Abstract
Purpose
To evaluate the effect of pelvic radiotherapy (RT) in patients with stage IV rectal cancer treated with resection of primary tumor with or without metastasectomy.
Materials and Methods
Medical records of 112 patients with stage IV rectal cancer treated with resection of primary tumor between 1990 and 2011 were retrospectively reviewed. Fifty-nine patients received synchronous or staged metastasectomy whereas fifty-three patients did not. Twenty-six patients received pelvic radiotherapy.
Results
Median overall survival (OS), locoregional recurrence-free survival (LRFS), and progression-free survival (PFS) of all patients was 27, 70, and 11 months, respectively. Pathologic T (pT), N (pN) classification and complete metastasectomy were statistically significant factors in OS (p = 0.040, 0.020, and 0.002, respectively). RT did not improve OS or LRFS. There were no significant factors in LRFS. pT and pN classification were also significant prognostic factors in PFS (p = 0.010 and p = 0.033, respectively). In the subgroup analysis, RT improved LRFS in patients with pT4 disease (p = 0.026). The locoregional failure rate of the RT group and the non-RT group were 23.1% and 33.7%, showing no difference in the failure pattern of both groups (p = 0.260).
Conclusion
Postoperative pelvic RT did not improve LRFS of all metastatic rectal cancer patients; however, it can be recommended to patients with pT4 disease. A complete resection of metastatic masses should be performed if possible.
Keywords: Rectal neoplasms, Neoplasm metastasis, Radiotherapy, Local neoplasm recurrence
Introduction
According to the data on age-standardized cancer incidence in 2009 from the Korea Central Cancer Registry, colorectal cancer is the second most common cancer in men (49.0/100,000) and the 3rd most common cancer in women (25.9/100,000). Colorectal cancer is shown to be one of the most sharply increased malignancies in Korea. Between 2000 and 2009, the incidence of colorectal cancer has increased by 6.8% in men and 5.1% in women, annually [1].
Stage IV colorectal cancer consists of 20% of colorectal cancer patients at the time of diagnosis, and shows a 11.9% of 5-year survival rates [2]. Some of stage IV rectal cancer patients with resectable metastatic disease in the liver or lung have a chance of curative surgical resection which can improve survival [3-6]. Additionally, even for the unresectable metastatic disease, the surgical resection of primary tumor can help them from symptoms, such as obstruction, perforation, pain, or bleeding [7,8].
As the survival rate of stage IV rectal cancer has improved, local control issues become more important. Despite an increased chance of survival following the resection of primary and metastatic liver tumors, the reported rate of pelvic failure is approximately 30-35% and the rate of extra-hepatic metastases is up to 67% [9,10].
Preoperative or postoperative pelvic radiotherapy (RT) can improve the local control of locally advanced rectal cancer [11-18]. Despite the consensus that adjuvant pelvic RT provides benefits for locally advanced rectal cancer, the role of preoperative/postoperative pelvic RT in rectal cancer with synchronous metastasis has not been clearly defined [19].
The aim of the present study is to evaluate the clinical implications of pelvic RT in rectal cancer patients with synchronous metastasis who received primary tumor resection.
Materials and Methods
Medical records of 112 patients with stage IV rectal cancer who received complete removal of primary tumors with or without metastasectomy in Seoul St. Mary's Hospital from March 1990 to February 2011 were retrospectively reviewed. All of the patients met the following eligibility criteria: histologically proven adenocarcinoma located within 12 cm from anal verge and no history of other malignancies. Synchronous metastasis was diagnosed during work-up or at the time of operation.
Each patient was evaluated through history, physical examination, routine blood tests, chest radiography, and other relevant studies. Pretreatment studies included computerized tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET).
1. Treatment
1) Surgical resection
All patients received a complete resection of primary tumor. Nine patients received anterior resection, 70 patients received low anterior resection, 17 patients received abdominoperineal resection, and 16 patients received Hartman's operation. Seventy-six patients received total mesorectal excision (TME) with pelvic lymph node dissection, whereas 12 patients did not. Fifty-nine patients received synchronous or staged complete resection of metastatic masses, and 53 patients did not. Pathologic report was re-classified by the 7th edition of the American Joint Committee on Cancer TNM classification.
2) Radiotherapy
Twenty-six patients received postoperative pelvic RT (RT group) and 86 patients did not receive RT (non-RT group). RT was delivered with a three- or four-field box technique with the patient in the prone position. The superior border of the RT field was L5/S1 junction and the inferior border was 3 cm below the distal extent of the primary tumor or at the bottom of the pubic bone. The lateral borders extended 1.5 cm lateral to the widest bony margins of the true pelvic side walls. The field also extended to the posterior aspect of the symphysis pubis. A total dose of 40 to 45 Gy was delivered to the whole pelvis and a boost dose of 5.4 to 10 Gy was delivered to the tumor bed and anastomosis site in a 1.8 to 2.0 Gy daily fraction. The radiation dose was modified by the physician's decision.
