Abstract
Our studies in HUVECs show that ox-LDL induced autophagy and damaged mtDNA leading to TLR9 expression. LOX-1 antibody or the ROS inhibitor apocynin attenuated ox-LDL-mediated autophagy, mtDNA damage and TLR9 expression, suggesting that these events are LOX-1 and ROS-dependent phenomena. Experiments using siRNA to DNase II indicated that DNase II digests mtDNA to protect the tissue from inflammation. Next, we studied and found intense autophagy, TLR9 expression and inflammatory signals (CD45 and CD68) in the aortas of LDLR knockout mice fed high cholesterol diet. Deletion of LOX-1 (LDLR/LOX-1 double knockout mice) attenuated autophagy, TLR9 expression as well as CD45 and CD68. Damaged mtDNA signal was also very high in LDLR knockout mice aortas, and this signal was attenuated by LOX-1 deletion. Thus, it appears that oxidative stress-mediated damaged mtDNA that escapes autophagy induces a potent inflammatory response in atherosclerosis.
Oxidized-low density lipoproteins (ox-LDL) accumulate in the arterial wall and activate signaling pathways that lead to inflammation and oxidative stress response1. Lectin-like oxidized low-density lipoprotein scavenger receptor-1 (LOX-1) is one of the major receptors responsible for binding, internalizing and degrading ox-LDL2. Activation of LOX-1 has been known to be related to many pathophysiological events, including endothelial cell and vascular smooth muscle cell proliferation, alteration in cell cycle signals, apoptosis and autophagy1,2,3,4.
As energy-producing organelles, mitochondria can suffer damage under oxidative stress that induces endothelial dysfunction and promotes leukocyte adhesion, inflammation, thrombosis and smooth muscle cell proliferation5. Damaged mitochondria are often degraded by autophagy, which is an evolutionarily conserved process for lysosomal recycling of cytoplasmic material6. Similar to bacterial DNA, mitochondrial DNA (mtDNA) contains inflammatogenic unmethylated CpG motifs, whereas nuclear DNA is modified by the addition of methyl groups on certain sequences known as CpG motifs6,7. This differential feature allows immune cells to recognize DNA of invading bacteria. mtDNA activates toll-like receptor 9 (TLR-9) that senses unmethylated CpG motifs and induces the synthesis of pro-inflammatory cytokines8.
Although mtDNA released from dying cells induces a TLR9-dependent inflammatory response6,7,8, there is no evidence to support that TLR9 pathway is relevant in atherosclerotic plaques that exhibit extensive inflammation in the absence of infection.
We hypothesized that DNA released during autophagy in endothelial cells (ECs) could trigger an inflammatory response similar to that generated by bacterial DNA during an infection. The present study was designed to test this hypothesis. We show for the first time autophagy, mtDNA release and TLR-9 expression in cultured human ECs exposed to ox-LDL. We also show that autophagy, mtDNA release, TLR9 expression and inflammatory response in atherosclerotic regions of LDLR-null mice aortas and the attenuation of these phenomena by LOX-1 abrogation.
Results
Ox-LDL induces autophagy and ROS generation
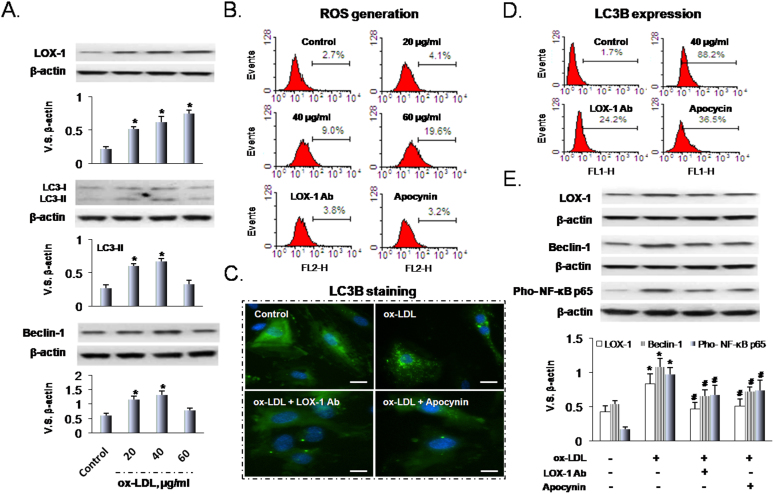
As observed previously9, LOX-1 expression in HUVECs increased in response to increasing ox-LDL concentration (0-60 μg/ml, incubation time 24 h) (Figure 1A top panel). We assessed autophagy in response to ox-LDL by measuring beclin-1 and LC3-I/LC3-II, both are recognized autophagosome markers10. As shown in Figure 1A, ox-LDL (20 to 40 μg/ml) treatment significantly increased beclin-1 and LC3-II. Higher concentration of ox-LDL (60 μg/ml), however, decreased both autophagy markers (Figure 1A middle and lower panels).
