Abstract
Background
reduced grip strength is associated with adverse health consequences, and there is interest in identifying modifiable influences. Cardiovascular drugs are commonly used by older people, but their effect on muscle strength is unclear.
Methods
we investigated associations between cardiovascular drug use and grip strength among 1,572 men and 1,415 women, aged 59-73, who participated in the Hertfordshire Cohort Study.
Results
Forty-five percent of participants were taking a cardiovascular drug. Furosemide was associated with average decreases in grip strength of 3.15 kg (95% confidence interval [CI] 0.90, 5.39, P<0.01) among men and 2.35 kg (95% CI 0.93, 3.77, P<0.01) among women after adjustment for age and height. Corresponding differences for nitrates were 1.84 kg (95% CI 0.29, 3.39, P=0.02) among men and 3.66 kg (95% CI 1.99, 5.33, P<0.01) among women. Calcium channel blockers and fibrates were associated with reduced grip among women. Statins were not associated with grip. The associations between grip strength and nitrate use in men and nitrate and fibrate use in women were robust to additional adjustment for comorbidity.
Conclusions
use of some cardiovascular drugs is associated with reduced grip strength in older people. These findings have potential implications for the functional ability of older people treated with these drugs.
Keywords: grip strength, sarcopenia, cardiovascular drugs, ageing, elderly
Introduction
Grip strength is a useful marker of muscle function and sarcopenia (the age-related decline in skeletal muscle mass, strength and quality) [1]. There is growing evidence that reduced grip strength in older people is associated with adverse outcomes including morbidity [2], disability [3], falls [3], higher fracture rates [4], increased length of hospital stay [5] and mortality [3]. There is therefore considerable interest in identification of modifiable life course influences on muscle strength.
Ageing is associated with an accumulation of prescribed medication, and the prevalence of polypharmacy (use of more than five medications) has increased [6]. Chronic disease is just one cause; new drugs, new indications, lower thresholds for treatment and inappropriate prescribing also contribute [7, 8]. Even with appropriate prescribing, the risk of adverse drug reactions, of which older people are at increased risk [9], rises with the use of more medications [10]. The National Service Framework for Older People identifies that use of four or more medications is a risk factor for falling and recommends discontinuation of excessive medication [11].
Cardiovascular drugs are the most commonly prescribed drugs by therapeutic group in the UK [12]. Some of these drugs are associated with known adverse effects on skeletal muscle and physical function; for example, diuretics are associated with falls through a number of mechanisms including postural hypotension [13], and statins are associated with myopathy [14] and possibly impaired muscle function [15]. However, there is also some evidence for beneficial effects of cardiovascular drugs on muscle, physical performance and frailty. For example, a study of 641 older women with hypertension but no heart failure participating in the longitudinal Women’s Aging and Health Study found that angiotensin converting enzyme (ACE) inhibitor treatment appeared to halt or slow decline in muscle strength [16]. The proposed mechanism is a direct beneficial effect on body composition through the actions of ACE inhibitors on inflammation and metabolic pathways rather than on lowering blood pressure [17].
Few studies have described the associations between the use of a range of commonly prescribed cardiovascular drugs and muscle strength in one group of older people. We therefore investigated the relationship between grip strength and use of cardiovascular drugs using data from the community-dwelling older men and women who participated in the Hertfordshire Cohort Study (HCS).
Methods
Study participants comprised 1,572 men and 1,415 women aged 59-73 years who participated in home interviews and clinic visits for the HCS between 1998 and 2004 [18]. Details of all currently used over the counter or prescription medications were coded to the British National Formulary. Grip strength was measured three times on each side using a Jamar handgrip dynamometer, and the best of the six measurements was used to characterise maximum muscle strength. Please see Appendix 1 on the journal website (http://www.ageing.oxfordjournals.org/) for a full description of the study population and methodology.