3) Chemotherapy
Neoadjuvant chemotherapy was delivered to 20 patients and adjuvant chemotherapy was delivered to all patients. Chemotherapy regimens were 5-fluorouracil (5-FU) only, 5-FU + leucovorin, FOLFOX, FOLFIRI, and others (Table 1).
Table 1.
Patients characteristics
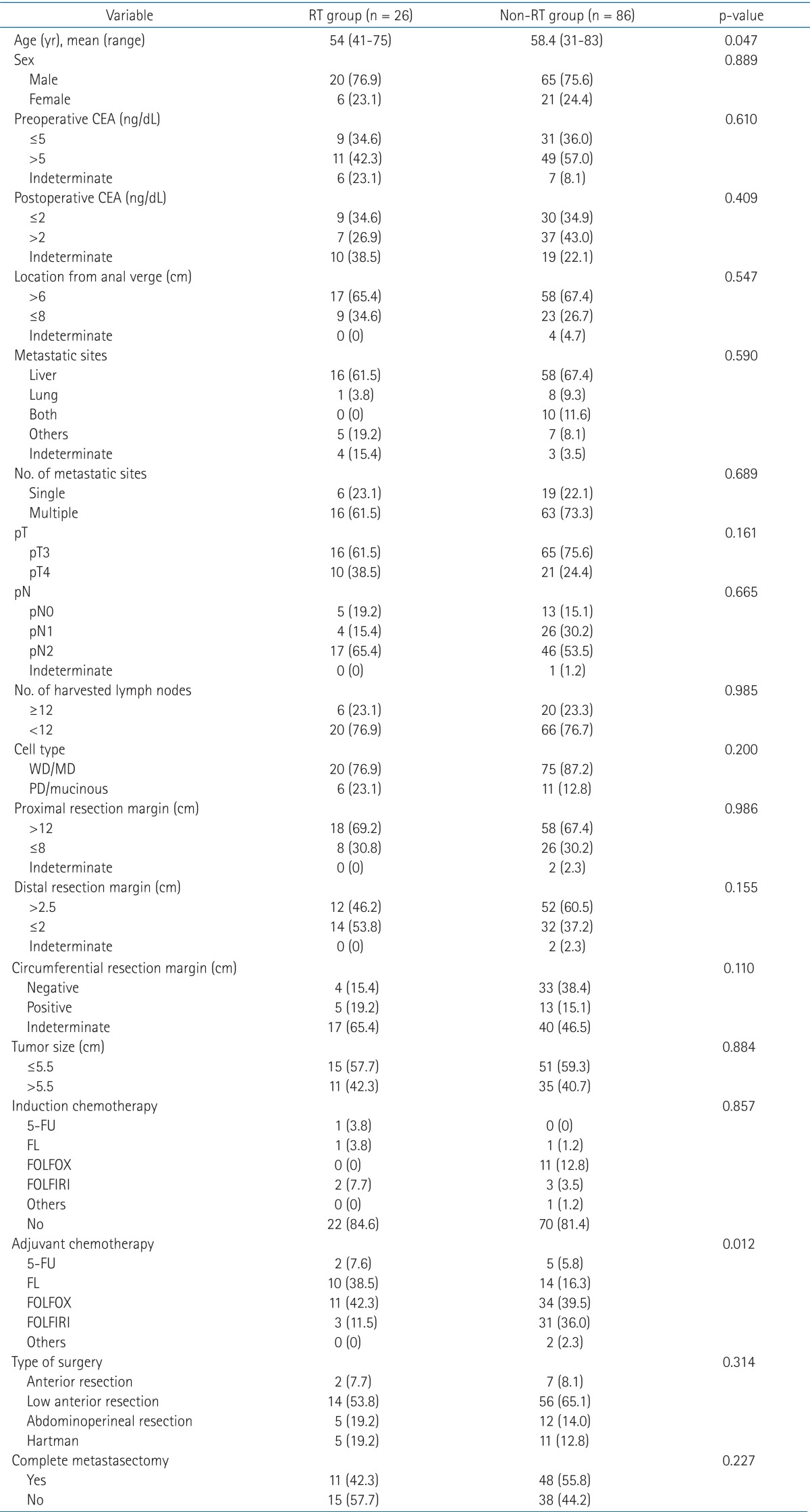
RT, radiotherapy; CEA, carcino-embryonic antigen; WD, well-differentiated; MD, moderate differentiated; PD, poorly differentiated; 5-fluorouracil, 5-FU; FL, 5-FU and leucovorin; FOLFOX, oxaliplatin, leucovorin, and 5-FU; FOLFIRI, irinotecan, leucovorin, and 5-FU.