Figure 1. Ox-LDL induces LOX-1, autophagy and ROS generation.
(A) Ox-LDL induces LOX-1 expression in a dose-dependent manner. Ox-LDL (20 to 40 μg/ml concentrations) induces expression of LC3 and beclin-1; however, 60 μg/ml ox-LDL decreases LC3 and beclin-1. (B) Ox-LDL in a dose-dependent manner induces ROS generation which is blocked by LOX-1 Ab and apocynin. (C) Immunofluorescence shows that ox-LDL enhances LC3B (fluorescent staining) which is blocked by LOX-1 Ab and apocynin. Scale bars: 20 μm. (D) Ox-LDL enhances LC3B expression (flow cytometry). (E) Ox-LDL increases the expression of LOX-1, beclin-1 and pho-NF-kB, and this effect can be blocked by LOX-1 Ab as well as apocynin. Bar graphs represent data in mean ± SD based on 3-5 experiments, * P<0.05 vs. Control, # P<0.05 vs. ox-LDL.
Ox-LDL is a potent inducer of ROS, and this was confirmed in the present study (Figure 1B). Further, pretreatment of HUVECs with LOX-1 Ab or the NADPH oxidase inhibitor apocynin significantly inhibited ROS generation in response to ox-LDL (Figure 1B).
As noted previously, some of the LC3 dissociates from the membrane after fusion with the lysosome during development of autophagy, and the LC3-GFP can serve as autolysosome and as a specific marker for autophagy9,10. We examined LC3B-GFP changes in HUVECs by fluorescence microscopy (Figure 1C). In normal cells, LC3B-GFP protein was distributed diffusely throughout the entire cell with almost no LC3B accumulation in the lysosomes. After incubating HUVECs with ox-LDL for 24 h, a large number of cells showed LC3B-GFP accumulation, suggesting activation of autophagic response. Pretreatment of cells with LOX-1 Ab or apocynin decreased LC3B accumulation induced by ox-LDL. The data from flow cytometry (Figure 1D) confirmed the results of fluorescence microscopy. Similarly to LC3B data, expression of LOX-1, beclin-1 and Pho-NF-kB p65 in cells treated with ox-LDL was inhibited by pretreatment with LOX-1 Ab or apocynin (Figure 1E).