Statistical methods
Multiple linear regression was used to analyse the association between maximum grip strength and total number of medications used, with and without adjustment for age and height. Subsequently, multiple linear regression was used to analyse differences in maximum grip strength between users versus non-users of each individual cardiovascular medication in turn. Analyses were conducted: without adjustment for potential confounders; with adjustment for age and height; with adjustment for age, height and also co-morbidity (ischaemic heart disease [IHD], hypertension, diabetes mellitus and history of stroke/transient ischaemic attack [TIA]) and walking speed as a marker of physical activity.
Multiple linear regression was also used to analyse the unadjusted, and age and height adjusted, associations between maximum grip strength and each co-morbidity and walking speed in turn. Data were analysed for men and women separately throughout using Stata release 10.
Results
Summary characteristics
The average age of HCS participants was 66.1 years (standard deviation [SD] 2.9). Average height was 174.2 cm for men and 160.8 cm for women. Average grip strength was higher among men (44.0 kg [SD 7.5]) than women (26.5 [SD 5.8]). Men used a median of one medication (inter-quartile range [IQR] 0, 3) and women a median of two (IQR 1, 4). The prevalences of IHD, stroke/TIA, diabetes, hypertension and slower than normal walking speed were 14.5, 5.0, 14.8, 39.9 and 28.6%, respectively, among men, and 9.1, 2.8, 14.3, 40.7 and 26.9%, respectively, among women. Grip strength was positively related to height (men r=0.29, P<0.01; women r=0.19, P<0.01) and inversely related to age (men r=−0.18, P<0.01; women r=−0.18, P<0.01).
The percentage of men and women who used at least one cardiovascular medication were 44.9 and 44.1%, respectively. The proportions of men using thiazide diuretics, loop diuretics, β-blockers, ACE inhibitors, angiotensin II antagonists, calcium channel blockers (CCBs), nitrates, α-blockers, aspirin, statins and fibrates were 10.2, 2.3, 12.9, 12.0, 2.9, 9.8, 5.0, 6.3, 21.2, 13.0 and 0.9%, respectively. Corresponding proportions for women were 18.9, 4.2, 14.4, 10.3, 4.4, 8.9, 3.0, 2.4, 12.7, 9.8 and 1.1%.
Grip strength in relation to total number of medications taken
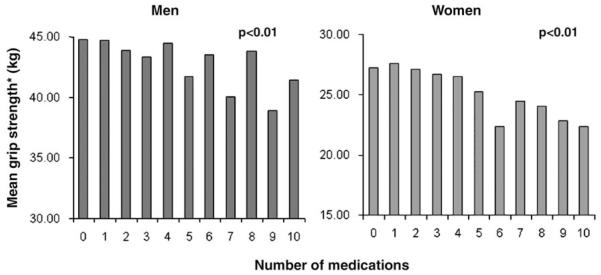
Grip strength decreased progressively with increasing numbers of medications used (Figure 1). After adjustment for age and height, each additional medication used was associated with an average reduction in grip strength of 0.36 kg (95% confidence interval [CI] 0.21, 0.52, P<0.01) among men and 0.42 kg (95% CI 0.31, 0.53, P<0.01) among women.
Figure 1.
Average grip strength according to number of medications used. *Adjusted for age and height. P values for trend from adjusted linear regression models.
