence of complications is observed by both genders in the BMI 30–39 kg/m2 group. Complication rate by BMI group is non-linear and is best estimated by a cubic root curve (black line). In this univariate analysis, P-value by Pearson Chi-square analysis equals 0.77 (males) and 0.21 (females).
As standard protocol at our institution all patients are admitted for observation overnight following AF ablation. Seventy-four percent (26/35) of the patients who experienced a complication required at least one extra day of inpatient hospitalization related to the complication. The median time of hospitalization was 4 days (Interquartile Range 2–8 days) with an absolute range of 1 to 23 days. There was one death that occurred 5 days after the procedure in a morbidly obese female in whom a femoral vein dissection was discovered at the time of sheath exchange. The patient was conservatively managed and demonstrated no hematoma or hematocrit drop over 48 hours of inpatient observation. Complete autopsy was unable to demonstrate the cause of death.
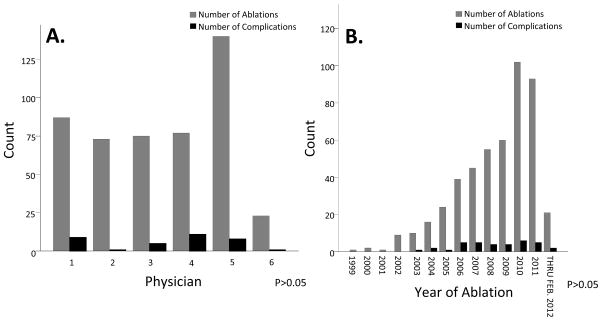
In univariate analysis, patients who experienced a complication were more likely to be female (49% v. 27%, P=0.01), possess a longer procedure time (322±94 v. 285±76 minutes, P=0.05), and have higher LVEF on MRI (68%±10 v. 62%±12, P=0.01). Complete results of univariate analysis are presented in Table 2. Year of ablation and physician were not found to increase rate of procedural complications (Figure 4).
Table 2.
Univariate Analysis of Complications
| Variable | Complication (n=35) | No Complication (n=477) | P-Value |
|---|---|---|---|
| Age (years) | 61±12 | 58±11 | 0.11 |
| Male | 18(51%) | 348(73%) | 0.01 |
| Body Mass Index (kg/m2) | 32±8 | 31±6 | 0.56 |
| Duration of AF (months) | 71±67 | 85±78 | 0.28 |
| Paroxysmal AF | 16(46%) | 222(47%) | 0.99 |
| Number of DCCV | 1±2 | 2±2 | 0.48 |
| Coronary Artery Disease | 4(11%) | 92(19%) | 0.36 |
| Obstructive Sleep Apnea | 7(20%) | 122(26%) | 0.46 |
| Clopidogrel | 1(3%) | 12(3%) | 0.90 |
| Aspirin | 11(31%) | 220(46%) | 0.09 |
| History of Amiodarone Use | 15(43%) | 170(36%) | 0.39 |
| Left Atrial Size from MRI (mm) | 39±8 | 39±8 | 0.77 |
| LVEF from MRI (%) | 68±10 | 62±12 | 0.01 |
| Redo Ablation | 5(14%) | 109(23%) | 0.24 |
| Cavotricuspid Isthmus Ablation | 8(23%) | 99(21%) | 0.77 |
| Roof Line | 15(43%) | 176(37%) | 0.48 |
| Mitral Valve Line | 7(20%) | 100(21%) | 0.89 |
| Complex Fractionated Atrial Electrogram Ablation | 3(9%) | 51(11%) | 0.69 |
| Cryoballoon | 0(0%) | 7(2%) | 0.47 |
| Procedure Time (min) | 322±94 | 285±76 | 0.05 |
Values are Mean ± SD when appropriate.
Figure 4. Physician (Panel A) and year of ablation (Panel B) were not associated with an increased rate of complications.
P-value by Chi-square analysis >0.05.
