Summary
In patients with diabetes, atherosclerosis is the main reason for impaired life expectancy, and diabetic nephropathy and retinopathy are the largest contributors to end-stage renal disease and blindness, respectively. An improved therapeutic approach to combat diabetic vascular complications might include blocking mechanisms of injury as well as promoting protective or regenerating factors, for example by enhancing the action of insulin-regulated genes in endothelial cells, promoting gene programs leading to induction of antioxidant or anti-inflammatory factors, or improving the sensitivity to vascular cell survival factors. Such strategies could help prevent complications despite suboptimal metabolic control.
Introduction
The vascular complications of diabetes are the most serious manifestations of the disease. Atherosclerosis is the main reason for impaired life expectancy in patients with diabetes whereas diabetic nephropathy and retinopathy are the largest contributors to end-stage renal disease and blindness, respectively. The most well-established clinical advances in preventing vascular complications of diabetes include intensive blood glucose lowering which decreases the risk of nephropathy and retinopathy, antihypertensive medicine which decreases the risk of cardiovascular disease, nephropathy, and retinopathy, panretinal photocoagulation and agents targeting vascular endothelial growth factor (VEGF) which slows the progression of diabetic retinopathy, and statin therapy which reduces the risk of cardiovascular disease. Despite these advances, diabetes complications remain an enormous problem. Its public health impact will continue to grow due to the expected increase in the prevalence of diabetes.
Although lowering blood glucose delays the onset of nephropathy and retinopathy, cardiovascular disease in diabetes shows less robust association with hyperglycemia and less benefit from glucose-lowering therapy. Moreover, it is clear that diabetes is associated with increased cardiovascular risk beyond what is explained by dyslipidemia or hypertension, both of which are more common in patients with diabetes. Accordingly, insulin resistance and its biological effects in various tissues may be more important factors than hyperglycemia in mediating atherothrombotic complications, particularly in type 2 diabetes. Despite these insights, there are few therapies targeting vascular abnormalities specific for diabetes.
Advances in understanding the vascular pathology of diabetes have made it clear that the pathogenesis of diabetic vascular complications is determined by a balance between molecular mechanisms of injury and endogenous protective factors (fig. 1). Both aspects of disease mechanisms provide targets for prevention even during suboptimal metabolic control. To emphasize these concepts, the current review will juxtapose some of the current knowledge about mechanisms of injury with selected literature describing protective factors.
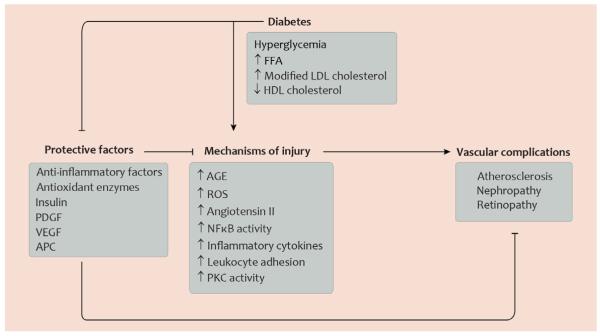
Fig. 1. Selected mechanisms of injury and protective factors determining development of diabetic vascular complications.
This diagram illustrates that in the normal state, factors with protective functions in the vasculature can render blood vessels less susceptible to vascular disease and can counteract mechanisms which promote vascular injury. In diabetes, however, glucose and lipid metabolites promote mechanisms of injury and, at the same time, inhibit factors with protective functions in the vasculature. Abbreviations: AGE, advanced glycation end-products; APC, activated protein C; FFA, free fatty acids; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NFκB, nuclear factor κB; PDGF platelet-derived growth factor; PKC, protein kinase C; ROS, reactive oxygen species; VEGF, vascular endothelial growth factor. Artwork by Leah A. Klein.
Pathogenesis of vascular complications
The complications of diabetes and the vascular components in their pathogenesis are so intertwined that often one aspect is not considered separately from the other. However, mechanisms unrelated to vascular dysfunction are a part of the pathogenesis of these complications, for example in renal tubules and in retinal neurons. These mechanisms will not be considered further here. Diabetic neuropathy, which traditionally is counted among microvascular complications, is likely caused by abnormalities in neuronal cells to a larger extent than by microvascular dysfunction; molecular mechanisms of diabetic neuropathy have been reviewed recently elsewhere (Vincent, 2011).
Atherosclerosis in diabetes
The pathology of atherosclerotic lesions in patients with diabetes is indistinguishable from lesions in patients in whom another characteristic, like hypercholesterolemia or smoking, is the major risk factor. Cholesterol, in particular LDL cholesterol, is usually considered the major contributor to atherosclerosis susceptibility. However, patients with type 2 diabetes typically do not have elevated cholesterol in low density lipoprotein (LDL), but rather a characteristic dyslipidemia of high triglycerides, low HDL, and small, dense LDL (Sniderman, 2001). Apolipoprotein B-containing and modified LDL retained in the arterial intima recruits monocyte-derived macrophages, which take up lipoproteins and differentiate into foam cells (fig. 2). Cytokines and chemokines released from macrophage foam cells and other immune cells recruit additional immune cells. Overproduction of reactive oxygen species (ROS) as a result of altered glucose metabolism and formation of advanced glycation end-products (AGE) further amplifies this process by activating nuclear factor κB (NFκB) and other proinflammatory pathways. In combination with endothelial cell insulin resistance, these changes cause endothelial dysfunction manifesting itself by increased expression of adhesion molecules and other changes. The resulting leukocyte recruitment, perhaps compounded by proliferation of plaque macrophages, further increases leukocyte numbers in the intima. Proliferating vascular smooth muscle cells are a major source of extracellular matrix in the atherosclerotic plaque and to the fibrous cap covering the plaque. Apoptosis of macrophages contribute to formation of a necrotic core in advanced plaques, and release of matrix metalloproteases and other enzymes from macrophages and other cells break down the fibrous cap and cause plaque rupture. This event can cause thrombosis when exposing the necrotic core and other extracellular components to circulating blood, precipitating the major life-threatening events in atherosclerosis, including myocardial infarction and stroke.