Grip strength in relation to use of individual cardiovascular drugs
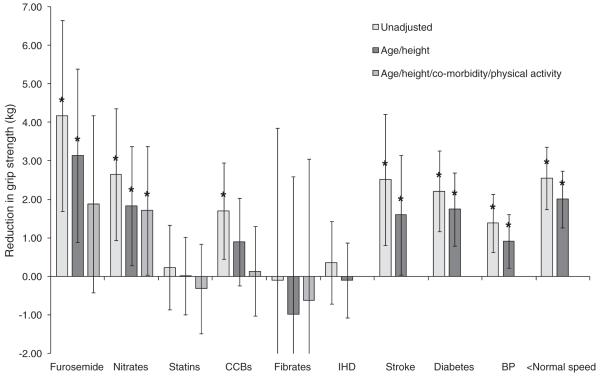
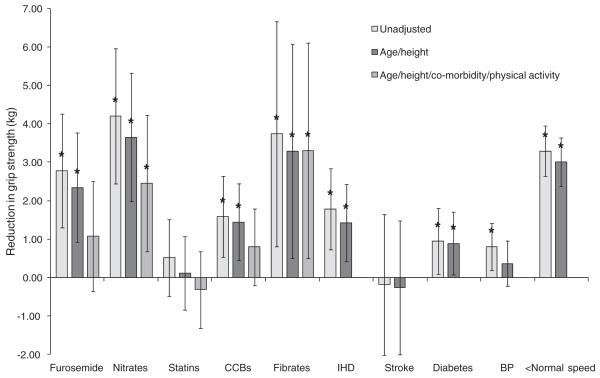
The associations between grip strength and furosemide, nitrate, statin, CCB and fibrate use, co-morbidity and physical activity are presented in Figures 2 (for men) and 3 (for women). There were no significant associations (P<0.05) between grip strength and thiazide diuretic, β-blocker, ACE inhibitor, angiotensin II antagonist, α-blocker or aspirin use in unadjusted or adjusted analyses, and these results have not been included in the figures.
Figure 2.
Association between grip strength, cardiovascular drug use, co-morbidity and physical activity among men. The figure shows the average reduction in grip strength in relation to medication use, co-morbidity and walking speed. Average reductions in grip strength were derived from linear regression models which were firstly unadjusted, then adjusted for age and height and then (for medication use analyses only) adjusted for age, height, co-morbidity and walking speed as a marker of physical activity. Error lines represent 95% confidence intervals. *P<0.05. CCBs, calcium channel blockers; IHD, ischaemic heart disease; BP, hypertension; <Normal speed, slower than normal walking speed.
Furosemide
Furosemide use was associated with a reduction in grip strength in men and women in unadjusted analyses (average reductions in grip strength [95% CI]: men 4.17 kg [1.69, 6.65], P<0.01; women 2.79 kg [1.31, 4.28], P<0.01). These associations were robust to adjustment for age and height (average age and height adjusted reductions in grip strength: men 3.15 kg [0.90, 5.39], P<0.01; women 2.35 kg [0.93, 3.77], P<0.01) but became non-significant after additional adjustment for co-morbidity and physical activity (average age, height, co-morbidity and physical activity adjusted reductions in grip strength: men 1.89 kg [−0.41, 4.19], P= 0.11; women 1.09 kg [−0.34, 2.52], P=0.14).
Nitrates
Nitrate use was associated with a significant reduction in grip strength in men and women in unadjusted analyses (average reductions in grip strength [95% CI]: men 2.65 kg [0.94, 4.36], P<0.01; women 4.21 kg [2.45, 5.96], P< 0.01). These associations remained after full adjustment for co-morbidity and physical activity (average age, height, co-morbidity and physical activity adjusted reductions in grip strength: men 1.72 kg [0.05, 3.41], P=0.04; women 2.46 kg [0.69, 4.23], P<0.01). Figures 2 and 3 show that the reductions in grip strength associated with nitrate use were of a similar, or greater, magnitude than the reductions in grip strength associated with diabetes (average age and height adjusted reductions in grip strength for diabetics compared with non-diabetics: men 1.75 kg [0.80, 2.70], P<0.01; women 0.89 kg [0.07, 1.71], P=0.03).
Figure 3.
Association between grip strength, cardiovascular drug use, co-morbidity and physical activity among women. The figure shows the average reduction in grip strength in relation to medication use, co-morbidity and walking speed. Average reductions in grip strength were derived from linear regression models which were firstly unadjusted, then adjusted for age and height and then (for medication use analyses only) adjusted for age, height, co-morbidity and walking speed as a marker of physical activity. Error lines represent 95% confidence intervals. *P<0.05. CCBs, calcium channel blockers; IHD, ischaemic heart disease; BP, hypertension; <Normal speed, slower than normal walking speed.