In multivariable analysis, BMI as a dichotomous variable (morbid obesity BMI >40 kg/m2) and female gender were significant predictors of complications after adjusting for age and coronary artery disease (CAD) (Table 3). Odds of a complication were increased 3.09-fold with morbid obesity (OR 3.09, 95% CI 1.14–8.36, P=0.03), and 2.13-fold with female gender (OR 2.13, 95% CI 1.04–4.38, P=0.04). When BMI was examined as a continuous variable, both BMI and female gender were found to be significant predictors of procedural complications after adjusting for age and CAD. Odds of a complication were increased 5% per 1 unit increase in BMI (OR 1.05, 95% CI 1.0–1.11, P=0.05), and 2.23-fold by female gender (OR 2.23, 95% CI 1.09–4.56, P=0.03). As seen when stratified by gender, the occurrence of complications among morbidly obese females was more common than among men although this did not reach statistical significance (25% v. 7.7%, P=0.120) (Figure 3).
Table 3.
Multivariable Analysis of Complications
| Model 1 | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Morbid Obesity | 3.09 | 1.14–8.36 | 0.03* |
| Female | 2.13 | 1.04–4.38 | 0.04* |
| Age | 1.02 | 0.99–1.06 | 0.20 |
| Coronary Artery Disease | 2.01 | 0.66–6.1 | 0.22 |
| Model 2
| |||
| Body Mass Index | 1.05 | 1.00–1.11 | 0.05* |
| Female | 2.23 | 1.09–4.56 | 0.03* |
| Age | 1.02 | 0.99–1.06 | 0.17 |
| Coronary Artery Disease | 1.98 | 0.66–5.95 | 0.23 |
Statistically significant given P<0.05. Model 1 includes morbid obesity dichotomized at 40 kg/m2. Model 2 includes BMI as a continuous variable.
Discussion
In this study, we observed that morbid obesity and female gender were significant predictors of procedural complications in multivariable analysis. Odds of experiencing a complication were increased over 3-fold in patients who were morbidly obese compared to non-morbidly obese patients and over 2-fold in female compared to male patients. A BMI cutoff >40 kg/m2 appeared to be a threshold at which the complication rate significantly increased. The occurrence of complications among morbidly obese patients was dominated by events occurring in female patients.
Many studies have examined the predictors for procedural complications with AF ablation, but only a few have included obesity or BMI in the analysis.1–4 The majority of previous work has not found an association between complications from AF ablation and obesity,5–7 which may be due to the non-linear effect of BMI that is recognized in a variety of other outcomes.8, 9 In studies examining the rate of complications in patients undergoing PCI, the rate of complications was found to paradoxically decrease in patients with mild/moderate obesity prior to increasing in patients with morbid obesity.8–12 Our finding that the odds of experiencing a complication was not increased in the BMI 30–39 kg/m2 group, but significantly increased in the morbidly obese group (BMI > 40 kg/m2) was in keeping with data on the occurrence of complications in patients undergoing PCI. Numerous studies have demonstrated a U-shaped effect of BMI on occurrence of complications in patients undergoing PCI such that complications increase in underweight patients, but paradoxically decrease in patients with mild/moderate obesity prior to increasing in patients with morbid obesity.8–12, 16, 17 It is possible that the absence of an association between obesity and complications in previous studies may be due to the need to analyze morbidly obese patients separately and/or an inadequate number of patients within the BMI >40 kg/m2 group.
Consistent with the findings of previous studies examining AF ablation1–3, 5, 18 as well as PCI,8, 19, 20 female gender was found to be a significant predictor of complications. In one of these studies, Patel et al. found a small (13%) increase in risk of complications among women with BMI >30 kg/m2.19 Our data, along with that from the PCI literature, would suggest the effect of moderate obesity (BMI 30–39.9) is minimal, and the increased risk of complications observed by Patel et al. using a BMI cutoff of >30 kg/m2 may have diluted a larger effect from the BMI >40 kg/m2 subset.