2. Statistical analysis
The primary endpoint of the study is the locoregional recurrence-free survival (LRFS) and the overall survival (OS). LRFS was defined as time from the date of the operation to the date of the first evidence of locoregional recurrence at follow-up. Progression-free survival (PFS) was defined as time from the date of the operation to the date of the first evidence of disease progression at any location or the last follow-up. Dead cases were censored in estimating LRFS or PFS. OS was defined as the time from the date of the operation to the date of death for any cause or the last follow-up. Recurrences within the pelvis, such as anastomosis site or regional lymph nodes, were considered as locoregional failures; newly onsets or progressions of metastatic tumor outside the pelvis were considered as distant failures. Chi-square test, Fisher's exact test, or linear by linear association were used to compare the characteristics between the two patient groups. The Kaplan-Meier method with log-rank tests were used to estimate LRFS, PFS, and OS. Tarone-Ware statistics were used to compare the survival curves in the sub-group analysis of patients with pathologic T4 (pT4) disease. Multivariate analysis was performed using the Cox-proportional hazard model (backward Wald model). The Factors that revealed p-values < 0.30 in the univariate analysis were included for the multivariate analysis. The p-values ≤ 0.05 were considered statistically significant. Data were analyzed using SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA).
Results
1. Patient characteristics
The median follow-up duration was 24 months (range, 1 to 174 months) for all patients and 31 months (range, 18 to 174 months) for the surviving patients (n = 39). There was no significant difference in the median follow-up durations of 20.5 months (range, 2 to 174 months) for the RT group and 24 months (range, 1 to 156 months) for the non-RT group.
The median age was 58 years (range, 31 to 83 years). Seventy-four (66.1%) patients had liver metastasis, 9 (8.0%) patients had lung metastasis, and 10 (8.9%) patients had both liver and lung metastasis. The circumferential resection margin (CRM) was evaluated in only 55 patients, 9 in the RT group and 46 in the non-RT group. Five patients of the RT group and 13 patients in the non-RT group had positive CRM, showing no statistical significance (p = 0.110). All variables were comparable between the two groups, except for the age and chemotherapy regimen. Patients in the non-RT group were older than those in the RT group (p = 0.006); moreover, those in the non-RT group received irinotecan based regimen more frequently (p = 0.012). The characteristics of patients are summarized in Table 1.
2. Survival
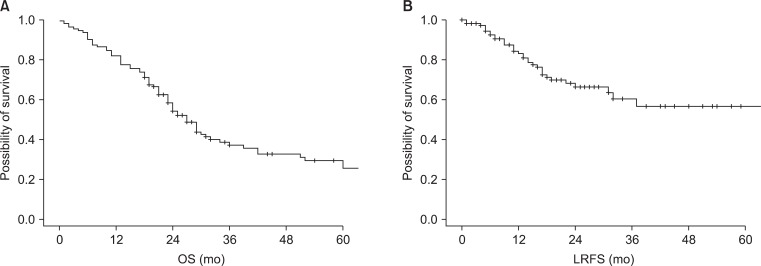
The median OS, LRFS, and PFS of all patients were 27, 70, and 11 months, respectively. Three-year OS, LRFS, and PFS were 37.2%, 60.4%, and 20.8%, respectively (Fig. 1).
Fig. 1.
(A) Overall survival (OS) of all patients and (B) locoregional recurrence-free survival (LRFS) of all patients.
