Abstract
Objectives
Despite adiponectin’s independent relationship with many markers of vascular disease risk, its association with clinical outcomes is unclear and results of studies have been inconsistent. We examined the association between adiponectin, an adipocytokine secreted by adipose tissue, and vascular events (stroke, myocardial infarction (MI), vascular death) in the multi-ethnic prospective population-based Northern Manhattan Study (NOMAS).
Methods
Adiponectin was measured at baseline among 2900 participants free of MI and stroke (mean age 69±10 years, 37% men, 21% white, 53% Hispanic, 24% black). Over a mean 10 years follow-up, 692 incident vascular events accrued.
Results
The mean adiponectin=11.4±6.2 μg/ml (median=9.8, range=2.1–53.3). In Cox models adjusting for demographics and vascular risk factors, a decreased risk of vascular events was suggested with lower adiponectin. Examination of quartiles suggested a non-linear relationship, with a reduction in risk observed among those in adiponectin quartiles 1–3 vs. 4, and the lowest effect estimates observed in quartile 2. Similar results were found when stroke, MI, and vascular death were examined separately. We saw no effect modification by baseline vascular health profile, but the positive association between adiponectin and vascular events was stronger among those with elevated waist circumference.
Conclusions
In NOMAS, low-moderate adiponectin was associated with a decreased risk of vascular events despite the fact that low adiponectin levels were associated with an elevated vascular risk profile. These counter-intuitive findings underscore the need for further research on adiponectin as a useful biomarker of vascular disease risk and mechanisms explaining the inconsistent observations in the literature.
Keywords: adiponectin, myocardial infarction, stroke, epidemiology
Introduction
Adiponectin is an adipocytokine secreted by adipose tissue, with insulin sensitizing effects as well as effects on triglyceride and high density lipprotein (HDL) cholesterol metabolism (1). Recently, adiponectin has received a great deal of attention due to its potential as a biomarker for cardiovascular disease risk, with low levels presumed to confer elevated risk.
In our large race/ethnically diverse population-based adult cohort, the Northern Manhattan Study (NOMAS), we recently confirmed that lower adiponectin levels are independently associated with several important vascular risk factors including smoking, hypertension, diabetes, waist circumference, body mass index (BMI), estimated glomerular filtration rate, high-sensitivity C-reactive protein (hsCRP), triglycerides, low density lipoprotein (LDL) cholesterol, low HDL cholesterol, and the metabolic syndrome (in press at Metabolic Syndrome and Related Disorders). We have also confirmed a now well-established association between greater adiponectin levels and older age, the underlying mechanisms of which have not been elucidated, that is inconsistent with adiponectin’s inverse association with many other vascular risk factors. Lastly, we have previously shown in NOMAS that lower adiponectin was associated with greater carotid intima-media thickness, a marker of atherosclerosis and stroke risk factor (2).
Despite mounting evidence of adiponectin’s independent relationship with many markers of vascular disease risk, its association with clinical outcomes, including stroke and myocardial infarction (MI), is unclear and the results of published studies to date have been inconsistent. Several epidemiologic studies have shown that adiponectin levels are lower in subjects with cardiovascular events, in some cases predicting events independently of established risk factors(3–5). However, other studies have failed to show an independent association between adiponectin and stroke and MI (6–8), and still others have demonstrated an increased risk of stroke (9), and cardiovascular disease and mortality (10–15) among those with greater adiponectin levels. Potential mechanisms underlying the latter positive associations are not well-understood. Nevertheless, adiponectin seems to be an attractive therapeutic target for prevention of CVD and diabetes, as it can be significantly increased by diet, exercise and weight reduction(16).
We examined the relationship between baseline serum adiponectin levels and incident vascular events (stroke, MI, and vascular death) in our prospective NOMAS cohort. Our recent findings that adiponectin levels vary across race-ethnic groups and are lower among non-Hispanic blacks and Hispanics compared to non-Hispanic whites (under review at Metabolic Syndrome and Related Disorders) underscore the importance of examining this relationship in a multi-ethnic community-based sample.
Methods
Study Population
NOMAS is a prospective cohort study designed to determine stroke incidence, risk factors, and prognosis in a multi-ethnic urban population. Study details have been published previously (17).
Eligible subjects: a) had never been diagnosed with ischemic stroke; b) were >40 years old; and c) resided in Northern Manhattan for ≥ 3 months, in a household with a telephone. Subjects were identified by random-digit dialing, and interviews were conducted by trained bilingual research assistants. The telephone response rate was 91%. Subjects were recruited from the telephone sample to have an in-person baseline interview and assessment. The enrollment response rate was 75%, the overall participation rate was 69%, and a total of 3,298 subjects were enrolled with an average annual contact rate of 95%. After excluding those participants with a MI prior to baseline and those with missing adiponectin 2900 NOMAS participants were included in the analytic sample. The study was approved by the Columbia University and University of Miami IRBs and all subjects provided informed consent.