Statins
Grip strength was not associated with statin use in men or women, in unadjusted (average unadjusted reductions in grip strength [95% CI]: men 0.24 kg [−0.86, 1.34], P=0.67; women 0.52 kg [−0.48, 1.53], P=0.31) or adjusted analyses.
CCBs
Use of CCBs was associated with a significant reduction in grip strength in men and women in unadjusted analyses (average reductions in grip strength [95% CI]: men 1.71 kg [0.46, 2.95], P<0.01; women 1.59 kg [0.54, 2.65], P< 0.01) but age and height adjustment attenuated this association in men (average age and height adjusted reductions in grip strength: men 0.90 kg [−0.24, 2.03], P=0.12; women 1.45 kg [0.45, 2.45], P<0.01) and full adjustment attenuated the association in women (average age, height, co-morbidity and physical activity adjusted reductions in grip strength: men 0.14 kg [−1.02, 1.31], P = 0.81; women 0.80 kg [−0.20, 1.80], P=0.12).
Fibrates
Fibrates were only taken by 0.9% men, and their use was not associated with grip strength (P=0.96 unadjusted; P=0.74 fully adjusted). However, the 1.1% of women who used fibrates had significantly lower grip strength than non-users (average unadjusted reductions in grip strength [95% CI]: 3.75 kg [0.82, 6.08], P=0.02; average fully adjusted reductions in grip strength [95% CI]: 3.31 kg [0.50, 6.11], P= 0.02). The magnitude of this difference was of similar or greater magnitude than the differences in grip strength associated with co-morbidity or physical activity among women (Figure 3).
Discussion
This study has demonstrated that the use of some cardiovascular drugs is associated with reduced grip strength in older people. Among community-dwelling older men and women who participated in the HCS, use of furosemide, nitrates and CCBs among men and women and fibrates among women was associated with reduced grip strength. Nitrate use in men and nitrate and fibrate use in women was associated with reduced grip strength, independent of age, height and comorbidity. These findings may reflect a direct adverse effect of specific drugs or the underlying process of cardiovascular disease on muscle function in older people. These novel findings have potential implications for the functional ability of older people treated with cardiovascular drugs, particularly people who are functionally impaired or frail.
The average reductions in grip strength associated with cardiovascular drug use in this study were sizeable and clinically relevant. For example, Ensrud et al. [19] have shown that a 5 kg reduction in grip strength is associated with an odds ratio of 1.5 for difficulty in performing three or more activities of daily living, and analysis of HCS data suggests that a 2 kg reduction in grip strength is equivalent to approximately 5 years of chronological ageing (data not shown). Other functional limitations associated with reduced grip strength include walking impairment, lower self-reported physical function and increased morbidity such as impaired metabolic function [20] and reduced quality of life [21].
Animal studies have indicated that furosemide can affect the membrane potential of skeletal muscle [22], but the only documented cases of furosemide-associated muscle weakness in humans have involved metabolic disturbances of magnesium [23] or potassium [24]. In this study, furosemide was associated with reduced grip strength in men and women after adjustment for age and height but not after additional adjustment for co-morbidity and physical activity. The possible additional confounding effect of heart failure could not be accounted for because an appropriate marker (e.g. systolic ejection fraction) was not available in the HCS study.
Nitrate use was robustly associated with reduced grip strength among men and women in this study. Nitrates exert their therapeutic effect through nitric oxide donation. They are generally regarded as selective to smooth muscle, and myopathy is not a recognised side effect [25]. However, nitric oxide synthase is present in skeletal muscle of all mammals, and experimental animal evidence suggests that exogenous nitrates can attenuate force production of limb muscles in situ [26].