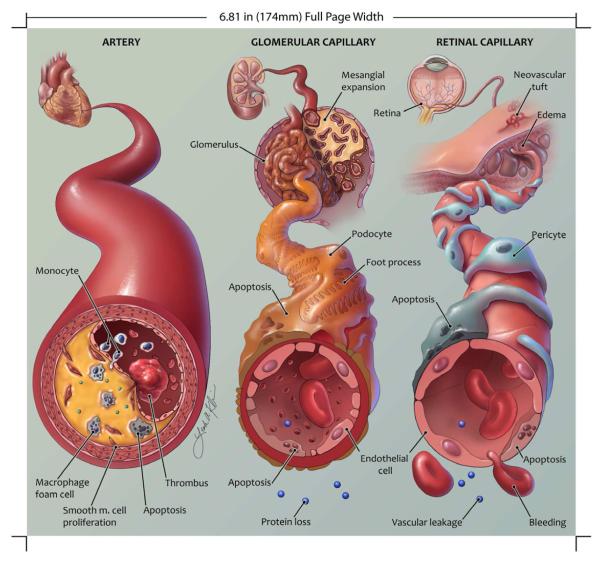
Fig. 2. Important histopathological changes during development of atherosclerosis, nephropathy, and retinopathy in diabetes.
This schematic illustration shows pathological changes in a coronary artery (left) and in glomerular and retinal capillaries (middle and right, respectively) in diabetes. Some of the main features are accumulation of lipid-laden macrophages in the atherosclerotic plaque and subsequent macrophage apoptosis (left); podocyte apoptosis, thickening of the glomerular basement membrane, and breakdown of the filtration barrier in the renal glomeruli (middle); and pericyte and endothelial cell apoptosis, vascular leakage , and hemorrhage in the retina (right). Proportions, in particular size of the artery relative to the capillaries, are not to scale. Artwork by Leah A. Klein.
Diabetic nephropathy
Patients with diabetes have increased glomerular perfusion of and plasma filtration owing to decreased resistance in both the afferent and efferent arteriole. The most important early clinical risk factor for diabetic nephropathy is albuminuria, which is caused by hemodynamic changes and by impairment of the glomerular filtration barrier. Changes to this barrier include thickening and changed composition of the glomerular basement membrane and regression of the cytoplasmic extensions, or foot processes, of podocytes (Dronavalli, 2008) (fig. 2). Proinflammatory and profibrotic signals from glomerular cells and infiltrating macrophages cause mesangial expansion consisting of accumulating extracellular matrix. Apoptosis of podocytes and glomerular endothelial cells can ensue, with glomerulosclerosis as the most advanced pathological change. Abnormal function of tubules and tubolointerstitial fibrosis develops in parallel with glomerular damage, possibly in response to albuminuria.
Diabetic retinopathy
The first pathological changes in diabetic retinopathy are decreased pericyte coverage of retinal capillaries and acellular capillaries representing apoptosis of pericytes and endothelial cells (Hammes, 2011) (fig. 2). Vascular cell apoptosis is caused by abnormal glucose metabolism, activation of protein kinase C (PKC), formation of AGE, increased production of ROS, release of proinflammatory cytokines from Müeller cells or microglia in the retina or from leukocytes adhering to capillary endothelium, loss of survival signaling stimulated by platelet-derived growth factor (PDGF) and other factors, and upregulation of angiostatic factors like Tie2 (Hammes, 2011). Impaired perfusion and retinal ischemia then causes upregulation of angiogenic molecules including VEGF, erythropoietin, and other vascular growth factors (Antonetti, 2012). These factors promote proliferative diabetic retinopathy and lead to increased vascular leakage, with contributions from the kallikrein-bradykinin signaling and other pathways (Antonetti, 2012). Vision loss can be secondary to preretinal angiogenesis which can cause bleeding into the vitreous or formation of an epiretinal membrane. Another major cause of impaired vision is macular edema.
Similarities and differences in vascular cell biology in diabetes
The alterations in cellular homeostasis and regulation of vascular physiology which leads to vascular complications affect all major functions of vascular cells (fig. 3). Increased vascular permeability and apoptosis of specific vascular cells, including retinal pericytes and glomerular podocytes, are prominent features of diabetic nephropathy and retinopathy. Macrophage apoptosis is important for plaque necrosis in atherosclerosis. Vasular beds in most tissues are affected by increased leukocyte adhesion which may participate in the pathogenesis of all these complications and is certainly pivotal for development of atherosclerosis. Proliferation of vascular smooth muscle cells determines remodeling of atherosclerotic plaques and formation of fibrous caps and proliferation of capillary endothelial cells drives diabetic proliferative retinopathy. Alterations in hemostasis are most significant during development of thrombi associated with atherosclerotic plaques and in microscopic bleeding from retinal vessels. Finally, blood flow is increased early in diabetic nephropathy, but decreased in the retina due to capillary occlusion and in large arteries as a result of development of occlusive plaques (fig. 3). These general changes reflect that similar systemic factors such as hyperglycemia, insulin resistance and dyslipidemia are present in all vascular tissue. However, they also highlight the importance of tissue-specific differences in the response to common metabolic abnormalities.