Although the precise etiology of the increased risk of complications in females is not clear, it is believed that the smaller vascular diameter in women compared to men is contributory. Also, an anatomic variation more common in women places the femoral artery and circumflex branches overtop the femoral vein which may increase the risk of accidental arterial puncture.18, 21, 22
This is a single center study which may limit the generalizability of our results to other populations. Patients included in this study were those enrolled in the Vanderbilt AF Registry, which represents only a subset of the total patients who undergo AF ablation at Vanderbilt. Average AF ablation volume at Vanderbilt is over 250 per year. Furthermore, the number of morbidly obese patients undergoing AF ablation was relatively small and limits the statistical power. Due to the observational nature of our study and extended period over which data was collected, ablation techniques and peri-procedural anticoagulation strategies evolved and consequently were not standardized. Furthermore, only complications that were detected clinically were recorded, and it is possible that subclinical complications occurred that were not recorded.
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health grants U19 HL65962, HL092217, UL1 RR024975, and UL1 TR000445 and American Heart Association Established Investigator (0940116N) and Clinical Research Program (11CRP7420009) Awards.
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–149. doi: 10.1016/j.jacc.2011.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baman TS, Jongnarangsin K, Chugh A, Suwanagool A, Guiot A, Madenci A, Walsh S, Ilg KJ, Gupta SK, Latchamsetty R, Bagwe S, Myles JD, Crawford T, Good E, Bogun F, Pelosi F, Jr, Morady F, Oral H. Prevalence and predictors of complications of radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electr. 2011;22:626–631. doi: 10.1111/j.1540-8167.2010.01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spragg DD, Dalal D, Cheema A, Scherr D, Chilukuri K, Cheng A, Henrikson CA, Marine JE, Berger RD, Dong J, Calkins H. Complications of catheter ablation for atrial fibrillation: Incidence and predictors. J Cardiovasc Electr. 2008;19:627–631. doi: 10.1111/j.1540-8167.2008.01181.x. [DOI] [PubMed] [Google Scholar]
- 4.Bertaglia E, Zoppo F, Tondo C, Colella A, Mantovan R, Senatore G, Bottoni N, Carreras G, Coro L, Turco P, Mantica M, Stabile G. Early complications of pulmonary vein catheter ablation for atrial fibrillation: A multicenter prospective registry on procedural safety. Heart Rhythm. 2007;4:1265–1271. doi: 10.1016/j.hrthm.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Hoyt H, Bhonsale A, Chilukuri K, Alhumaid F, Needleman M, Edwards D, Govil A, Nazarian S, Cheng A, Henrikson CA, Sinha S, Marine JE, Berger R, Calkins H, Spragg DD. Complications arising from catheter ablation of atrial fibrillation: Temporal trends and predictors. Heart Rhythm. 2011;8:1869–1874. doi: 10.1016/j.hrthm.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 6.Cha YM, Friedman PA, Asirvatham SJ, Shen WK, Munger TM, Rea RF, Brady PA, Jahangir A, Monahan KH, Hodge DO, Meverden RA, Gersh BJ, Hammill SC, Packer DL. Catheter ablation for atrial fibrillation in patients with obesity. Circulation. 2008;117:2583–2590. [Google Scholar]
- 7.Letsas KP, Siklody CH, Korantzopoulos P, Weber R, Burkle G, Mihas CC, Kalusche D, Arentz T. The impact of body mass index on the efficacy and safety of catheter ablation of atrial fibrillation. Int J Cardiol. 2011 doi: 10.1016/j.ijcard.2011.06.092. [DOI] [PubMed] [Google Scholar]