Ox-LDL dependent mtDNA damage and regulation of TLR9
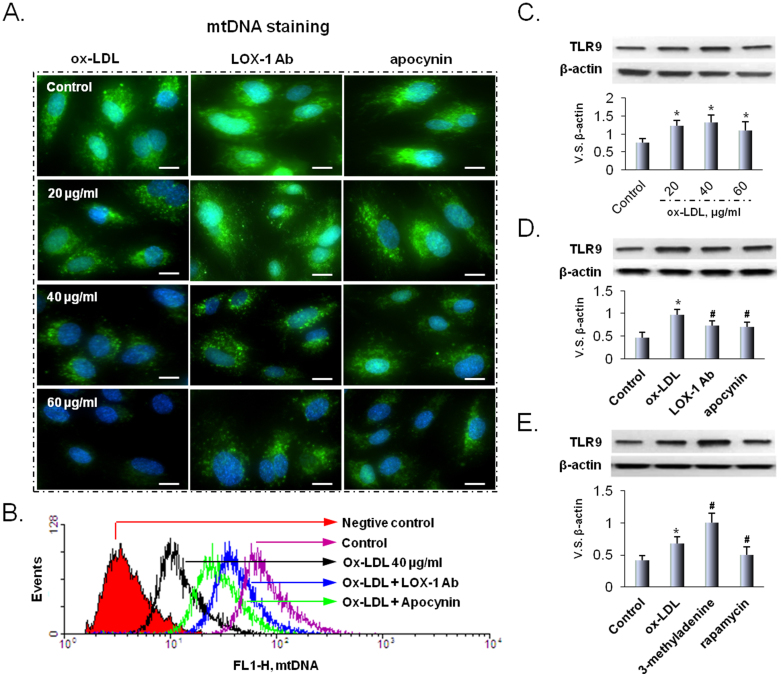
Recent clinical studies show accumulation of mtDNA in atherosclerotic lesions5. There is also evidence for accumulation of ox-LDL in atherosclerotic tissues1,2,3,4. However, there is no direct evidence of correlation of ox-LDL and mtDNA that escapes autophagy. We looked for and found that ox-LDL in a dose-dependent manner induced mtDNA leakage in HUVECs (Figure 2). Further, pretreatment of cells with LOX-1 Ab or apocynin reduced mtDNA leakage (Figure 2A). PicoGreen staining based flow cytometry also confirmed the presence of mtDNA leakage (Figure 2B) and the data were similar to the immunocytochemistry observations.
Figure 2. Ox-LDL damages mtDNA and induces TLR9 expression.
(A) Ox-LDL damages mtDNA in a dose-dependent manner, and LOX-1 Ab as well as apocynin block the effect of ox-LDL. Scale bars: 20 μm. (B) The results of mtDNA staining are confirmed by flow cytometry. In addition, LOX-1 Ab as well as apocynin block the formation of mtDNA. (C) Ox-LDL (20 to 40 μg/ml) induces TLR9 expression. (D) The effects of ox-LDL on the expression of TLR9 are blocked by LOX-1 Ab as well as apocynin. (E) 3-methyladenine (an autophagy inhibitor) enhances and rapamycin (an autophagy inducer) inhibits TLR9 expression. Bar graphs represent data in mean ± SD based on 3-5 experiments, * P<0.05 vs. Control, #P<0.05 vs. ox-LDL.
Localized to intracellular compartments, TLR9 recognizes viral and bacterial DNA that contains unmethylated CpG dinucleotides, which induces the production of pro-inflammatory cytokines8. We examined the relationship between ox-LDL and TLR9. As shown in Figure 2C, ox-LDL (20 to 40 μg/ml) increased TLR9 expression. Higher concentration of ox-LDL (60 μg/ml), however, reduced TLR9 expression. As with mtDNA leakage data, pretreatment with LOX-1 Ab or apocynin blocked TLR9 expression in response to ox-LDL (40 μg/ml).
As further evidence for the link between mtDNA leakage and TLR9 expression, we treated HUVECs with 3-methyladenine (an autophagy inhibitor) or rapamycin (an autophagy inducer). We observed that 3-methyladenine enhanced and rapamycin inhibited TLR9 expression, indicating that mtDNA that escapes autophagy cell-autonomously leads to TLR9 expression.