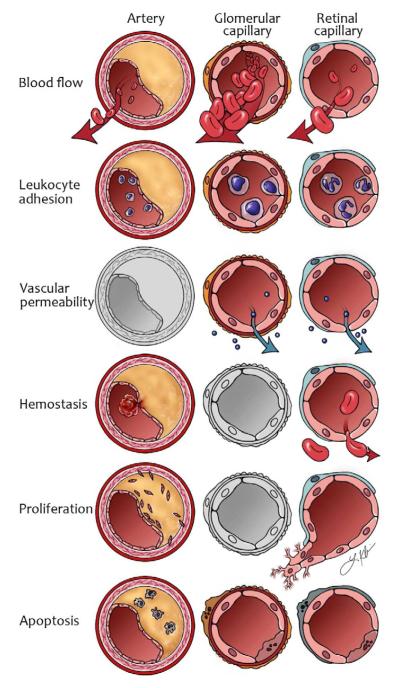
Fig. 3. General abnormalities of vascular function in diabetes.
Diagram showing changes in some of the main functions of blood vessels. The diagram should be read like a table, with the affected vessel in each of 3 columns and functions row by row. In arteries, blood flow may be reduced because of atherosclerosi. Glomerular perfusion is increased in early diabetes and the retina becomes ischemic because of insufficient blood flow. Leukocyte adhesion is present in all three vascular beds, but has a particularly important role in atherogenesis. Increased vascular permeability has a prominent role in the glomerular and retinal capillary. Cellular proliferation occurs for vascular smooth muscle cells in the atherosclerotic plaques and for endothelial cells in proliferative diabetic retinopathy. Apoptosis has important implications when it occurs in macrophages in atherosclerosis and is a major characteristic of the histopathology in diabetic nephropathy and retinopathy. Vessels in gray represent a less prominent abnormality for the vessel in question. Artwork by Leah A. Klein.
Molecular mechanisms of injury
A host of abnormalities in cell signaling, gene expression and regulation of cell biology and physiology has been described in diabetes, and it appears likely that many of these abnormalities operate concurrently during development of diabetic vascular complications. Some of these mechanisms may be active preferentially in the vascular tissue in one organ, but generally they are relevant for development of complications in several organs. Therefore, they are described here without a strict association with a specific pathology.
PKC
PKC is a ubiquitously expressed enzyme which participates in a wide range of intracellular signaling. Its activity is upregulated in vascular tissue in diabetes, including in large arteries, the renal glomeruli, and the retina. Among the 10 mammalian isoforms of PKC, the α, β and δ isoforms have been most consistently implicated in diabetic vascular complications. Gene knockout of PKCβ in a mouse model of retinal ischemia reduces proliferative retinopathy (Suzuma, 2002) and knockout of PKCδ in mice with streptozotocin-induced diabetes prevents retinal pericyte apoptosis (Geraldes, 2009). Mesangial expansion and albuminuria in mice with streptozotocin-induced diabetes is improved in both PKCβ (Ohshiro, 2006) and PKCδ (Mima, 2012) knockout mice and deletion of PKCβ in apoE null mice results in a large reduction of atherosclerosis (Harja, 2009).
The β isoform of PKC can be selectively inhibited with the bisindolylmaleimide compound ruboxistaurin and oral treatment with this drug ameliorates vascular complications in animal models of diabetes. For example, ruboxistaurin improves glomerular and retinal hemodynamics in experimental diabetes (Ishii, 1996), prevents albuminuria and mesangial expansion (Koya, 2000), blocks retinal vascular permeability (Aiello, 1997), and reduces atherosclerosis (Harja, 2009). Effects of PKCβ inhibition were established in preclinical models of early-stage complications, but so far this treatment has only been tested in patients with late-stage complications. Ruboxistaurin was shown to be effective in the treatment of patients with advanced nephropathy (Tuttle, 2005) and late-stage retinopathy (Aiello, 2011). These trials showed that the PKCβ inhibitor reduced albuminuria, prevented a decline in glomerular filtration rate (Tuttle, 2005) and improved the incidence of vision loss (Aiello, 2011). However, further phase III clinical trials of ruboxistaurin or other PKCβ inhibitors are needed and inhibitors of PKCδ for clinical use are warrented.
PKC isoforms are grouped into classic, novel and atypical based on structure and modes of activation. PKCβ and δ belong to the classic and novel groups, respectively, which can both be activated by the lipid diacyl glycerol (DAG). In diabetes, intracellular DAG abundance is increased in vascular tissue through de novo synthesis via glyceraldehyde 3-phosphate and phosphatidic acid or from non-esterified fatty acids. Alternatively, increased DAG synthesis can occur from the glycolytic intermediate dihydroxyacetone phosphate accumulating because of inhibition of the glycolytic enzyme glyceraldehyde 3-phosphate dehydrogenase (GAPDH) after poly(ADP-ribosyl)ation during high glucose concentrations (Du, 2003). Increased DAG mass causes a relatively low, but sustained, level of PKC activation, quite different from the transient activation of PKC seen after activation of G-protein coupled receptors. High glucose concentrations can also increase PKC activity through increases in transcriptional upregulation, as seen in the case of increased expression of PKCδ in retinal vascular cells (Geraldes, 2009) and renal glomeruli (Mima, 2012) (fig. 4).