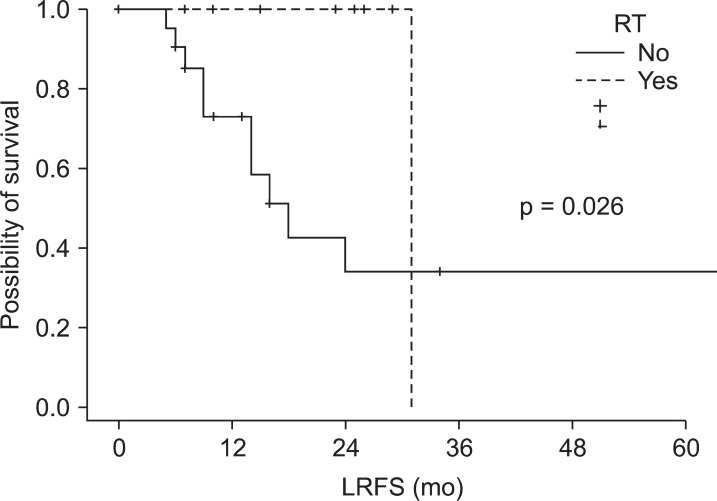
In the univariate analysis, patients with higher pN stage, incomplete metastasectomy, and pelvic RT were poor prognostic factors in the OS (p = 0.028, p < 0.001, and p = 0.038, respectively) (Table 2). Low pT and pN classification and complete metastasectomy improved OS significantly in the multivariate analysis (p = 0.040, 0.020, and 0.002, respectively) (Table 2). There were no factors influencing LRFS (Table 3). There was no difference between the RT group (58.2%) and the non-RT group (59.8%) in a 3-year LRFS (p = 0.563). In the subgroup analysis of pT4 disease, LRFS was significantly high in the RT group compared with the non-RT group (p = 0.026) (Fig. 2). In PFS, pT, and pN, the classifications were significant prognostic factors in the univariate analysis (p = 0.010 and 0.033, respectively) (Table 4), but not in the multivariate analysis (p = 0.125 and 0.159, respectively).
Table 2.
Univariate and multivariate analysis for overall survival
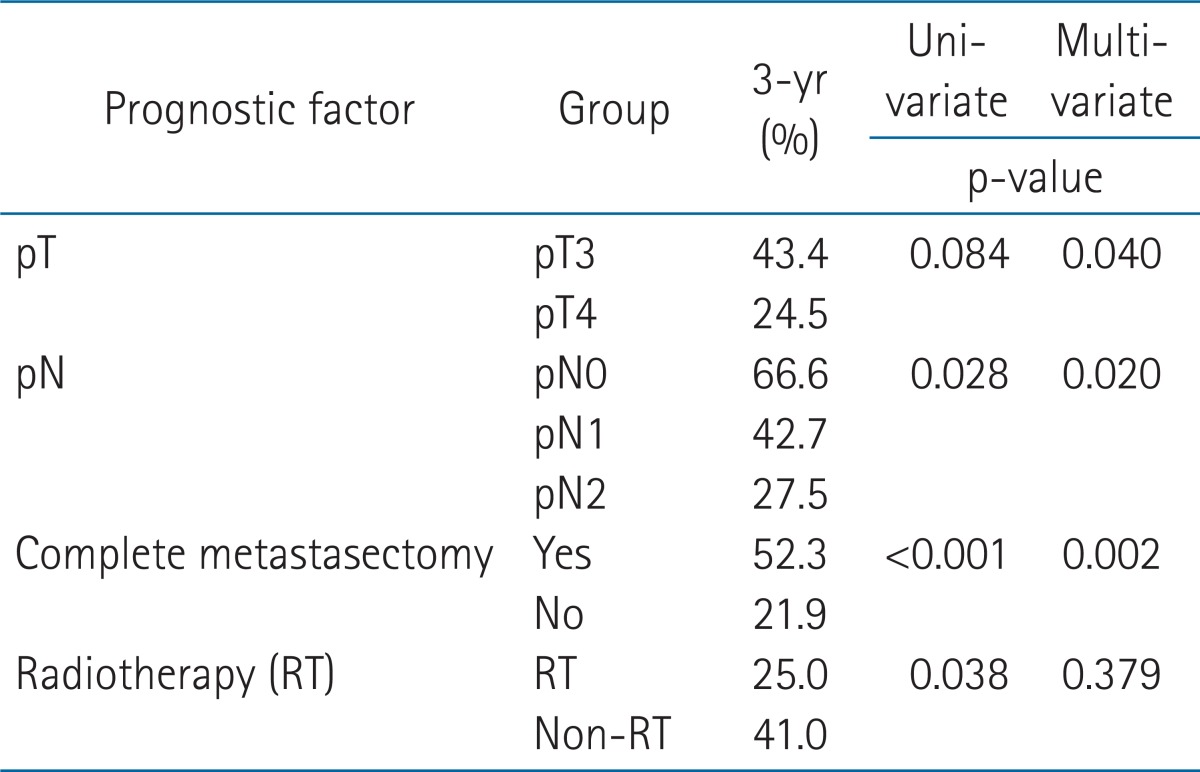
Table 3.