The exact type of CCB used was not specified in the HCS data, but it is likely that most people were taking dihydropyridine types (e.g. amlodipine). The findings in this study are consistent with a previous longitudinal study in which CCBs were found to be a risk factor for loss of muscle strength [27]. In a case-control study, amlodipine and verapamil were associated with altered neuromuscular transmission as indicated by Electromyography (EMG) [28].
Fibrates were associated with reduced grip strength in HCS women. This could be a true direct effect because fibrates have known myopathic potential which may be caused by calcium-mediated apoptosis of myocytes [29]. The gender difference in this association could arise from gender specific differences in drug handling or effects on skeletal muscle. Alternatively, these medications may only have an appreciable effect on muscle of lower strength.
Statin use was prevalent (13.0% men, 9.8% women) but was not associated with impaired grip strength in men or women. Findings from other observational studies have been conflicting. For example, a longitudinal study of community-dwelling older men and women in Australia found that statin use was associated with lower leg strength [15]. However, a study of older people from the United States demonstrated no difference between statin users and non-users with regard to muscle strength [30]. A recent small randomised controlled trial of a high dose statin in 10 men and women aged 55-76 years found that 12 week treatment was associated with a decrease in Low Density Lipoprotein (LDL) and total cholesterol levels but no myalgic or myopathic symptoms and no significant change in maximal muscle strength, power or endurance [31]. The link however between statin use, myopathy and muscle strength remains to be fully explored [32].
ACE inhibitor use was also prevalent in this study (men 12.0%, women 10.3%) but we were unable to demonstrate any beneficial effect on muscle strength in either gender. This contrasts with findings from the Women’s Aging and Health Study where longitudinal data were available [16]. There is also evidence from a randomised controlled trial of 120 functionally impaired older people with no heart failure that ACE inhibitor treatment is associated with improved 6 minute walking distance. However, there was no associated change in chair rises used as a marker of lower leg strength and the trial did not include any direct measures of muscle strength [33].
Our study had several limitations. Firstly, the data were cross-sectional and causality cannot be implied. However, the average reductions in grip strength were sizeable and of similar or greater magnitude than differences in grip strength according to co-morbidity, suggesting that the drugs may have a direct effect on muscle. Secondly, many statistical tests were conducted which raises the possibility of false positive results. We have addressed this by replicating the findings for nitrates and furosemide in the smaller Hertfordshire Ageing Study (data not shown) [34]. Thirdly, although we have adjusted for potentially important co-morbidities, we were not able to allow for disease severity or duration, and residual confounding by other chronic diseases is a possibility. However, the non-significant association between grip strength and use of statins argues against the possibility that our results were simply due to residual confounding by comorbidity because users of statins will have a similar co-morbidity profile as users of the other cardiovascular drugs (e.g. furosemide and nitrates) that were associated with grip strength.
Our study also had many strengths. Firstly, we analysed a large dataset of community-dwelling older men and women whose cardiovascular drug use was ascertained by a trained research nurse at a face-to-face home interview. Secondly, grip strength was directly measured at clinic as a marker of sarcopenia. Thirdly, the data were rigorously collected according to strict protocols by trained research nurses and doctors [18]. Finally, we are confident that our results are generalisable to the wider population of older people in England, because the cohort have been shown to be broadly comparable with participants in the nationally representative Health Survey for England [18].
In conclusion, the use of some cardiovascular drugs is associated with reduced grip strength in older people. The associations identified in this study may ref lect a direct adverse effect of specific drugs or the underlying process of cardiovascular disease on muscle function in older people. These novel findings have potential implications for the functional ability of older people treated with cardiovascular drugs, particularly people who are functionally impaired or frail. Further research is needed to replicate our findings and to elucidate causal relationships. If our findings are replicated, the risk-to-benefit ratio of using these drugs in frail old people would need to be re-evaluated and where they have to be prescribed, the place of interventions to minimise their impact would require investigation.
Key points.
Reduced grip strength is associated with adverse health consequences, and there is interest in identifying modifiable influences. Cardiovascular drugs are commonly used by older people, but their effect on muscle strength is unclear.