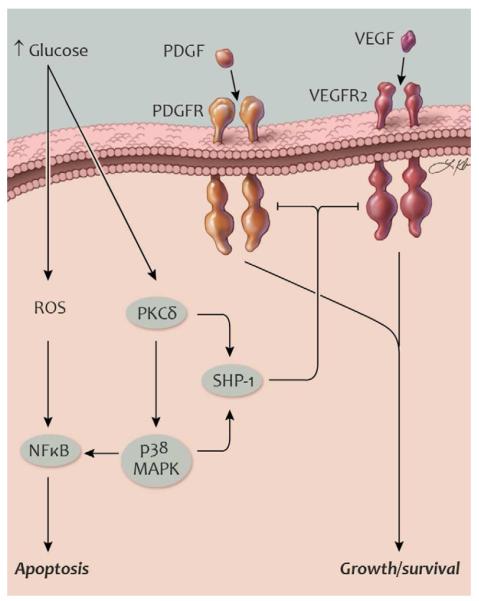
Fig. 4. Activation of SHP-1 and inhibition of survival pathways in diabetes.
Schematic illustration of mechanisms promoting apoptosis in retinal pericytes and glomerular podocytes. PDGF and VEGF receptor signaling promotes cell survival. In diabetes, activation of SHP-1 can dephosphorylate these receptors and contribute to apoptosis. Abbreviations: MAPK, mitogen-activated protein kinase; NFκB, nuclear factor κB; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; ROS, reactive oxygen species; SHP-1, Src homology-2 domain-containing phosphatase-1; VEGF, vascular endothelial growth factor; VEGFR2, VEGF receptor-2. Artwork by Leah A. Klein.
Once activated by hyperglycemia or free fatty acids, PKC promotes retinopathy through several different downstream signaling pathways. For example, the mechanism for reduced retinal blood flow mediated by PKCβ involves endothelin-1 (ET-1) which is upregulated in the retina of diabetic rats (Yokota, 2003). This induction of retinal ET-1 can be blocked by treating the animals with ruboxistaurin (Yokota, 2003). Diabetic macular edema is mediated in part by VEGF through signaling involving PKCβ (Aiello, 1997), in part by increasing phosphorylation of occludin, a component of tight junctions, leading to increased vascular permeability (Murakami, 2012). High glucose concentrations may also increase endothelial cell permeability through PKCα (Hempel, 1997). In addition, activation of PKCδ can enhance the apoptosis of retinal pericytes via multiple mechanisms including activation of p38 MAPK, NADPH oxidase, and NF-κB.
Several isoforms of PKC are involved in the development of diabetic kidney disease. PKCα and β1 are activated in renal glomeruli in mice with streptozotocin-induced diabetes, and 50% of the increase in PKC activity in renal glomeruli is prevented in mice with knockout of PKCβ (Ohshiro, 2006). In wildtype mice, diabetes increases activity of NADPH oxidase and induce expression of ET-1, VEGF, transforming growth factor-β (TGF-β), connective tissue growth factor (CTGF), and collagen IV and VI. These changes are partly prevented in PKCβ knockout mice (Ohshiro, 2006). Supporting the relevance of these findings for human disease, polymorphisms in the PKCβ1 gene have been associated with end-stage renal disease in Chinese patients with type 2 diabetes (Ma, 2010). Activation of PKCα causes upregulation of VEGF through activation of NADPH (Thallas-Bonke, 2008), and PKCα knockout mice are protected against loss of basement membrane proteoglycans and induction of VEGF (Menne, 2004). Surprisingly, PKCε may have effects on diabetic nephropathy opposite of PKCα, β, and δ. One study showed that knockout of PKCε upregulates renal TGFβ1 and its downstream signaling and increases expression of fibronectin and collagen IV, causing glomerular and tuboloinsterstitial fibrosis and development of albuminuria (Meier, 2007). These changes are further aggravated by diabetes (Meier, 2007). Therefore, PKCε may act as a protective factor by reducing kidney damage. Further studies are necessary to understand the role of PKCε in complications of diabetes.
As shown by these and other studies, the evidence for activation of PKC in the diabetic retina and kidney and the causal relationship between PKC activation and vascular complications is very strong. The interest in developing new PKC inhibitors is growing because of recent studies showing roles for PKCδ and PKCε in liver insulin resistance in obesity. However, to achieve clinically significant effects in the prevention or treatment of diabetic retinopathy and nephropathy, inhibition of multiple PKC isoforms, including α, β, and δ, may be needed.
Hyperactive metabolic pathways
Increased cellular glucose uptake increases the flux of glucose through the polyol pathway (also known as the sorbitol pathway). This pathway consumes NADPH in the aldose reductase reaction and reduces NAD+ in the sorbitol reductase reaction (Brownlee, 2001). Activation of the polyol pathway may result not only from increased availability of glucose, the upstream substrate for the pathway, but also from inactivation of GAPDH. This can occur through addition of ADP-ribose moieties to GAPDH by the enzyme poly(ADP-ribose) polymerase (PARP) after its activation by reactive oxygen (Du, 2003). GAPDH is necessary for the glycolytic conversion of glyceraldehyde 3-phosphate. Therefore, GAPDH inactivation leads to increased levels of glyceraldehyde 3-phosphate which in turn causes increased production of methylglyoxal, an AGE precursor, or de novo synthesis of DAG, a PKC activator (Brownlee, 2001).
A hyperactive polyol pathway can also adversely affect cellular homeostasis by depleting cytosolic NADPH, which is necessary to maintain the primary intracellular antioxidant, glutathione, in its reduced state. In addition, pools of intracellular NADPH can be diminished by a different mechanism whereby increased glucose concentrations inhibits glucose 6-phosphate dehydrogenase. This enzyme catalyzes the first intermediary reaction in the pentose phosphate pathway, the primary source of NADPH (Xu, 2009).