Univariate and multivariate analysis for locoregional recurrence-free survival
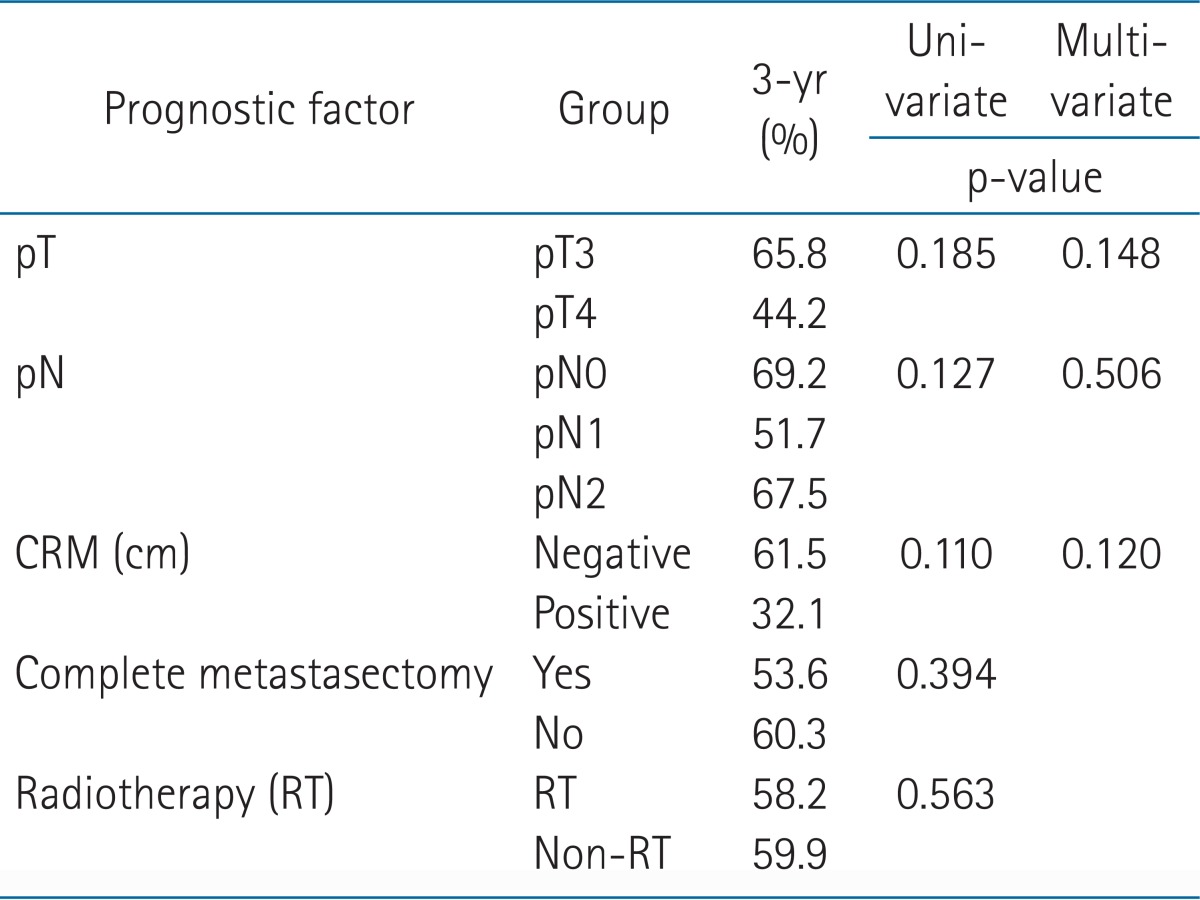
CRM, circumferential resection margin.
Fig. 2.
Locoregional recurrence-free survival (LRFS) of pT4 disease. RT, radiotherapy.
Table 4.