Deoxyribonuclease II (DNase II) inhibition induces autophagy and TLR9 expression
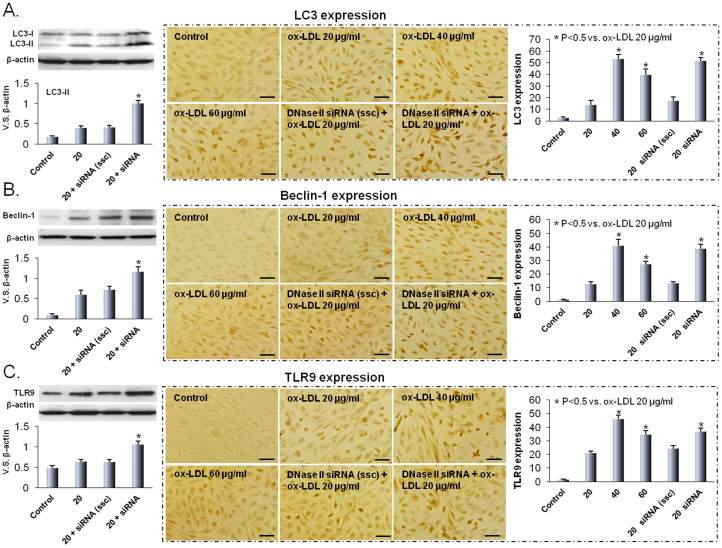
DNases are found in the lysosome7,8. DNase II in particular has an essential role in the degradation of DNA of apoptotic cells after macrophages engulf them11. We hypothesized that DNase II in HUVECs would digest mtDNA and protect the cultured cells from inflammatory response (TLR9 expression) to ox-LDL. To confirm this hypothesis, HUVECs were transfected with DNase II siRNA and the cells were then exposed to ox-LDL. As shown in Figure 3A and 3B, inhibition of DNase II induced LC3 and beclin-1 expression in response to ox-LDL. Consistent with our hypothesis, inhibition of DNase II also induced TLR9 expression (Figure 3C). These observations support previous data on ox-LDL induced autophagy and TLR9 expression ( Figure 1 and 2).
Figure 3. DNase II inhibition induces autophagy and TLR9 expression.
Western blot and immunohistochemical analyses indicate that ox-LDL increases the expression of LC3 (A), beclin-1 (B) and TLR9 (C). Modest concentrations of ox-LDL (20 to 40 μg/ml) enhance LC3, beclin-1 and TLR9 expression, while 60 μg/ml concentration somewhat decreases this effect. DNase II inhibition by DNase II siRNA transfection induces the expression of LC3, beclin-1 and TLR9. Scrambled siRNA has no effect. Scale bars: 50 μm. Bar graphs represent data in mean ± SD based on 3-5 experiments, * P<0.05 vs. ox-LDL 20 μg/ml.
LOX-1 deletion reduces autophagy and TLR9 mediated inflammation response
Previous studies from our laboratory3 showed that both LDL-cholesterol and ox-LDL were higher in the LDLR KO and double KO mice than corresponding controls (WT and LOX-1 KO mice, respectively). The extent of atherosclerosis was also reduced by LOX-1 abrogation in the LDLR KO mice.
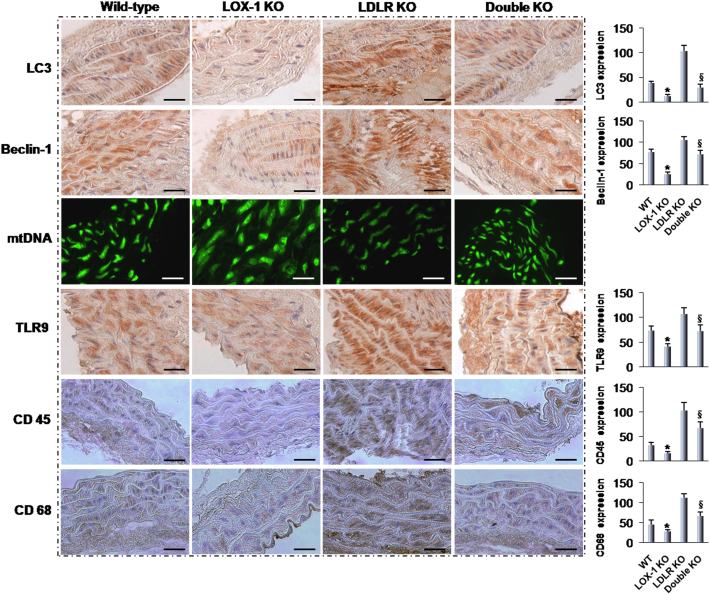
To further study the relationship between autophagy, mtDNA leakage and TLR9 in atherosclerosis, we checked atherosclerotic aorta sections from different groups of mice (Figure 4). Immunohistochemistry showed a very high expression of LC3 and beclin-1 in LDLR KO mice compared with WT mice. Concomitant with increased autophagy, mtDNA leakage and TLR9 were highly expressed in the LDLR KO mice aortas. The abrogation of LOX-1 in LDLR KO mice (double KO mice) reduced autophagy, mtDNA leakage and TLR9 expression in the LDLR KO mice aortas. Of note, autophagy markers (LC3 and beclin-1), mtDNA leakge and TLR9 expression all were lower in LOX-1 KO mice than in the WT mice aortas.