Early animal studies of aldose reductase inhibition showed promise with regard to an effect on diabetic retinopathy or nephropathy but such effects have not been demonstrated in patients with diabetes. A renewed interest in aldose reductase has emerged from studies of atherosclerosis in diabetes models. Ubiquitous overexpression of aldose reductase in mice increased atherosclerosis (Vikramadithyan, 2005). Paradoxically, increased atherosclerosis was also described in mice with knockout of the aldose reductase gene or treatment with an aldose reductase inhibitor (Srivastava, 2009). Therefore, further study is necessary to determine the contribution of aldose reductase pathway to atherosclerosis development in diabetes.
Oxidative stress
Production of superoxide and other ROS in the vascular wall plays a prominent role in the pathogenesis of vascular disease in general and has characteristic features in diabetes. A major source of superoxide in vascular cells is thought to be an oxidase which resembles the phagocytic NADPH oxidase, but favors NADH as a substrate (Lassegue, 2012). It is expressed in endothelial cells and vascular smooth muscle cells (Lassegue, 2012). Expression and activity of vascular NADH oxidase are increased in rat models of type 1 (Hink, 2001) and type 2 (Kim, 2002) diabetes. This enzyme may be activated by an increase in the NADH/ NAD+ ratio, which in diabetes may be caused by an increased flux through the polyol pathway (see above) or activation of PARP (Garcia Soriano, 2001). NADH oxidase activity may also be increased by elevated glucose and free fatty acid concentrations through PKC activation (Inoguchi, 2000). Clinical trials have been initiated to test the value of isoform-specific inhibitors of NADPH oxidases in prevention of diabetic complications (Lassegue, 2012).
Mitochondria are another important source of ROS. In mitochondria, the citric acid cycle provides NADH and FADH2 that can act as electron donors for the electron transport chain, creating a proton gradient over the inner mitochondrial membrane (Brownlee, 2001). When intracellular glucose concentration increases in diabetes and thereby yields excessive reducing equivalents for this process, the proton gradient increases and inhibits the transfer of electrons from reduced co-enzyme Q (ubiquinone) to complex III of the electron transport chain (Brownlee, 2001). Instead, electrons is transferred to molecular oxygen causing production of superoxide.
Nitric oxide (NO) can neutralize ROS, but paradoxically endothelial NO synthase (eNOS) can become a source of ROS if an already pro-oxidant redox state favors oxidation of the eNOS cofactor tetrahydrobiopterin (BH4). This leads to uncoupling of electron transport in eNOS and release of superoxide (Laursen, 2001). By promoting DNA strand breaks, oxidative stress can activate PARP, which in turn can activate NFκB and cause endothelial dysfunction (Garcia Soriano, 2001). Oxidative stress can also inhibit the proteasomal degradation of homeo-domain interacting protein kinase 2 (HIPK2) which promotes kidney fibrosis through activation of p53, TGF-β, and Wnt (Jin, 2012).
Glycemic modification of proteins
Modification of extracellular and intracellular proteins by sugars can result in the formation of AGE which can alter protein structure or function and activate proinflammatory and other signaling through cell-surface receptors (Yan, 2010). It is thought that increased concentrations of AGE in diabetes is directly correlated with the level of hyperglycemia although it has been proposed that the rate of AGE formation could also be determined by changes in the metabolism of glucose-derived reactive intermediates, irrespective of glucose concentrations (Fleming, 2012). AGE formation can occur a non-enzymatic reaction between glucose and protein through the Amadori product (1-amino-1-deoxyfructose adducts to lysine). However, much faster reactions take place between proteins and intracellularly formed dicarbonyls including 3-deoxyglucosone, glyoxal, and methylglyoxal. These processes are accelerated by reactive oxygen species.
The most prevalent AGE is carboxymethyl-lysine. Additional non-enzymatic modification results in cross-linking of proteins. Due to their long turn-over rate, structural extracellular proteins such as collagen are particularly susceptible to AGE modification. AGE has been demonstrated in numerous tissues in both types of diabetes, such as the retina, the glomeruli, and the aorta.
AGE modification of extracellular matrix proteins and signaling molecules may alter their function. In addition, AGE-modified extracellular proteins may act by binding to receptors, the most well-characterized being receptor for AGE (RAGE) (Yan, 2010). The ability of RAGE signaling to cause diabetic complications has been directly demonstrated in a study where transgenic mice overexpressing iNOS targeted to β cells, providing a model for type 1 diabetes, were crossbred with transgenic mice overexpressing RAGE. These double transgenic mice developed accelerated glomerular lesions (Yamamoto, 2001), which could be prevented by an AGE inhibitor (Yamamoto, 2001). A soluble receptor for RAGE prevents development of increased vascular permeability and atherosclerosis (Park, 1998) in experimental diabetes. Clinical trials are ongoing for small molecule antagonists of RAGE (Yan, 2010).
The renin-angiotensin system
A large number of clinical trials have unequivocally shown that treatment with angiotensin converting enzyme (ACE) inhibitors, angiotensin type 1 (AT1) receptor blockers, or their combination can prevent the incidence of renal disease or delay the progression to renal failure (Burnier, 2006). However, analysis of renal biopsies from type 1 diabetic patients treated with these drugs showed no improvement in glomerular pathology, indicating that inhibition of the renin-angiotensin system may slow down only the progression of functional impairment in diabetic nephropathy (Mauer, 2009). Many studies also support that treatment with ACE inhibitors can prevent diabetic retinopathy.