Univariate and multivariate analyses for progression-free survival
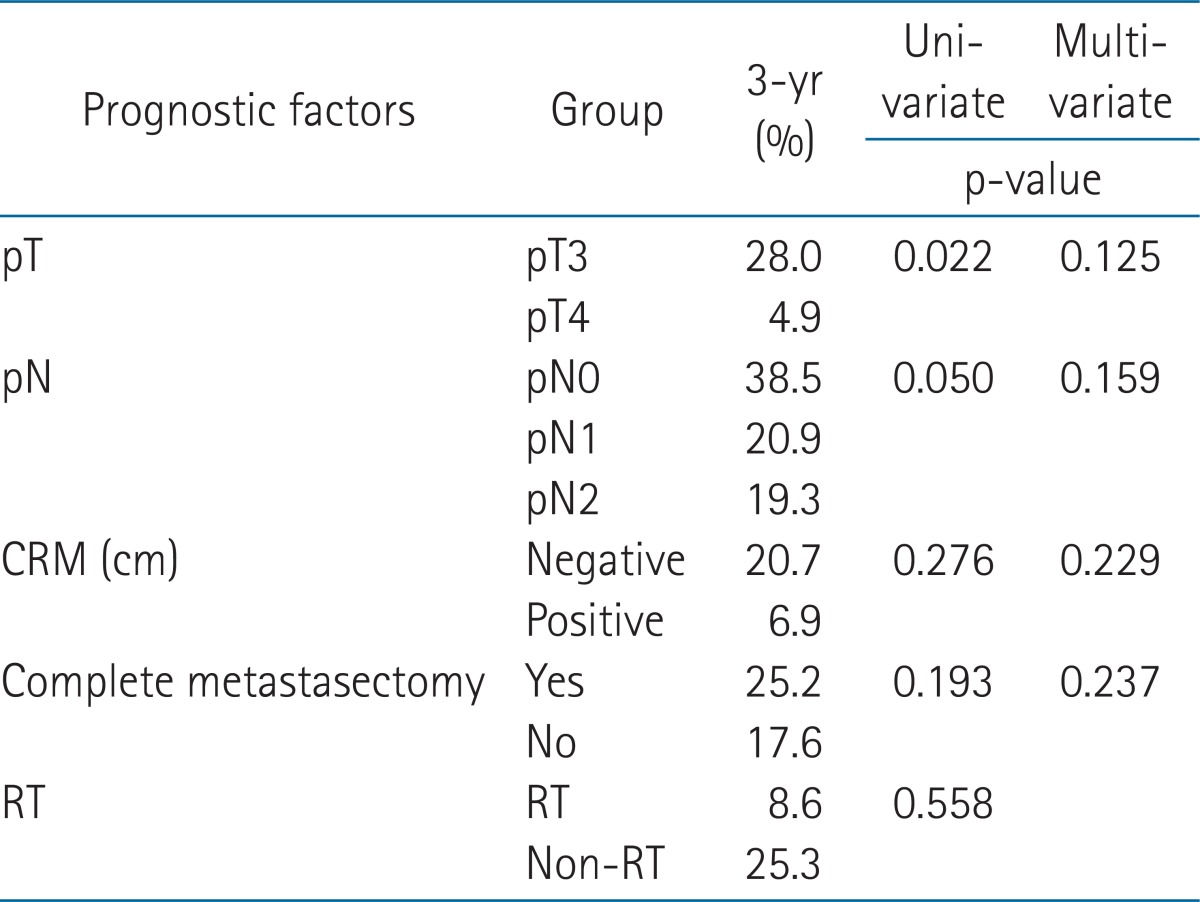
CRM, circumferential resection margin.
3. Recurrence
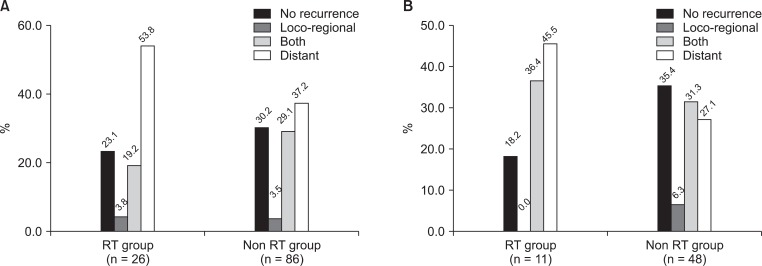
Profiles for the pattern of recurrence are depicted in Fig. 3A. During the follow-up period, no differences in the patterns and rate of recurrences were evident between the two groups. The recurrence rate in the RT group and the non-RT group was 76.9% and 69.8%, respectively (p = 0.260). For the RT and the non-RT groups, the locoregional failure occurred in 6 (23.1%) patients and 28 (32.6%) patients, respectively, locoregional failure alone occurred in 1 (3.8%) patient and 3 (3.5%) patients, respectively, and distant failure developed in 19 (73.1%) patients and 57 (66.3%) patients, respectively.
Fig. 3.
(A) Recurrence patterns of all patients and (B) recurrence patterns of the patients whose metastatic sites were completely resected. RT, radiotherapy.
In 59 patients with complete tumor removal, including the metastatic site, the overall recurrence rate of the RT group and the non-RT group was 81.8% and 60.4%, respectively. The locoregional failure of both groups was 4 (36.4%) patients and 18 (37.5%) patients, respectively. There were no patients with locoregional failure only in the RT group (Fig. 3B).
Discussion and Conclusion
In managing rectal cancer, local control is one of the most important issues for improving the treatment result. TME has diminished the locoregional recurrence rate [20,21]. In addition, preoperative or postoperative pelvic RT has shown better results in rectal cancer in terms of local control [14,22-25]. However, pelvic RT can induce several complications, including proctitis, enteritis, stricture, obstruction, and perforation, and delay systemic chemotherapy. For these reasons, there is debate as to whether or not to perform pelvic RT to stage IV rectal cancer patients.