Among HCS participants, use of furosemide, nitrates and CCBs among men and women and fibrates among women was associated with reduced grip strength. Nitrate use in men and nitrate and fibrate use in women was associated with lower grip strength, independent of age, height and co-morbidity.
Use of some cardiovascular drugs is associated with reduced grip strength in older people. These findings have potential implications for the functional ability of older people treated with these drugs.
Acknowledgements
We thank the men and women who participated in the Hertfordshire Cohort Study, the Hertfordshire General Practitioners and the nurses and doctors who conducted the fieldwork.
Funding
This work was supported by the Medical Research Council and University of Southampton, UK.
Footnotes
Conflicts of interest
There is no conflict of interest to declare.
Supplementary data
Supplementary data mentioned in the text is available to subscribers at the journal website http://ageing.oxfordjournals.org
References
- 1.Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000:4140–2. [PubMed] [Google Scholar]
- 2.Sayer AA, Syddall HE, Dennison EM, et al. Grip strength and the metabolic syndrome: findings from the Hertfordshire Co-hort Study. QJM. 2007;100:707–13. doi: 10.1093/qjmed/hcm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohannon RW. Hand-grip dynamometry predicts future out-comes in aging adults. J Geriatr Phys Ther. 2008:313–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sirola J, Rikkonen T, Tuppurainen M, et al. Association of grip strength change with menopausal bone loss and related fractures: a population based follow-up study. Calcif Tissue Int. 2006;78:218–26. doi: 10.1007/s00223-005-0298-y. [DOI] [PubMed] [Google Scholar]
- 5.Kerr A, Syddall HE, Cooper C, et al. Does admission grip strength predict length of stay in hospitalised older patients? Age Ageing. 2006;35:82–4. doi: 10.1093/ageing/afj010. [DOI] [PubMed] [Google Scholar]
- 6.Rumble RH, Morgan K. Longitudinal trends in prescribing for elderly patients: two surveys four years apart. Br J Gen Pract. 1994;44:571–5. [PMC free article] [PubMed] [Google Scholar]
- 7.Gorard DA. Escalating polypharmacy. QJM. 2006;99:797–800. doi: 10.1093/qjmed/hcl109. [DOI] [PubMed] [Google Scholar]
- 8.Stuck AE, Beers MH, Steiner A, et al. Inappropriate medication use in community-residing older persons. Arch Intern Med. 1994:1542195–200. [PubMed] [Google Scholar]
- 9.McGavock H. The Scientific Basis of Prescribing in the Elderly. Prescriber. 2002:1386–9. http://www.escriber.com/Prescriber/Features.asp?ID=384&GroupID=37&Action=View (accessed 13 Nov 2007) [Google Scholar]
- 10.Tanner LA, Baum C. Spontaneous adverse reaction reporting in the elderly. Lancet. 1988;2:580. doi: 10.1016/s0140-6736(88)92713-4. [DOI] [PubMed] [Google Scholar]
- 11.Department of Health . National Service Framework for Older People. Department of Health; London: 2001. [Google Scholar]
- 12.Office of National Statistics . Prescriptions Dispensed in the Community Statistics for 1996 to 2006: England. 2007. URL: http://www.ic.nhs.uk/webfiles/publications/PrescDispensed%2096to06/Bulletin%20220807%20version%20for%202006.pdf (accessed 8 Dec 2008) [Google Scholar]
- 13.Lawlor DA, Patel R, Ebrahim S. Association between falls in elderly women and chronic diseases and drug use: cross sectional study. BMJ. 2003;327:712–7. doi: 10.1136/bmj.327.7417.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasternak RC, Smith SC, Jr., Bairey-Merz CN, et al. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Stroke. 2002:332337–41. doi: 10.1161/01.str.0000034125.94759.41. [DOI] [PubMed] [Google Scholar]