Angiotensin I and II are produced locally in the kidney and part of the renoprotective effects of ACE inhibition is through a decrease of glomerular capillary pressure rather than lowering systemic blood pressure. It has been shown that angiotensin II actions may lead to kidney damage through induction of local factors, including extracellular matrix protein synthesis via TGF-β (Kagami, 1994). A similar mechanism may contribute to cardiac fibrosis (Kawano, 2000). Apart from promoting extracellular matrix protein synthesis in cardiac fibroblasts, angiotensin II can induce myocardial PAI-1 expression, which can inhibit extracellular matrix breakdown through inhibition of metalloproteinases (Kawano, 2000).
Plasma kallikrein and bradykinin
Retinal vascular permeability is regulated by activation of bradykinin B1 and B2 receptors and B1 receptor numbers are increased in diabetes. The proteolytic cleavage of high-molecular weight kininogen mediated by plasma kallikrein (PK) results in the increased extracellular expression in the retina of several isoforms of bradykinin. One mechanism by which this kallikrein-kinin system is activated in diabetes is in response to the release of carbonic anhydrase I (CA-I) by red blood cells after microscopic hemorrhage in the retina and into the vitreous (Gao, 2007). In a proteomic analysis, CA-I was found to be increased vitreous samples from patients with proliferative diabetic retinopathy compared to samples from patients without diabetes or diabetic patients without retinopathy (Gao, 2007). Injection of CA-I into the vitreous in rats increased intraocular pH, which in turn activated PK and increased retinal vascular permeability. These effects could be blocked by inhibitors of PK or bradykinin receptors (Gao, 2007). Intravitreal injection of PK increased retinal vascular permeability acutely in diabetic rats by a greater extent than nondiabetic controls and caused retinal thickening (Clermont, 2011). PK inhibitors (Clermont, 2011) or bradykinin receptor blockers (Abdouh, 2008) reduce retinal vascular permeability in rats with streptozotocin-induced diabetes. Inhibitors of PK are currently being developed as a possible treatment of diabetic macular edema.
SHP-1
High glucose concentrations and diabetes can activate Src homology-2 domain-containing phosphatase-1 (SHP-1), a tyrosine phosphatase, in several tissues including the retina and renal glomeruli. This leads to the dephosphorylation and deactivation of specific growth factor receptors critical for survival of pericytes in the retina and podocytes in the kidney (Geraldes, 2009). In the diabetic retina, SHP-1 activation can desensitize pericytes to PDGF and cause pericyte apoptosis, an initiating step in the development of diabetic retinopathy (Geraldes, 2009). In the renal glomeruli, impairment of VEGF survival signaling by upregulation of SHP-1 expression can lead to increased podocyte apoptosis and endothelial dysfunction (Mima, 2012).
Upregulation of SHP-1 in diabetes is dependent on activation of PKCδ and p38 MAPK (Geraldes, 2009; Mima, 2012) (fig. 4). The diabetes-induced upregulation of p38 MAPK and SHP-1 is prevented in PKCδ knockout mice and these animals are protected from apoptosis of retinal pericytes and from mesangial expansion and albuminuria (Geraldes, 2009; Mima, 2012). Retinal pericyte apoptosis has been shown to involve activation of NFκB. However, upregulation of SHP-1 in the diabetic retina and glomerulus is independent of NFκB activation (Geraldes, 2009; Mima, 2012) (fig. 4). Therefore, inhibition of SHP-1 is a potential novel approach to preserve survival signaling in vascular cells.
ER stress
The endoplasmatic reticulum (ER) plays important roles in Ca2+ and redox homeostasis, lipid biosynthesis, and protein folding. Increases in protein synthesis, protein misfolding, or perturbations in Ca2+ and redox balance can disturb ER function, leading to the development of ER stress. In response, a coordinated program referred the unfolded protein response (UPR) is initiated to reduce translation and increase protein folding capacity in an attempt to restore ER homeostasis. Under conditions of chronic, unresolved ER stress the UPR can also initiate signaling events that promote apoptosis.
ER stress has emerged as an important mechanism linking obesity and the development of insulin resistance. It is also involved in pancreatic β-cell failure due to chronic activation of this pathway in the hyperinsulinemic state associated with obesity and insulin resistance. Although the underlying mechanisms that cause ER stress in the context of obesity have only begun to be elucidated, likely causes include increases in protein synthesis in response to nutrient excess, elevated levels of lipids, and changes in ER calcium homeostasis due to alterations in sarco(endo)plasmic reticulum Ca(2+)-ATPase (SERCA) function (Park, 2010b)
Recently, a central role has been described for PI3K regulatory subunits as modulators of the UPR by virtue of their ability to regulate the nuclear translocation of X-box binding protein 1 (XBP-1). The regulatory p85 subunit of PI3K promote nuclear translocation of XBP-1 by binding to this transcription factor as a p85α or p85β monomer (Park, 2010a; Winnay, 2010). However, this interaction competes with formation of p85α/p85β heterodimers (Park, 2010a). Insulin stimulation leads to a disruption of p85α/β heterodimers (Park, 2010a), thereby allowing monomeric p85 to promote XBP-1 nuclear translocation and subsequent upregulation of UPR target genes (Park, 2010a; Winnay, 2010). In the ob/ob mouse model of obesity, this effect of insulin is lost and nuclear translocation of XBP-1 is profoundly impaired, thereby preventing activation of the UPR and resolution of ER stress. Collectively, these data suggest that insulin resistance may fundamentally alter the cellular response to ER stress.
A variety of studies have also implicated ER dysfunction in the pathogenesis of atherosclerosis. For example, UPR target genes are upregulated in areas of the aorta susceptible to atherosclerosis (Civelek, 2009). Moreover, it has been demonstrated that ER stress is induced in endothelial cells by pro-atherosclerotic factors including oxidized LDL or increased intracellular concentrations of glutamine. In addition, macrophage ER stress promotes apoptosis of atherosclerotic plaque macrophages which can lead to necrotic core formation and thrombosis. Accordingly, knockout of the UPR target gene C/EBPα-homologous protein (CHOP) in macrophages prevents macrophage apoptosis (Thorp, 2009). Macrophage apoptosis and plaque rupture can be partly prevented by a pharmacological chaperone, implicating ER stress as a major mechanism regulating these processes (Erbay, 2009).