Baseline Evaluation
Data were collected through interviews with trained bilingual research assistants in English or Spanish. Physical and neurological examinations were conducted by study neurologists. Race-ethnicity was based upon self-identification through a series of questions modeled after the US census and conforming to standard definitions outlined by Directive 15 (18).
Standardized questions were adapted from the Behavioral Risk Factor Surveillance System by the Centers for Disease Control regarding hypertension, diabetes, smoking, and cardiac conditions (19). Smoking was categorized as never smoking, former smoking, and current (within the past year) smoking. Moderate alcohol use was defined as current drinking of >1 drink per month and ≤2 drinks per day. Hypertension was defined as a blood pressure ≥140/90 mmHg (based on the average of two measurements during one sitting), anti-hypertensive medication use, or the patient’s self-reported hypertension. Diabetes mellitus was defined by the patient’s self-reported diabetes, use of insulin or oral anti-diabetic medication, or fasting glucose ≥126 mg/dl. Fasting lipid profile was measured at enrollment. Physical activity was defined as the frequency and duration of 14 different recreational activities during the 2-week period before the interview, as described previously (20). Waist measurements were determined to the nearest inch. Elevated waist circumference was defined as >35.2 inches for women and >40.8 inches for men (the Third Report of the National Cholesterol Education Program: Adult Treatment Panel III (NCEP ATP III)(21). hsCRP was collected at baseline from 2091 of the study participants. Carotid plaque presence and carotid intima-media thickness (cIMT), two distinct markers of atherosclerosis, were measured by B-mode ultrasound, as described previously (2).
Adiponectin
Baseline adiponectin was measured from stored plasma using a commercially available sandwich ELISA (Mercodia, Winston Salem NC; Catalogue No. 10-1193-01). The assay uses standards in the range of 5 to 300 ng/mL; because human sera adiponectin levels are in the microgram per milliliter range, samples were diluted (approximately 1:100) before assay. The intra- and interassay coefficients of variation were < 4% and <7%, respectively.
Prospective Follow-up
Annual telephone screening was conducted to determine changes in vital status, detect neurologic events, document interval hospitalizations, and review risk factor status, medication changes, and changes in functional status. Persons who screened positive had an in-person assessment, including chart review and physician examination. Outcome events were detected through ongoing hospital surveillance of admission and discharge data from all area hospitals, including screening of International Classification of Diseases-9 codes.
Definition of Outcomes
The outcomes were (1) a combined incident vascular event (incident stroke, MI, or vascular death) as well as (2) incident stroke, (3) incident MI, and (4) vascular death. Vascular death included death due to stroke, MI, heart failure, pulmonary embolus, cardiac arrhythmia, or other vascular cause. These are ICD-9 codes 390–459. Follow-up procedures and outcome classifications were detailed previously (22, 23). Briefly, all hospitalization medical records were reviewed to confirm the details of suspected events. Outcome events were reviewed by a specially trained research assistant and, when available, medical records were reviewed for all outcome events. Two neurologists independently classified the strokes after review of the data, and one of the principal investigators (RLS, MSVE) adjudicated disagreements.
Statistical Analysis
Cox proportional hazards models were used to examine the association between adiponectin and vascular events, and hazard ratios (HR) and 95% confidence intervals (CI) were calculated. Person-time of follow-up was accrued from baseline to the end of follow-up (March, 2012), outcome event, death or loss to follow-up, whichever came first. Adiponectin was examined continuously and in quartiles, with the fourth quartile as the referent. The following sequence of models was constructed: (1) Univariate; (2) Adjusted for demographics (age, sex, race/ethnicity); (3) Adjusted for demographics, smoking, hypertension, diabetes, LDL cholesterol, HDL cholesterol, triglycerides, waist circumference, moderate alcohol use, moderate-heavy physical activity, and previous cardiac disease history. We examined possible interactions between adiponectin and age, sex, race/ethnicity, waist circumference, diabetes, hypertension, lipid levels, previous cardiac disease history, plaque, and cIMT in model 3, and stratified analyses were conducted when effect modification was suggested (interaction p<0.05).
Several sensitivity analyses were conducted. First, we included estimated glomerular filtration rate (eGFR), carotid plaque presence, and cIMT as additional covariates to model 3. Second, we ran a series of analyses excluding participants who were underweight (BMI<18.5, N=59) to eliminate the possibility of bias due to frailty. Third, due to concern that the effect of adiponectin may differ based on prevalent cardiovascular disease risk, we stratified the study sample based on their cardiovascular health at baseline, defined as: healthy (free of diabetes, hypertension, metabolic syndrome, and previous cardiac disease history: N=491) vs. not (having any one or more of the listed conditions: N=2409). Due to the strong potential confounding by age, we regressed adiponectin on age and examined the residual (observed adiponectin level – predicted) as the primary exposure of interest in model 3 to confirm our findings. A sensitivity analysis model 4 was constructed, additionally controlling for hsCRP among the subset of participants with available data (N=2091). Lastly, in place of the individual demographic and vascular risk factors, we ran models only controlling for the NOMAS global vascular risk score, to calculate the effect estimate of adiponectin independent of this established score to quantify risk of vascular events in our study cohort. Details regarding the creation of the NOMAS global vascular risk score have been previously described (24).