Figure 4. Immunohistochemistry markers of autophagy, mtDNA damage, and inflammation.
The expression of LC3 and beclin-1 (autophagy markers) is much higher in the LDLR KO and LDLR/LOX-1 DKO mice than in their counterpart WT and LOX-1 KO mice. Staining for mtDNA damage, TLR9 and inflammatory cell accumulation (CD45 and CD68) show similar pattern. The pictures are representative of multiple sections of aortas from different groups of mice (at least 5 in each group). Bar graphs represent data in mean ± SD based on 3–5 experiments. * P<0.05: LOX-1 KO vs. WT; §P<0.05: Double KO vs. LDLR KO. Scale bars: 80 μm.
Next, we evaluated inflammatory cell infiltration using antibodies against CD45 and CD68 (common leukocyte antigens) as markers for macrophages and leukocytes, respectively7. Immunohistochemistry showed that CD45 and CD68 were highly expressed in the LDLR KO mice aortas and their expression was reduced by LOX-1 abrogation (double KO mice). Consistent with TLR9 data, CD45 and CD68 expression was much less in the LOX-1 KO mice compared with WT mice aorta.
Discussion
Ox-LDL transport into the subendothelial space at the sites of endothelial damage is considered a seminal initiating event for atherogenesis12. LOX-1 is present in ECs in much greater amount than any other scavenger receptor and serves to activate these cells and internalize ox-LDL. The role of LOX-1 as a key scavenger receptor became evident is studies showing significant abrogation of atherosclerosis in the LDLR KO mice3.
Autophagy is a potent adaptive mechanism to protect cells from stress-induced injury, which removes superfluous and damaged mitochondrial organelles13. Studies have shown that mtDNA released from dying cells into the extracellular space induces a TLR9-dependent inflammatory response in some cells of the immune system14,15. However, the mechanism of TLR9 pathway involved in atherosclerotic areas that exhibit extensive inflammation in the absence of infection has not been well studied. In the present study, we show that mtDNA leakage that escapes from autophagy leads to TLR-9 mediated inflammatory responses in cultured HUVECs. We also show that mtDNA leakage and subsequent TLR9 expression are LOX-1 mediated phenomena.
Autophagy is an evolutionary conserved process involved in the degradation of long-lived proteins, which becomes manifest during state of increased stress, and may be considered a cell survival mechanism16,17. We now show that HUVECs when exposed to 20 μg/ml of ox-LDL show autophagy (measured as expression of LC3 and beclin-1), suggesting that autophagy in HUVECs in response to circulating concentrations of ox-LDL reflects a stress response that allows HUVECs to survive during harsh conditions. Importantly, in the present study the ox-LDL induced autophagy was blocked by apocynin as well as LOX-1 antibody suggesting that the autophagic response in HUVECs is mediated by ROS-LOX-1 pathway. Atherosclerosis involves oxidative stress and inflammation3,4. Keeping with this concept we found that the expression of NF-κB was reduced by pretreatment with LOX-1 Ab and apocynin, indicating that ROS-LOX-1 pathway is important in the expression of this transcription factor.