- 8.Byrne J, Spence MS, Fretz E, Mildenberger R, Chase A, Berry B, Pi D, Janssen C, Klinke P, Hilton D. Body mass index, periprocedural bleeding, and outcome following percutaneous coronary intervention (from the british columbia cardiac registry) Am J Cardiol. 2009;103:507–511. doi: 10.1016/j.amjcard.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Ellis SG, Elliott J, Horrigan M, Raymond RE, Howell G. Low-normal or excessive body mass index: Newly identified and powerful risk factors for death and other complications with percutaneous coronary intervention. Am J Cardiol. 1996;78:642–646. doi: 10.1016/s0002-9149(96)00386-4. [DOI] [PubMed] [Google Scholar]
- 10.Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J., Jr The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: The obesity paradox? J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 11.Gurm HS, Brennan DM, Booth J, Tcheng JE, Lincoff AM, Topol EJ. Impact of body mass index on outcome after percutaneous coronary intervention (the obesity paradox) Am J Cardiol. 2002;90:42–45. doi: 10.1016/s0002-9149(02)02384-6. [DOI] [PubMed] [Google Scholar]
- 12.Minutello RM, Chou ET, Hong MK, Bergman G, Parikh M, Iacovone F, Wong SC. Impact of body mass index on in-hospital outcomes following percutaneous coronary intervention (report from the new york state angioplasty registry) The Am J Cardiol. 2004;93:1229–1232. doi: 10.1016/j.amjcard.2004.01.065. [DOI] [PubMed] [Google Scholar]
- 13.Darbar D, Motsinger AA, Ritchie MD, Gainer JV, Roden DM. Polymorphism modulates symptomatic response to antiarrhythmic drug therapy in patients with lone atrial fibrillation. Heart Rhythm. 2007;4:743–749. doi: 10.1016/j.hrthm.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. Hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the heart rhythm society (hrs) task force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Gurm HS, Whitlow PL, Kip KE. The impact of body mass index on short- and long-term outcomes inpatients undergoing coronary revascularization. Insights from the bypass angioplasty revascularization investigation (bari) J Am Coll Cardiol. 2002;39:834–840. doi: 10.1016/s0735-1097(02)01687-x. [DOI] [PubMed] [Google Scholar]
- 17.Powell BD, Lennon RJ, Lerman A, Bell MR, Berger PB, Higano ST, Holmes DR, Jr, Rihal CS. Association of body mass index with outcome after percutaneous coronary intervention. Am J Cardiol. 2003;91:472–476. doi: 10.1016/s0002-9149(02)03252-6. [DOI] [PubMed] [Google Scholar]
- 18.Patel D, Mohanty P, Di Biase L, Sanchez JE, Shaheen MH, Burkhardt JD, Bassouni M, Cummings J, Wang Y, Lewis WR, Diaz A, Horton RP, Beheiry S, Hongo R, Gallinghouse GJ, Zagrodzky JD, Bailey SM, Al-Ahmad A, Wang P, Schweikert RA, Natale A. Outcomes and complications of catheter ablation for atrial fibrillation in females. Heart Rhythm. 2010;7:167–172. doi: 10.1016/j.hrthm.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Kawamura A, Piemonte TC, Nesto RW, Bilazarian SD, Riskalla NS, Chauhan MS. Impact of gender on in-hospital outcomes following contemporary percutaneous intervention for peripheral arterial disease. J Invasive Cardiol. 2005;17:433–436. [PubMed] [Google Scholar]
- 20.Farouque HM, Tremmel JA, Raissi Shabari F, Aggarwal M, Fearon WF, Ng MK, Rezaee M, Yeung AC, Lee DP. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein iib/iiia inhibitors and vascular closure devices. J Am Coll Cardiol. 2005;45:363–368. doi: 10.1016/j.jacc.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 21.Siddharth P, Smith NL, Mason RA, Giron F. Variational anatomy of the deep femoral artery. The Anatomical record. 1985;212:206–209. doi: 10.1002/ar.1092120216. [DOI] [PubMed] [Google Scholar]
- 22.Hughes P, Scott C, Bodenham A. Ultrasonography of the femoral vessels in the groin: Implications for vascular access. Anaesthesia. 2000;55:1198–1202. doi: 10.1046/j.1365-2044.2000.01615-2.x. [DOI] [PubMed] [Google Scholar]