Results
Among a study sample of 2900 NOMAS participants, the mean age at baseline was 69±10 years, mean BMI was 28.0±5.6, 37% were men, 21% white, 53% Hispanic, and 24% black, 21% had diabetes, 73% had hypertension, and 42% had the metabolic syndrome. The distribution of sample characteristics did not differ from the overall cohort (N=3,298). The mean adiponectin was 11.4±6.2 μg/ml, median was 9.8, and the range was 2.1–53.3 μg/ml (1st quartile 2.1–7.0, 2nd quartile 7.0–9.8, 3rd quartile 9.8–13.8, 4th quartile 13.8–53.3). Over a mean follow-up of 10 years, 692 incident vascular events accrued, including 269 strokes, 230 MI’s, and 410 vascular deaths.
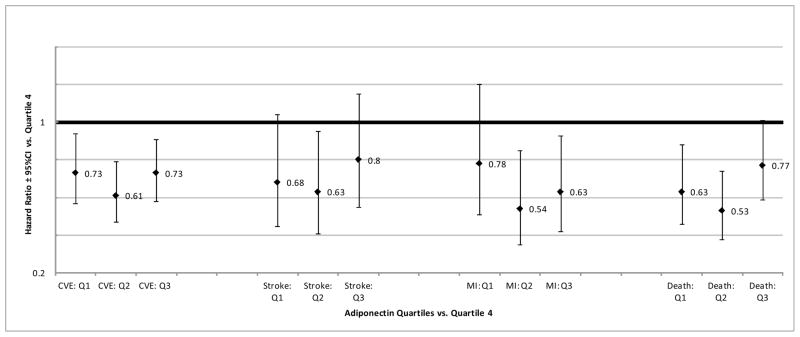
Figure 1 is a graphical representation of the association between adiponectin quartiles and risk of the four study outcomes (combined vascular events, stroke, MI, and vascular death) in multivariable-adjusted model 3.
Figure 1. Adiponectin Quartiles and Vascular Outcomes.
Abbreviations: CVE=combined vascular events, MI=myocardial infarction, Death=Vascular death, Q1=Adiponectin Quartile1, Q2= Adiponectin Quartile 2, Q3= Adiponectin Quartile 3, CI=confidence interval
Table 1 shows the association between adiponectin and risk of combined vascular events in the three primary Cox models, as well as the sensitivity model 4 controlling for hsCRP and model 5 controlling for the NOMAS global vascular risk score. In models 1, 3, 4, and 5 a decreased risk of vascular events is suggested with lower adiponectin levels. However, examination of the quartile analysis suggested a non-linear relationship with a reduction in risk observed among those in adiponectin quartiles 1–3 vs. quartile 4, and the lowest effect estimates observed in quartile 2. The consistency of effect estimates between models 1 and 3 and the attenuation of effects in model 2 suggests that residual confounding by age, sex, and race/ethnicity in model 1 is counterbalanced by residual confounding by vascular risk factors included in model 3. The results remained consistent when we controlled for eGFR, carotid plaque, or cIMT in model 3 (data not shown). The results also remained generally consistent when we controlled for hsCRP in model 4, and in fact the effect estimates became slightly stronger for quartiles 1 and 2. The results of model 5 suggest a significant decreased risk of vascular events among those with low adiponectin levels, particularly those in quartiles 2 and 3, independent of the NOMAS global vascular risk score.
Table 1.
Adiponectin and combined vascular events (Hazard Ratio, 95% Confidence Interval), N=692 events (overall N=2900)
| Adiponectin | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| Continuous | 1.04 (1.03–1.05) | 1.00 (0.99–1.02) | 1.03 (1.01–1.04) | 1.03 (1.01–1.05) | 1.02 (1.01–1.03) |
| Quartile 1 | 0.63 (0.51–0.77) | 1.05 (0.84–1.31) | 0.73 (0.57–0.94) | 0.64 (0.47–0.87) | 0.83 (0.67–1.03) |
| Quartile 2 | 0.50 (0.41–0.62) | 0.82 (0.65–1.03) | 0.61 (0.47–0.79) | 0.49 (0.44–0.80) | 0.69 (0.54–0.87) |
| Quartile 3 | 0.66 (0.54–0.81) | 0.88 (0.71–1.07) | 0.73 (0.58–0.91) | 0.75 (0.58–0.98) | 0.75 (0.60–0.93) |
| Quartile 4 | ref | ref | ref | ref | ref |
Model 1: Univariate
Model 2: Adjusting for age, sex, race/ethnicity
Model 3: Adjusting for age, sex, race/ethnicity, smoking, hypertension, diabetes, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, waist circumference, moderate alcohol use, moderate-heavy physical activity, previous cardiac disease history
Model 4: Adjusting for the variables in model 3 + hsCRP (N=2091)
Model 5: Adjusting for the NOMAS global vascular risk score
When adiponectin was regressed on age and the residual (examined continuously) was included in model 3 instead of adiponectin and age, the conclusions remained the same – higher than age-predicted adiponectin was associated with an increased risk of vascular events (data not shown). We did not observe a significant interaction between age and adiponectin in model 3. However, when we stratified the analysis of adiponectin with combined vascular events by age strata (Figure 2) the association between high adiponectin and increased risk of vascular events was stronger among those under the age of 65, with protective effects observed among those in the first three quartiles of adiponectin vs. quartile 4 controlling for all vascular risk factors in model 3. Again, examination of the quartiles did not suggest a dose-response relationship among this younger subset of the population.