The National Comprehensive Cancer Network (NCCN) Clinical Practice Guideline recommends pelvic RT to resectable stage IV [26]; however, there has yet to be a prospective study result. Some retrospective study reported the effect of the preoperative or postoperative pelvic RT for patients with stage IV rectal cancer. Kim et al. [27] reported that postoperative pelvic RT could improve LRFS in patients who had rectal cancer with resectable liver metastasis. Eighty-nine patients with synchronous distant metastases from rectal cancer were treated with TME alone or TME followed by pelvic RT. A 2-year pelvic failure-free survival rate of the RT group and the non-RT group was 80.8% and 64.8%, respectively (p = 0.028). There was no benefit in the OS. On the other hand, Chang et al. [28] reported that postoperative pelvic RT after the complete removal of tumors could not improve local control, DFS, or OS in patients with resectable liver or lung metastasis. Pasetto et al. [29] also reported no benefits of postoperative pelvic RT. In the case of unresectable metastatic rectal cancer, the palliative complete resection of primary tumor can be performed for symptom palliation. Further, pelvic chemoradiation can help local control. Crane et al. [30] have reported that pelvic RT has a palliative role in treating rectal cancer with liver metastasis. Symptomatic pelvic control rates were 81% and 91% in the chemoradiation and chemoradiation combined with surgery groups, respectively.
The purpose of this study was to evaluate the effect of pelvic RT in stage IV rectal cancer patients who received primary tumor resection; the results showed that pelvic RT could improve LRFS in T4 disease. However, pelvic RT could not improve the local control rate of stage IV rectal cancer who received a complete resection of primary tumor. However, a 2-year LRFS of the RT group and the non-RT group was 77.5% and 65.8%, respectively (p = 0.563), showing similar results with the report by Kim et al. [27] (80.8% and 64.8%, respectively; p = 0.028), which could reach a statistical significance.
The present study had several limitations. First, there were no strict criteria for radiation. The decision for RT was made by an individual surgeon or oncologist, which may be the main cause of selection bias. High risk patients for local recurrence were recommended to pelvic radiation more frequently. There was no significant difference in pathologic parameters including resection margin and staging, which are important factors for local recurrence between the two groups. However, a microscopic review of CRM was conducted in only a half of all patients, and in those patients, a positive CRM rate of the RT group was higher than that of the non-RT group, though statistically insignificant. The second limitation was heterogeneity of the study group in terms of stage and treatment modality; hence, statistical power decreased.
In conclusion, pelvic RT could not improve local control or OS in patients with metastatic rectal cancer who were treated by resection of primary tumor in this study. In patients with pT4 disease, pelvic RT could improve LRFS, thus, RT may be recommended to reduce pelvic failure. To further investigate the effect and candidates of pelvic RT more accurately, a prospective study should be conducted.
Acknowledgments
This work was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (0820010).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Institute SEER cancer statistics review 1975-2009 [Internet] Bethesda, MD: National Cancer Institute; c2012. [cited 2012 Nov 25]. Available from: http://seer.cancer.gov/csr/1975_2009_pops09/results_merged/sect_06_colon_rectum.pdf. [Google Scholar]
- 3.Tepper JE, O'Connell M, Hollis D, et al. Analysis of surgical salvage after failure of primary therapy in rectal cancer: results from Intergroup Study 0114. J Clin Oncol. 2003;21:3623–3628. doi: 10.1200/JCO.2003.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Wanebo HJ, Semoglou C, Attiyeh F, Stearns MJ., Jr Surgical management of patients with primary operable colorectal cancer and synchronous liver metastases. Am J Surg. 1978;135:81–85. doi: 10.1016/0002-9610(78)90014-4. [DOI] [PubMed] [Google Scholar]
- 5.Cady B, Monson DO, Swinton NW. Survival of patients after colonic resection for carcinoma with simultaneous liver metastases. Surg Gynecol Obstet. 1970;131:697–700. [PubMed] [Google Scholar]
- 6.Adson MA, van Heerden JA, Adson MH, Wagner JS, Ilstrup DM. Resection of hepatic metastases from colorectal cancer. Arch Surg. 1984;119:647–651. doi: 10.1001/archsurg.1984.01390180015003. [DOI] [PubMed] [Google Scholar]