UPR genes are upregulated in kidney tissue from patients with diabetes and ER stress may be a mediator of diabetic nephropathy. Mice with streptozotocin-induced diabetes and knockout of CHOP are protected from diabetic nephropathy (Wu, 2010). In the retina of rats with streptozotocin-induced diabetes, ER stress is also involved in upregulation of inflammatory genes and VEGF and in mediating increased vascular permeability (Zhong, 2012). These and other findings have prompted development of drugs which can ameliorate ER stress in patients, including synthetic chaperones to promote protein folding and inhibitors of CHOP and other molecules enabling the UPR.
Protective factors
As knowledge about homeostasis of vascular cells has accumulated, it has become clear that some factors play a protective role for the function and survival of cells involved in the vascular complications of diabetes (fig. 1). In the Medalist Study from the Joslin Diabetes Center, more than 40% of a large group of insulin-requiring diabetic patients with disease duration of 50 years or longer were free from significant retinal and renal dysfunction (Keenan, 2007; Sun, 2011). The presence of microvascular complications did not correlate with glycemic control suggesting the presence of endogenous protective factors in this unusual group of patients with diabetes of extreme duration. The possibility that endogenous protective factors are common in the general population of patients with diabetes is supported by the finding that over half of diabetic patients with microalbuminuria have regression of this marker over 6 years of follow-up (Perkins, 2003). Some factors with well-established function have only recently been perceived as protective. For example, the focus on insulin action has traditionally been its metabolic actions, but studies now show that this hormone counteracts excessive leukocyte-endothelial cell adhesion and atherosclerosis development (Rask-Madsen, 2010) and is an important survival factor for glomerular podocytes (Welsh, 2010). As another example, activated protein C (APC) have been known for roles in hemostasis but has recently been identified as a survival factor for renal glomerular cells in diabetes (Isermann, 2007).
Insulin
Insulin receptors are present on vascular cells and cells recruited to the vascular wall, among them endothelial cells, vascular smooth muscle cells, pericytes, and macrophages. Insulin stimulates signal transduction in these cells but does not regulate cell metabolism in the same manner as classic insulin-sensitive cell types. For example, endothelial cells express the insulin-unresponsive glucose transporter GLUT1, but not GLUT4 which increases cellular glucose uptake in response to insulin. However, insulin has important effects on endothelial cell homeostasis. Insulin can activate and upregulate gene expression of eNOS (Kuboki, 2000) which is considered to have an antiatherosclerotic effect. Conversely, insulin can induce expression of ET-1 (Oliver, 1991) which can promote atherosclerosis. The overall effect, however, of endothelial cell insulin resistance is acceleration of atherosclerosis, as demonstrated in apoE null mice with knockout of the insulin receptor (Insr) gene targeted to vascular endothelium (EIRAKO mice) (Rask-Madsen, 2010). In these mice, atherosclerosis increased by up to 2.9-fold compared to apoE null controls with intact insulin receptors. Accelerated atherosclerosis in EIRAKO mice occurred without changes in insulin levels, lipid metabolism, insulin sensitivity, glucose tolerance, or blood pressure. They had, however, increased leukocyte-endothelial cell adhesion to endothelial cells, with up to a 4-fold increase in leukocyte adhesion as measured during in vivo microscopy (Rask-Madsen, 2010). There was no difference in atherosclerosis development in apoE null mice after replacing their bone marrow with grafts from EIRAKO compared to their controls. Furthermore, increased leukocyte adhesion could be recapitulated by injecting labeled monocytes from EIRAKO donors into wildtype recipients, but not by injecting monocytes from wildtype donors into EIRAKO recipients. These results indicated that hematopoietic cells did not contribute significantly to the increased leukocyte adhesion and atherosclerosis development in EIRAKO mice. Additional experiments showed that insulin downregulates VCAM1, an adhesion molecule critical for firm leukocyte adhesion, and that VCAM1 was upregulated in primary endothelial cells from EIRAKO mice (Rask-Madsen, 2010).
FoxO nuclear factors mediate many actions of insulin which require changes in gene transcription. Insulin inhibits FoxO activity by activating Akt, which directly phosphorylates FoxO, causing it to be excluded from the nucleus. Knockout of 3 major FoxO isoforms in endothelial cells had a dramatic effect on atherosclerosis in LDLR knockout mice while reducing oxidative stress, increasing NO production, and decreasing endothelial cells apoptosis (Tsuchiya, 2012). Therefore, FoxO-regulated genes in endothelial cells are likely responsible for the increased susceptibility to atherosclerosis caused by endothelial cell insulin resistance and may prove to be useful targets for prevention of cardiovascular disease.
Hypercholesterolemic mice with conditional knockout of the Insr gene in myeloid cells (primarily monocytes and granulocytes) (Baumgartl, 2006) or with bone marrow replaced with hematopoietic cells from Insr null mice (Han, 2006) had a modest decrease or increase in atherosclerosis, respectively. Mice with bone marrow replaced with grafts from mice with heterozygous knockout of the insulin receptor and IRS-1 showed no change in atherosclerosis development (Galkina, 2012). Therefore, macrophage insulin signaling appears to have little effect on atherosclerosis development. However, impairment of insulin signaling in macrophages causes increased rates of macrophage apoptosis, at least in part by enhancing ER stress. Insulin can inhibit apoptosis by downregulating macrophage scavenger receptors (Liang, 2004), by activating Akt (Han, 2006), inhibiting FoxO1 (Senokuchi, 2008), or inhibiting XBP-1 (Park, 2010a; Winnay, 2010).