Figure 2. Adiponectin Quartiles and Combined Vascular Events Stratified by Age, Waist Circumference, and Race/Ethnicity.
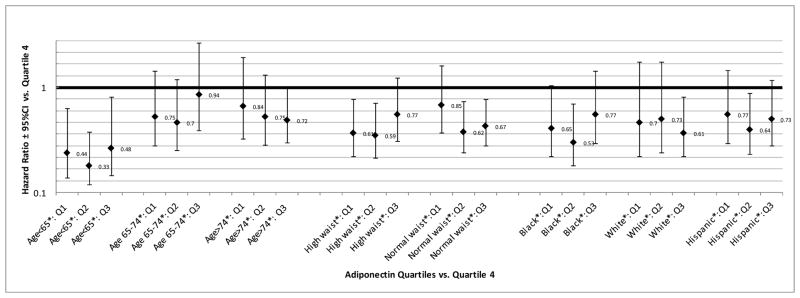
Abbreviations: Q1=Adiponectin Quartile1, Q2= Adiponectin Quartile 2, Q3= Adiponectin Quartile 3, CI=confidence interval
* Sample size and HR (95% CI) for continuous adiponectin: Age<65(N=1070, 119 events)=1.07 (1.03–1.11); Age 65–74(N=983, 257 events)=1.03 (1.01–1.06); Age>74(N=847, 316 events)=1.01 (0.99–1.03); High waist (sex-specific)(N=1205, 297 events)=1.05 (1.03–1.08); Normal waist (sex-specific)(N=1695, 395 events)=1.02 (1.00–1.04); Black(N=706, 212 events)=1.03 (1.01–1.06); White(N=584, 178 events)=1.02 (1.00–1.05); Hispanic(N=1545, 291 events)=1.03 (1.01–1.06)
Effect modification for the adiponectin and vascular events relationship was not observed for sex, race/ethnicity, diabetes, hypertension, lipid levels, previous cardiac disease history, plaque, or cIMT. The association between adiponectin quartiles and combined vascular events stratified by race/ethnicity is shown in Figure 2. However, we did find an interaction between waist circumference and adiponectin, and therefore a stratified analysis was conducted by the sex-specific guidelines for elevated waist circumference, as shown in Figure 2. The positive association between adiponectin and combined vascular events appeared to be stronger among those with elevated waist circumference, and this difference by waist circumference appeared to be most pronounced for the protective effect seen in the first quartile of adiponectin.
Out of the 491 participants classified as healthier at baseline, 64 vascular events were accrued over follow-up. In this group, after adjustment for demographics, smoking, alcohol, physical activity, waist circumference, and lipids, greater adiponectin was associated with an increased risk of vascular events (per μg/ml, RR=1.05, 95% CI 1.01–1.09). Among the 2409 who were less healthy at baseline, 628 experienced a vascular event during follow-up, and in a similar multivariable-adjusted model greater adiponectin was also associated with an increased risk of vascular events (per μg/ml, RR=1.02, 95% CI 1.01–1.04). Although we saw no significant effect modification by vascular health profile at baseline (p>0.05), the effect estimates for adiponectin quartile 1–3 vs. quartile 4 were more protective among those classified as healthy at baseline (healthy: q1 vs. q4 RR=0.46, p=0.11, q2 vs. q4 RR=0.45, p=0.10, q3 vs. q4 RR=0.63, p=0.20; unhealthy: q1 vs. q4 RR=0.78, p=0.06, q2 vs. q4 RR=0.64, p=0.001, q3 vs. q4 RR=0.74, p=0.01).
The relationships between adiponectin and risk of stroke, MI, and vascular death considered individually are shown in Table 2, 3, and 4 respectively. For all three outcomes, when adiponectin was examined continuously we observed a significant positive association, with similar magnitude. For all three outcomes, the effect estimates were most protective for the second adiponectin quartile as compared to quartile 4. For MI, the trend appeared almost j-shaped with protective associations observed for quartiles 2 and 3 vs. quartile 4. The risk of vascular death was significantly reduced for those in the first two quartiles of adiponectin. As in the analysis of combined vascular events, the results and conclusions remained consistent when eGFR was included in model 3, when those who were underweight at baseline were excluded, and when adiponectin was regressed on age and the residual was included as the primary exposure (data not shown). In general, the conclusions remained the same in models 4 controlling for hsCRP, and in fact, in some analyses the effect estimates became stronger (more protective) for adiponectin in relation to the outcomes controlling for hsCRP.