TLR9, localized in the endolysosome, senses DNA with unmethylated CpG motifs derived from bacteria and viruses, which is the major contributor to acute inflammation induced by microbial infection18,19. To investigate the role of ox-LDL in mtDNA mediated TLR9 expression, HUVECs were colabeled with green-fluorescing PicoGreen and Hoechst 33342. PicoGreen is a DNA-intercalating fluorophore that penetrates mitochondrial membranes and labels mtDNA, while Hoechst 33342 stains the nuclei20,21. Our data show that ox-LDL in a dose-dependent manner damaged mtDNA expression; importantly, pretreatment of cells with LOX-1 Ab or apocynin blocked this ox-LDL effect. These effects of ox-LDL on mtDNA damage were assessed by two different methods, immunocytochemistry and flow cytometery, and both methods gave similar results.
Ox-LDL induced TLR9 expression at modest concentrations (20 nd 40 μg/ml) but inhibited it at 60 μg/ml concentration. It is likely that high concentartions of ox-LDL resulted in death of some cells resulting in lower TLR9 expression; a similar phenomenon was reported initially by Mehta et al while studying ox-LDL-mediated LOX-1 expression2,3,4. Importantly, treatment of cells with 3-methyladenine enhanced TLR9 expression and treatment with rapamycin inhibited TLR9 expression. DNase II has been suggested to protect the heart from inflammation by digesting mtDNA and thereby block or attenuate the inflammatory response6,7. In keeping with this postulate, inhibition of DNase expression by DNase II siRNA aggravated autophagy (enhanced expression of LC3 II and beclin-1) and TLR9 expression. These data were confirmed by immunocytochemistry and Western blotting. It has been suggested that mtDNA released from the dying cells into the extracellular space induces a TLR9-dependent inflammatory response in some cells of the immune system6. Oka et al. found that mtDNA that escapes degradation can trigger a similar response in the cell where the initial reaction started7. From the aforementioned observations, it can be concluded that LOX-1 mediated mtDNA damage increases its leakage into the cells, and induces autophagy. As leaked mtDNA exceeds the ability of autophagy to degrade, expression of TLR9 increases.
As reported previously3, VLDL- and LDL-cholesterol levels in plasma were much higher in the LDLR KO and LDLR/LOX-1 DKO mice than in the plasma of their counterpart WT and LOX-1 KO mice. Of interest, there was no difference in the cholesterol levels in LDLR KO and LDLR/LOX-1 DKO mice. However, extent of atherosclerosis (areas of sudanophilia) was much less in the LDLR/LOX-1 DKO than in LDLR KO mice3. Presumably, either the ox-LDL generation was much less in the LDLR/LOX-1 DKO than in LDLR KO mice or LOX-1 deletion resulted in reduced uptake of ox-LDL resulting in attenuation of extent of sudanphilic areas. Previous studies from our group suggested a decrease in oxidant stress in the DKO mice as compared with LDLR KO mice.
We now extended the study by examining multiple sections of aorta from different groups of mice, and observed evidence for significant autophagy (expression of LC3 and beclin-1) and inflammation (expression of TLR9, CD45 and CD68) in the LDLR KO mice and these phenomena were attenuated by LOX-1 deletion. In keeping with the data from in vitro studies in HUVECs, mtDNA damage (and leakage) was extensive in LDLR KO mice and it was reduced by LOX-1 abrogation.