- 7.Wood CB, Gillis CR, Blumgart LH. A retrospective study of the natural history of patients with liver metastases from colorectal cancer. Clin Oncol. 1976;2:285–288. [PubMed] [Google Scholar]
- 8.Lyass S, Zamir G, Matot I, Goitein D, Eid A, Jurim O. Combined colon and hepatic resection for synchronous colorectal liver metastases. J Surg Oncol. 2001;78:17–21. doi: 10.1002/jso.1117. [DOI] [PubMed] [Google Scholar]
- 9.Bozzetti F, Doci R, Bignami P, Morabito A, Gennari L. Patterns of failure following surgical resection of colorectal cancer liver metastases: rationale for a multimodal approach. Ann Surg. 1987;205:264–270. doi: 10.1097/00000658-198703000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekberg H, Tranberg KG, Andersson R, et al. Pattern of recurrence in liver resection for colorectal secondaries. World J Surg. 1987;11:541–547. doi: 10.1007/BF01655821. [DOI] [PubMed] [Google Scholar]
- 11.Song JH, Jang HS, Kim YS, et al. The pathological and clinical effects of preoperative chemoradiation in rectal cancer. J Korean Soc Ther Radiol Oncol. 2011;29:11–19. [Google Scholar]
- 12.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results-EORTC 22921. J Clin Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 13.Yoon MS, Nam TK, Kim HR, et al. Results of preoperative concurrent chemoradiotherapy for the treatment of rectal cancer. J Korean Soc Ther Radiol Oncol. 2008;26:247–256. [Google Scholar]
- 14.Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Kim SH, Kim JG, Cho HM, Shim BY. Preoperative chemoradiotherapy (CRT) followed by laparoscopic surgery for rectal cancer: predictors of the tumor response and the long-term oncologic outcomes. Int J Radiat Oncol Biol Phys. 2011;81:431–438. doi: 10.1016/j.ijrobp.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Jang HS, Kim JG, et al. Lymphovascular invasion is a significant prognosticator in rectal cancer patients who receive preoperative chemoradiotherapy followed by total mesorectal excision. Ann Surg Oncol. 2012;19:1213–1221. doi: 10.1245/s10434-011-2062-z. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Kim DY, Nam TK, et al. Long-term follow-up of preoperative pelvic radiation therapy and concomitant boost irradiation in locally advanced rectal cancer patients: a multi-institutional phase II study (KROG 04-01) Int J Radiat Oncol Biol Phys. 2012;84:955–961. doi: 10.1016/j.ijrobp.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 18.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 19.O'Neil BH, Tepper JE. Current options for the management of rectal cancer. Curr Treat Options Oncol. 2007;8:331–338. doi: 10.1007/s11864-007-0048-7. [DOI] [PubMed] [Google Scholar]
- 20.Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995;181:335–346. [PubMed] [Google Scholar]
- 21.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 22.Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don't know. JAMA. 1988;259:3571–3578. [PubMed] [Google Scholar]
- 23.Randomised trial of surgery alone versus surgery followed by radiotherapy for mobile cancer of the rectum. Medical Research Council Rectal Cancer Working Party. Lancet. 1996;348:1610–1614. [PubMed] [Google Scholar]
- 24.Goldberg PA, Nicholls RJ, Porter NH, Love S, Grimsey JE. Long-term results of a randomised trial of short-course low-dose adjuvant pre-operative radiotherapy for rectal cancer: reduction in local treatment failure. Eur J Cancer. 1994;30A:1602–1606. doi: 10.1016/0959-8049(94)00312-s. [DOI] [PubMed] [Google Scholar]
- 25.Stockholm Colorectal Cancer Study Group. Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol. 1996;3:423–430. doi: 10.1007/BF02305759. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology [Internet] Fort Washington, PA: National Comprehensive Cancer Network; c2012. [cited 2012 Jul 26]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [Google Scholar]
- 27.Kim JW, Kim YB, Kim NK, et al. The role of adjuvant pelvic radiotherapy in rectal cancer with synchronous liver metastasis: a retrospective study. Radiat Oncol. 2010;5:75. doi: 10.1186/1748-717X-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang CY, Kim HC, Park YS, et al. The effect of postoperative pelvic irradiation after complete resection of metastatic rectal cancer. J Surg Oncol. 2012;105:244–248. doi: 10.1002/jso.22109. [DOI] [PubMed] [Google Scholar]
- 29.Pasetto LM, Friso ML, Pucciarelli S, et al. Primary rectal carcinoma in patients with stage IV resectable disease at diagnosis. Anticancer Res. 2007;27:1079–1085. [PubMed] [Google Scholar]
- 30.Crane CH, Janjan NA, Abbruzzese JL, et al. Effective pelvic symptom control using initial chemoradiation without colostomy in metastatic rectal cancer. Int J Radiat Oncol Biol Phys. 2001;49:107–116. doi: 10.1016/s0360-3016(00)00777-x. [DOI] [PubMed] [Google Scholar]