Table 2.
Adiponectin and stroke (Hazard Ratio, 95% Confidence Interval), N=269 events
| Adiponectin | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Continuous | 1.03 (1.01–1.04) | 1.01 (0.99–1.03) | 1.03 (1.01–1.06) | 1.03 (1.01–1.06) |
| Quartile 1 | 0.73 (0.53–1.01) | 0.88 (0.62–1.27) | 0.68 (0.45–1.04) | 0.61 (0.38–0.99) |
| Quartile 2 | 0.61 (0.43–0.86) | 0.75 (0.52–1.09) | 0.63 (0.41–0.95) | 0.61 (0.38–0.99) |
| Quartile 3 | 0.79 (0.57–1.10) | 0.91 (0.66–1.27) | 0.80 (0.55–1.15) | 0.57 (0.36–0.92) |
| Quartile 4 | ref | ref | ref | ref |
Model 1: Univariate
Model 2: Adjusting for age, sex, race/ethnicity
Model 3: Adjusting for age, sex, race/ethnicity, smoking, hypertension, diabetes, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, waist circumference, moderate alcohol use, moderate-heavy physical activity, previous cardiac disease history
Model 4: Adjusting for the variables in model 3 + hsCRP (N=2091)
Table 3.
Adiponectin and MI (Hazard Ratio, 95% Confidence Interval), N=230 events
| Adiponectin | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Continuous | 1.03 (1.01–1.05) | 0.99 (0.97–1.01) | 1.02 (1.00–1.05) | 1.03 (1.00–1.06) |
| Quartile 1 | 0.74 (0.53–1.04) | 1.32 (0.90–1.93) | 0.78 (0.51–1.20) | 0.54 (0.32–0.92) |
| Quartile 2 | 0.54 (0.37–0.78) | 0.91 (0.61–1.36) | 0.54 (0.35–0.85) | 0.41 (0.24–0.71) |
| Quartile 3 | 0.64 (0.44–0.91) | 0.85 (0.59–1.22) | 0.63 (0.42–0.93) | 0.67 (0.42–1.06) |
| Quartile 4 | ref | ref | ref | ref |
Model 1: Univariate
Model 2: Adjusting for age, sex, race/ethnicity
Model 3: Adjusting for age, sex, race/ethnicity, smoking, hypertension, diabetes, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, waist circumference, moderate alcohol use, moderate-heavy physical activity, previous cardiac disease history
Model 4: Adjusting for the variables in model 3 + hsCRP (N=2091)
Table 4.
Adiponectin and vascular death (Hazard Ratio, 95% Confidence Interval), N=410 events
| Adiponectin | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Continuous | 1.06 (1.04–1.07) | 1.01 (1.00–1.03) | 1.03 (1.02–1.05) | 1.03 (1.01–1.05) |
| Quartile 1 | 0.43 (0.33–0.56) | 0.88 (0.65–1.18) | 0.63 (0.46–0.88) | 0.52 (0.35–0.78) |
| Quartile 2 | 0.38 (0.29–0.51) | 0.73 (0.54–0.99) | 0.53 (0.38–0.74) | 0.56 (0.38–0.82) |
| Quartile 3 | 0.63 (0.50–0.81) | 0.91 (0.71–1.17) | 0.77 (0.59–1.01) | 0.79 (0.56–1.09) |
| Quartile 4 | ref | ref | ref | ref |
Model 1: Univariate
Model 2: Adjusting for age, sex, race/ethnicity
Model 3: Adjusting for age, sex, race/ethnicity, smoking, hypertension, diabetes, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, waist circumference, moderate alcohol use, moderate-heavy physical activity, previous cardiac disease history
Model 4: Adjusting for the variables in model 3 + hsCRP (N=2091)
Discussion
Low and moderate adiponectin levels at baseline were associated with a significant decreased risk of incident vascular events in our race/ethnically diverse population-based cohort study in Northern Manhattan. Low-moderate adiponectin was also associated specifically with decreased risk of stroke, MI, and vascular death when these outcomes were considered separately. These trends persisted in models that controlled for demographic variables, behavioral risk factors such as smoking and physical activity, as well as traditional vascular risk factors including waist circumference, diabetes, hypertension, lipid levels, and markers of atherosclerosis. The associations were observed among those with and without an elevated waist circumference, and among those with and without a healthy cardiovascular disease risk profile at baseline. Our results did not suggest a dose-response relationship.