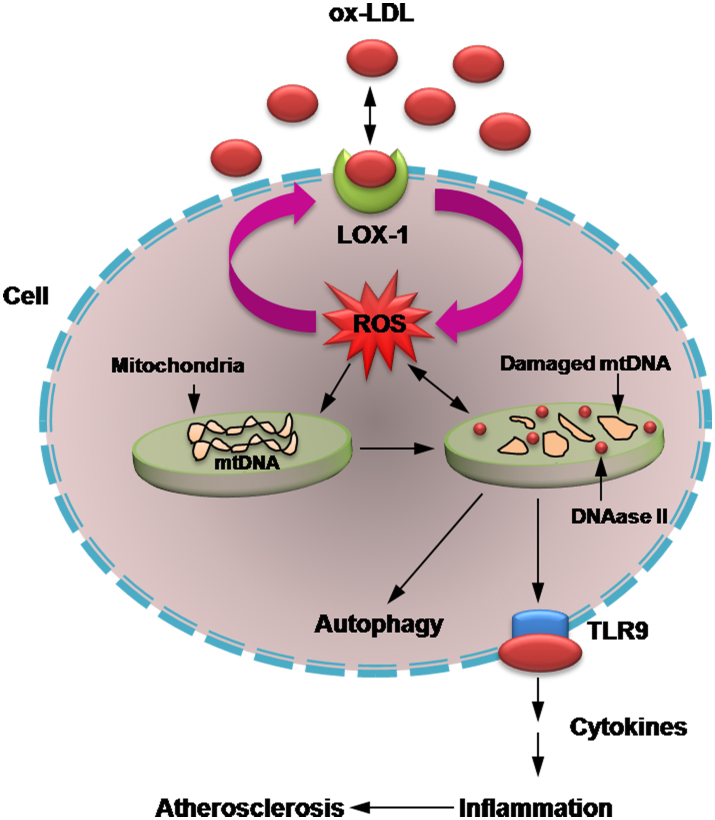
In conclusion, the present study confirmed our hypothesis that during oxidant stress damaged mtDNA leaks into cytoplasm, and mtDNA that escapes autophagy can trigger a TLR9-related inflammatory response. This phenomenon seems to occur in a LOX-1 dependent fashion, and may well be a basis of inflammation in atherosclerosis (Fig. 5).
Figure 5. Possible link between LOX-1-mediated mtDNA damage and autophagy.
mtDNA that escapes autophagy induces a potent inflammatory response in atherosclerosis.
Methods
Cell culture
Primary human umbilical vein endothelial cells (HUVECs) were obtained from ATCC (Manassas, VA), and maintained in vascular cell basal medium. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
High TBAR ox-LDL (90 nmoles MDA/mg Protein) was purchased from Biomedical Technologies Inc., Stoughton, MA. Anti-human LOX-1 neutralizing antibody (LOX-1 Ab) JTX92 was a gift of Dr. T. Sawamura (Osaka, Japan). ROS inhibitor (Apocynin, 1 mmol/l) was obtained from Sigma (St. Louis, MO). To examine the receptor specificity of ox-LDL action, HUVECs were pretreated with human LOX-1 blocking antibody (10 μg/ml) and then exposed to ox-LDL.
For mtDNA detection, HUVECs or aortic sections were incubated in PicoGreen (Molecular Probes, Carlsbad, CA) for 1 h. Then fluorescent microscopy and flow cytometry were used to assess mtDNA expression.
Western blot
Details of Western blotting have been published recently4. The antibodies used were anti-rabbit LOX-1 (Abcam, CA), LC3 (Cell Signaling, Boston, MA), beclin-1 (Novus Biologicals, Littleton, CO), TLR9, CD45 and CD68 (last 3 from Santa-Cruz Biotechnology, Inc., CA).
Measurement of intracellular reactive oxygen species
Intracellular ROS was measured with the use of the fluorescent signal dihydroethidium (DHE), a cell-permeable indicator for ROS generation. Essentially, cells were cultured in 6-well plate and incubated with 10 μmol/L DHE in PBS for 30 min. The ROS-mediated fluorescence was measured by flow cytometry (Becton Dickinson, Franklin Lakes, NJ), and the results were analyzed with the software WinMDI29, as described9.
DNase II siRNA transfection
In order to knock down DNase II, cells were transfected with small interfering RNA (siRNA) duplexes using siRNA transfection reagent (Santa Cruz, CA) as per manufacturer supplied protocols. Parallel aliquot of cells was transfected with sequence scrambled siRNA (ssc) duplexes as control.
Immunohistochemical analysis
HUVECs and aortic sections were stained with different antibodies using Mouse/Rabbit Specific HRP/DAB detection IHC kit (Abcam, San Francisco, CA) as per the manufacturer's instructions. Image J. v 1.46 (NIH, MD) was used to quantify immunohistochemical staining according to the manufacturer's instructions.