- 15.Scott D, Blizzard L, Fell J, et al. Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM. 2009;102:625–33. doi: 10.1093/qjmed/hcp093. [DOI] [PubMed] [Google Scholar]
- 16.Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359:926–30. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 17.Carter CS, Onder G, Kritchevsky SB, et al. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60:1437–46. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- 18.Syddall HE, Aihie SA, Dennison EM, et al. Cohort profile: the Hertfordshire Cohort Study. Int J Epidemiol. 2005;34:1234–42. doi: 10.1093/ije/dyi127. [DOI] [PubMed] [Google Scholar]
- 19.Ensrud KE, Nevitt MC, Yunis C, et al. Correlates of impaired function in older women. J Am Geriatr Soc. 1994;42:481–9. doi: 10.1111/j.1532-5415.1994.tb04968.x. [DOI] [PubMed] [Google Scholar]
- 20.Sayer AA, Dennison EM, Syddall HE, et al. Type 2 diabetes, muscle strength and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28:2541–2. doi: 10.2337/diacare.28.10.2541. [DOI] [PubMed] [Google Scholar]
- 21.Sayer AA, Syddall HE, Martin HJ, et al. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35:409–15. doi: 10.1093/ageing/afl024. [DOI] [PubMed] [Google Scholar]
- 22.van Mil HG, Geukes Foppen RJ, Siegenbeek vH. The influence of bumetanide on the membrane potential of mouse skeletal muscle cells in isotonic and hypertonic media. Br J Pharmacol. 1997;120:39–44. doi: 10.1038/sj.bjp.0700887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsau YK, Tsai WY, Lu FL, et al. Symptomatic hypomagnesemia in children. Chung Hua Min Kuo Hsiao Erh Ko I Hsueh Hui Tsa Chih. 1998;39:393–7. [PubMed] [Google Scholar]
- 24.Shintani S, Shiigai T, Tsukagoshi H. Marked hypokalemic rhabdomyolysis with myoglobinuria due to diuretic treatment. Eur Neurol. 1991;31:396–8. doi: 10.1159/000116702. [DOI] [PubMed] [Google Scholar]
- 25.Joint Formulary Committee . British National Formulary. 55th ed. British Medical Association and Royal Pharmaceutical Society of Great Britain; London: 2008. [Google Scholar]
- 26.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–37. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 27.Forrest KY, Zmuda JM, Cauley JA. Patterns and determinants of muscle strength change with aging in older men. Aging Male. 2005;8:151–6. doi: 10.1080/13685530500137840. [DOI] [PubMed] [Google Scholar]
- 28.Ozkul Y. Influence of calcium channel blocker drugs in neuromuscular transmission. Clin Neurophysiol. 2007;118:2005–8. doi: 10.1016/j.clinph.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Matzno S, Yasuda S, Kitada Y, et al. Clofibrate-induced apoptosis is mediated by Ca2+-dependent caspase-12 activation. Life Sci. 2006;78:1892–9. doi: 10.1016/j.lfs.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Agostini JV, Tinetti ME, Han L, et al. Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc. 2007;55:420–5. doi: 10.1111/j.1532-5415.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- 31.Traustadóttir T, Stock AA, Harman SM. High-dose statin use does not impair aerobic capacity or skeletal muscle function in older adults. Age (Dordr) 2008;30:283–91. doi: 10.1007/s11357-008-9070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grundy SM. Can statins cause chronic low-grade myopathy. Ann Intern Med. 2002;137:617–8. doi: 10.7326/0003-4819-137-7-200210010-00015. [DOI] [PubMed] [Google Scholar]
- 33.Sumukadas D, Witham MD, Struthers AD, et al. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007:9177867–74. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Syddall HE, Simmonds SJ, Martin HJ, et al. Hertford-shire Cohort Study Group Cohort profile: the Hertfordshire Ageing Study (HAS) Int J Epidemiol. 2009 Jan 8; doi: 10.1093/ije/dyn275. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]