The observed association between adiponectin levels and risk of vascular events was not expected because we and others have previously shown a strong and independent relationship between low adiponectin and many important factors associated with an increased risk of vascular events, including elevated blood pressure, insulin resistance, obesity, lipid levels, renal function, the metabolic syndrome, and carotid atherosclerosis (in press at Metabolic Syndrome and Related Disorders) (2). In addition, several studies have reported an increased risk of vascular events among those with lower adiponectin levels. In some studies this association persisted after controlling for vascular risk factors, while in others the association was attenuated and lost statistical significance (5)(3, 4). Of note, in the current study we found that the other vascular risk factors included as covariates were associated with the risk of vascular events as expected. Specifically, an elevated risk of vascular events was associated with increased age, male sex, smoking, hypertension, diabetes, previous cardiac disease, and low HDL, and a decreased risk of vascular events was associated with moderate alcohol use and moderate-heavy physical activity (data not shown).
However, ours is not the first study to suggest that, in fact, high adiponectin may not be a reliable biomarker for a decreased risk of stroke and MI, but rather may be associated with an increase in risk. In a nested case-control study within the all male PRIME study, each standard deviation increase in adiponectin was associated with an approximate two-fold increased risk of ischemic stroke during 10 years of follow-up after adjusting for vascular risk factors (9). Another prospective study conducted among an older group of men in the British Regional Heart Study also reported that those in the top tertile of adiponectin had a significantly increased risk of coronary heart disease, CVD mortality, as well as all-cause mortality (10). However, they found that controlling for N-terminal pro-brain natriuretic peptide (NT-proBNP) attenuated the association, and concluded that a concomitantly elevated NT-proBNP may account for some of the observed relationship between adiponectin and CVD morbidity and mortality. Unfortunately, we do not have data on NT-proBNP to test this hypothesis in NOMAS.
In the Dutch prospective Hoorn Study, high adiponectin was associated with an increased risk of CVD mortality (12). However, they reported possible effect modification by the presence of prevalent CVD, as there was a nonsignificant inverse trend between adiponectin and CVD mortality among those without prevalent CVD, and a nonsignificant positive trend among those with prevalent CVD. The authors suggested that among individuals with prevalent CVD, adiponectin may be up-regulated as a compensatory mechanism due to cardiovascular damage or frailty. While we are unable to adequately test this hypothesis in our cohort free of stroke and MI prior to baseline, we did not observe effect modification by previous cardiac disease history, atherosclerosis at baseline, or baseline vascular disease risk profile. In addition, we found that our conclusions remained the same when we excluded those who were underweight at baseline. In opposition to the hypothesis that the association between high adiponectin and cardiovascular disease risk may be due to age-related decreases in fat-free mass, studies have also found that high adiponectin levels remained associated with increased CVD risk after adjusting for weight changes (11, 13). In NOMAS we also found that the association was strongest among those in the youngest age strata, in which frailty is presumed to be less common.
Of note, all of the studies described above were conducted in predominantly Caucasian populations. In NOMAS, we have previously shown that adiponectin levels varied across race/ethnic groups, and in multivariable-adjusted models adiponectin levels were elevated among non-Hispanic blacks and Hispanic compared to non-Hispanic whites, confirming some previous studies suggesting race/ethnic variability in adiponectin levels. Race/ethnic disparities in risk of vascular events are also well-established. Incidence data from NOMAS have demonstrated that blacks had a 2.4 fold increased annual stroke incidence, and Hispanics a 2-fold increased annual stroke incidence compared to whites (25, 26). These findings underscore the need for studies on the relationship between adiponectin and risk of vascular events in racially and ethnically diverse communities, a strength of NOMAS. However, the Health, Aging, and Body Composition study also included a large population of older blacks and whites age 70–79. In this study, the relationships between adiponectin and prevalent and incident CHD were modified by race, such that among blacks elevated adiponectin was associated with an increased risk of prevalent and incident CHD, independent of covariates, while in whites an inverse association was attributed to confounding by HDL and glucose (14). While we did not observe significant effect modification by race/ethnicity for the relationship between adiponectin and vascular outcomes in NOMAS, the power to detect such interactions was low, and further study in similar race/ethnically diverse cohort studies is needed. The association between high adiponectin and risk of CHD limited to blacks in the Health, Aging, and Body Composition study suggests that the unique race/ethnic makeup of the NOMAS population may account, in part, for why we observed such a strong positive relationship between adiponectin and risk of vascular events in our cohort as compared to some other predominantly Caucasian cohort studies.
Mechanisms underlying a potential positive association between adiponectin and vascular disease risk are not known. While confounding by kidney disease has been suggested, our conclusions remained consistent when we controlled for eGFR, and we did not find eGFR to be an effect modifier as well. The concept of adiponectin resistance has been suggested in some animal and human studies, and has been hypothesized as a possible underlying mechanism to explain the unexpected increased risk of cardiovascular disease with high adiponectin levels (27–29). The possibility that elevated adiponectin may in fact have deleterious effects in all or a subset of the population requires further investigation, despite the fact that it is inversely associated with so many vascular disease risk factors including insulin resistance, lipid levels, inflammation, and atherosclerosis.