Animal protocol
The generation of LOX-1 KO and LOX-1/LDLR double KO mice on C57BL/6 background (also referred to as wild-type mice) has been described previously3. All animals were housed in the breeding colony at University of Arkansas for Medical Sciences, Little Rock, AR, USA. All male animals were given high-cholesterol diet (4% cholesterol/10% cocoa butter) for 18 weeks from the age of 6 weeks. All experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Usage Committee, and conform to the Guidelines for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Autophagy detection in HUVECs and rabbit thoracic aortas
Autophagy was detected by Western blot, fluorescent microscopy, immunohistochemistry (PremoTM Autophagy Sensors (L3B-GFP) (Invitrogen, Grand Island, NY), and flow cytometry9.
Statistical analysis
Data from at least three independent experiments were used for statistical analysis. Results are shown as mean ± SD. Multiple means were compared using a one-way analysis of variance (ANOVA). Paired Student's t-test was used to assess significant differences. P value <0.05 was considered significant.
Author Contributions
Z.D. and S.L. developed the concept of this study and performed the experiments; X.W. and M.K. performed immunohistochemical analyses; Y.D. helped with autophagy experiments; J.L.M. designed the study, analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This study was supported in part by funds from the Department of Veterans Affairs.
References
- Sawamura T. et al. An endothelial receptor for oxidized low-density lipoprotein. Nature 386, 73–77 (1997). [DOI] [PubMed] [Google Scholar]
- Chen J. W. et al. Role of caspases in ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ. Res. 94, 370–376 (2004). [DOI] [PubMed] [Google Scholar]
- Mehta J. L. et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 100, 1634–1642 (2007). [DOI] [PubMed] [Google Scholar]
- Li D. & Mehta J. L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol 20, 1116–1122 (2000). [DOI] [PubMed] [Google Scholar]
- Madamanchi N. R. & Runge M. S. Mitochondrial dysfunction in atherosclerosis. Circ Res 100, 460–473 (2007). [DOI] [PubMed] [Google Scholar]
- Konstantinidis K. & Kitsis R. N. Cardiovascular biology: Escaped DNA inflames the heart. Nature 485, 179–180 (2012). [DOI] [PubMed] [Google Scholar]
- Oka T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe Y. Okabe Y., Kawane K., Akira S., Taniguchi T. & Nagata S. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp Med 202, 1333–1339 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z. et al. Degradation of heparan sulfate proteoglycans enhances oxidized-LDL-mediated autophagy and apoptosis in human endothelial cells. Biochem Biophys Res Commun 426, 106–11 (2012). [DOI] [PubMed] [Google Scholar]
- Levine B., Mizushima N. & Virgin H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. J. & Aguilera R. J. DNase II: genes, enzymes and function. Gene 322, 1–15 (2003). [DOI] [PubMed] [Google Scholar]
- Li D. & Mehta J. L. Oxidized LDL, a critical factor in atherogenesis. Cardiovasc Res 68, 353–354 (2005). [DOI] [PubMed] [Google Scholar]
- Garber K. Autophagy. Explaining exercise. Science 335, 281 (2012). [DOI] [PubMed] [Google Scholar]
- Collins L. V., Hajizadeh S., Holme E., Jonsson I. M. & Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J Leukoc Biol 75, 995–1000 (2004). [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Immunology. Eating in to avoid infection. Science 315, 1376–1377 (2007). [DOI] [PubMed] [Google Scholar]
- Mizushima N., Levine B., Cuervo A. M. & Klionsky D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 12, 222–230 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M. CpG motifs: the active ingredient in bacterial extracts? Nat Med 9, 831–835 (2003). [DOI] [PubMed] [Google Scholar]
- Medzhitov R. CpG DNA: security code for host defense. Nat Immunol 2, 15–16 (2001). [DOI] [PubMed] [Google Scholar]
- Ashley N., Harris D. & Poulton J. Detection of mitochondrial DNA depletion in living human cells using PicoGreen staining. Exp Cell Res 303, 432–446 (2005). [DOI] [PubMed] [Google Scholar]
- Saffran H. A., Pare J. M., Corcoran J. A., Weller S. K. & Smiley J. R. Herpes simplex virus eliminates host mitochondrial DNA. EMBO Rep 8, 188–193 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]