The current study is the first to our knowledge to examine the association between adiponectin and combined vascular events, including stroke and MI, in the same race/ethnically diverse cohort. The strength of the associations was similar for stroke and MI, but the shape of the relationship with MI appeared j-shaped, which was not as apparent for stroke.
In addition to the racially and ethnically diverse population, study strengths include the high follow-up rate, confirmation of outcome events, and comprehensive data on other established vascular disease risk factors. However, some limitations are notable. We did not have information on leptin, another important adipose-derived hormone that regulates energy intake and expenditure. It has been suggested that the ratio of leptin and adiponectin may be etiologically relevant (30–31). Although we did not measure the high-molecular weight form of adiponectin, total adiponectin levels have been shown to be highly correlated with the high-molecular weight form (32). However, it has been suggested that the different forms of adiponectin measured across studies may account for some of the discrepancies in the literature, and racial differences in the proportion of high-molecular-weight-form may account for racial differences in the relationship between adiponectin and CHD (14). We also lacked information on changes in adiponectin levels and weight during the course of follow-up. Data have suggested that plasma total adiponectin levels remain stable over a shorter time period of one year (33). The power to detect effect modification was only modest in the current study. Although we did not observe effect modification by baseline vascular disease risk profile or race/ethnicity, the findings may not be generalizable to other study populations. Lastly, residual confounding by both measured and unmeasured vascular risk factors remains a potential source of bias.
In the multi-ethnic population-based NOMAS cohort, moderate-low adiponectin was associated with a significantly decreased risk of vascular events, including stroke and MI, during follow-up, despite the fact that low adiponectin levels were associated with an elevated vascular risk profile. These counter-intuitive findings have now been observed in several studies, underscoring the need for further research on the potential role of adiponectin as a useful biomarker of vascular disease risk and the mechanisms explaining the inconsistent observations in the literature.
Highlights.
Low-moderate adiponectin was associated with a decreased risk of vascular events.
A dose-response relationship was not suggested.
The association was independent of demographics and vascular risk factors.
The association was stronger among those with elevated waist circumference.
Adiponectin was positively associated with stroke, MI, and vascular death.
Acknowledgments
Funding: Funding for this study was provided by a grant from the NINDS (R37 NS 29993). The funding source had no involvement in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lara-Castro C, Fu Y, Chung BH, Garvey WT. Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol. 2007 Jun;18(3):263–270. doi: 10.1097/MOL.0b013e32814a645f. [DOI] [PubMed] [Google Scholar]
- 2.Gardener H, Sjoberg C, Crisby M, Goldberg R, Mendez A, Wright CB, et al. Adiponectin and Carotid Intima-Media Thickness in the Northern Manhattan Study. Stroke. 2011 Dec 22; doi: 10.1161/STROKEAHA.111.641761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003 Jan 1;23(1):85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 4.Frystyk J, Berne C, Berglund L, Jensevik K, Flyvbjerg A, Zethelius B. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007 Feb;92(2):571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 5.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004 Apr 14;291(14):1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 6.Rajpathak SN, Kaplan RC, Wassertheil-Smoller S, Cushman M, Rohan TE, McGinn AP, et al. Resistin, but not adiponectin and leptin, is associated with the risk of ischemic stroke among postmenopausal women: results from the Women’s Health Initiative. Stroke. 2011 Jul;42(7):1813–1820. doi: 10.1161/STROKEAHA.110.607853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto M, Ishikawa S, Kajii E. Association of adiponectin with cerebrovascular disease: a nested case-control study. Stroke. 2008 Feb;39(2):323–328. doi: 10.1161/STROKEAHA.107.497552. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005 Oct;90(10):5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 9.Prugger C, Luc G, Haas B, Arveiler D, Machez E, Ferrieres J, et al. Adipocytokines and the risk of ischemic stroke: the PRIME Study. Ann Neurol. 2012 Apr;71(4):478–486. doi: 10.1002/ana.22669. [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Welsh P, Whincup PH, Sawar N, Thomas MC, Gudnarsson V, et al. High adiponectin and increased risk of cardiovascular disease and mortality in asymptomatic older men: does NT-proBNP help to explain this association? Eur J Cardiovasc Prev Rehabil. 2011 Feb;18(1):65–71. doi: 10.1097/HJR.0b013e32833b09d9. [DOI] [PubMed] [Google Scholar]
- 11.Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007 Jul 23;167(14):1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 12.Dekker JM, Funahashi T, Nijpels G, Pilz S, Stehouwer CD, Snijder MB, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008 Apr;93(4):1489–1496. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 13.Kizer JR, Barzilay JI, Kuller LH, Gottdiener JS. Adiponectin and risk of coronary heart disease in older men and women. J Clin Endocrinol Metab. 2008 Sep;93(9):3357–3364. doi: 10.1210/jc.2008-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaya AM, Wassel Fyr C, Vittinghoff E, Havel PJ, Cesari M, Nicklas B, et al. Serum adiponectin and coronary heart disease risk in older Black and White Americans. J Clin Endocrinol Metab. 2006 Dec;91(12):5044–5050. doi: 10.1210/jc.2006-0107. [DOI] [PubMed] [Google Scholar]
- 15.Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of adiponectin with coronary heart disease and mortality: the Rancho Bernardo study. Am J Epidemiol. 2007 Jan 15;165(2):164–174. doi: 10.1093/aje/kwk001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva FM, de Almeida JC, Feoli AM. Effect of diet on adiponectin levels in blood. Nutr Rev. 2011 Oct;69(10):599–612. doi: 10.1111/j.1753-4887.2011.00414.x. [DOI] [PubMed] [Google Scholar]
- 17.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, et al. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001 Aug;32(8):1725–1731. doi: 10.1161/01.str.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 18.Wallman KK, Hodgdon J. Race and ethnic standards for Federal statistics and administrative reporting. Stat Report. 1977;(77–110):450–4. [PubMed] [Google Scholar]
- 19.Sacco RL, Elkind M, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, et al. The protective effect of moderate alcohol consumption on ischemic stroke. JAMA. 1999 Jan 6;281(1):53–60. doi: 10.1001/jama.281.1.53. [DOI] [PubMed] [Google Scholar]
- 20.Sacco RL, Gan R, Boden-Albala B, Lin IF, Kargman DE, Hauser WA, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke. 1998 Feb;29(2):380–387. doi: 10.1161/01.str.29.2.380. [DOI] [PubMed] [Google Scholar]
- 21.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 22.Boden-Albala B, Sacco RL, Lee HS, Grahame-Clarke C, Rundek T, Elkind MV, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke. 2008 Jan;39(1):30–35. doi: 10.1161/STROKEAHA.107.496588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boden-Albala B, Cammack S, Chong J, Wang C, Wright C, Rundek T, et al. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS) Diabetes Care. 2008 Jun;31(6):1132–1137. doi: 10.2337/dc07-0797. [DOI] [PubMed] [Google Scholar]
- 24.Sacco RL, Khatri M, Rundek T, Xu Q, Gardener H, Boden-Albala B, et al. Improving global vascular risk prediction with behavioral and anthropometric factors. The multiethnic NOMAS (Northern Manhattan Cohort Study) J Am Coll Cardiol. 2009 Dec 8;54(24):2303–2311. doi: 10.1016/j.jacc.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998 Feb 1;147(3):259–268. doi: 10.1093/oxfordjournals.aje.a009445. [DOI] [PubMed] [Google Scholar]
- 26.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005 Mar 15;111(10):1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 27.Van Berendoncks AM, Garnier A, Beckers P, Hoymans VY, Possemiers N, Fortin D, et al. Functional adiponectin resistance at the level of the skeletal muscle in mild to moderate chronic heart failure. Circ Heart Fail. 2010 Mar;3(2):185–194. doi: 10.1161/CIRCHEARTFAILURE.109.885525. [DOI] [PubMed] [Google Scholar]
- 28.Bruce CR, Mertz VA, Heigenhauser GJ, Dyck DJ. The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes. 2005 Nov;54(11):3154–3160. doi: 10.2337/diabetes.54.11.3154. [DOI] [PubMed] [Google Scholar]
- 29.Mullen KL, Smith AC, Junkin KA, Dyck DJ. Globular adiponectin resistance develops independently of impaired insulin-stimulated glucose transport in soleus muscle from high-fat-fed rats. Am J Physiol Endocrinol Metab. 2007 Jul;293(1):E83–90. doi: 10.1152/ajpendo.00545.2006. [DOI] [PubMed] [Google Scholar]
- 30.Kotani K, Sakane N, Saiga K, Kurozawa Y. Leptin: adiponectin ratio as an atherosclerotic index in patients with type 2 diabetes: relationship of the index to carotid intima-media thickness. Diabetologia. 2005 Dec;48(12):2684–2686. doi: 10.1007/s00125-005-0015-4. [DOI] [PubMed] [Google Scholar]
- 31.Norata GD, Raselli S, Grigore L, Garlaschelli K, Dozio E, Magni P, et al. Leptin:adiponectin ratio is an independent predictor of intima media thickness of the common carotid artery. Stroke. 2007 Oct;38(10):2844–2846. doi: 10.1161/STROKEAHA.107.485540. [DOI] [PubMed] [Google Scholar]
- 32.Komura N, Kihara S, Sonoda M, Kumada M, Fujita K, Hiuge A, et al. Clinical significance of high-molecular weight form of adiponectin in male patients with coronary artery disease. Circ J. 2008 Jan;72(1):23–28. doi: 10.1253/circj.72.23. [DOI] [PubMed] [Google Scholar]
- 33.Pischon T, Hotamisligil GS, Rimm EB. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003 Apr;49(4):650–652. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]