Insulin receptor signaling also has a surprisingly profound effect on podocyte survival. A study examined mice with gene knockout of the insulin receptor targeted to podocytes (Welsh, 2010) using promoter activity of either podocin or nephrin, genes not expressed by other kidney cells. From 5 weeks of age, these mice developed albuminuria, effacement of podocyte foot processes, and increased apoptosis together with more deposition of basal membrane components compared to control animals. This pathology was quite similar to that seen in diabetic nephropathy. Some animals also developed shrunken kidneys with prevalent scar tissue, similar to the macroscopic appearance of kidneys in late-stage diabetic nephropathy. These changes were accompanied by mild worsening of kidney function, which is notable because kidney function is not affected by streptozotocin-induced diabetes, the most commonly studied rodent model of diabetes, despite albuminuria and histopathological changes.
Insulin increases expression of VEGF in several cell types and VEGF expression is decreased in the heart muscle of animals with diabetes (Chou, 2002). Therefore, insulin could upregulate VEGF, which in turn could act as a survival factor by autocrine or paracrine signaling to podocytes, endothelial cells, and mesangial cells. Insulin could also prevent apoptosis by other mechanisms, including inhibition of the pro-apoptotic molecule caspase-9 (Hermann, 2000), by inhibition of the transcription factor FoxO (Tsuchiya, 2012), or by upregulation of antioxidant activity of heme oxygenase-1 (HO-1) (Geraldes, 2010) (fig. 5).
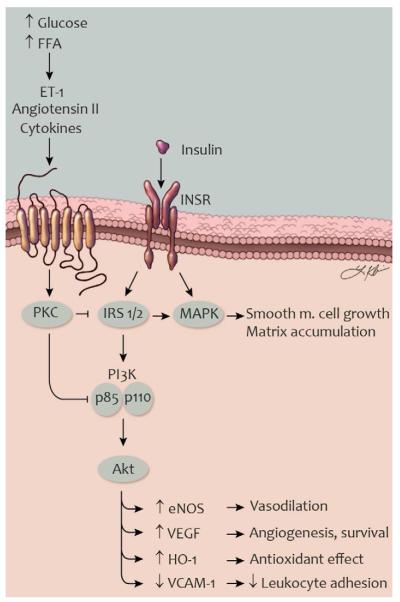
Fig. 5. Selective insulin resistance in vascular cells in type 2 diabetes.
Schematic illustration of mechanisms causing impaired insulin signaling in vascular endothelial and smooth muscle cells. ET-1, angiotensin II, and other factors can be increased by the metabolic milieu and activate PKC. Activated PKC, in turn, can phosphorylate IRS proteins, the p85 subunit of PI3K, and other signaling molecules. In this state, insulin-stimulated activation of the PI3K signaling is inhibited while signaling through the MAPK pathway is preserved or enhanced. Selective insulin resistance in vascular cells cause impaired vasodilation and angiogenesis, reduced antioxidant effects and increased leukocyte adhesion. Abbreviations (see also fig. 3): eNOS, endothelial nitric oxide synthase; ET-1, endothelin-1; FFA, free fatty acids; HO-1, heme oxygenase-1; INSR, insulin receptor; IRS, insulin receptor substrate; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1. Artwork by Leah A. Klein.
Impairment of insulin action on arteries and glomeruli as described above may contribute to formation and destabilization of atherosclerotic plaques and to diabetic nephropathy in patients with type 2 diabetes. Many of insulin’s protective effects are mediated via the IRS/PI3K/Akt pathway, including upregulation of eNOS (Naruse, 2006) and HO-1 (Geraldes, 2010). In contrast, some mechanisms of injury stimulated by insulin are mediated by the Ras/MAPK pathway, such as induction of ET-1 (Oliver, 1991).
In diabetes or other conditions of insulin resistance, elevated concentrations of glucose and free fatty acids can activate PKC, causing selective inhibition of insulin signaling through the PI3K pathway (Naruse, 2006)(fig. 5). Certain serine residues on IRS-2 and on the p85 regulatory subunit of PI3K have recently been identified as substrates for PKC and phosphorylation of these sites inhibits insulin signaling through the PI3K pathway (Maeno, 2012). Despite these changes, insulin signaling through the Ras/MAPK pathway is preserved or increased in insulin resistance (Jiang, 1999). This state of selective insulin resistance has been demonstrated in animal models (Jiang, 1999) as well as in human endothelial cells (Gogg, 2009). Hyperinsulinemia in type 2 diabetes could conceivably promote vascular disease through induction of ET-1 or other factors induced by MAPK signaling. However, a mouse model of hyperinsulinemia with preserved vascular tissue insulin signaling did not show increased atherosclerosis (Rask-Madsen, 2012), suggesting that the vascular wall may be less affected by hyperinsulinemia than by vascular cell insulin resistance. Future studies will be needed to determine whether enhancement of signaling through the PI3K pathway can ameliorate endothelial dysfunction and atherosclerosis development in type 2 diabetes and other insulin resistant states.
Antioxidant enzymes
Despite overwhelming preclinical evidence showing that oxidative stress is involved in vascular complications, including all the diabetic vascular complications discussed in this review, an amount of resignation has dominated clinical translation of this knowledge because most large trials of antioxidants have failed to show significant advantages. Researchers with an unperturbed optimism about the importance of oxidative stress mechanisms argued that antioxidant activity needed to be induced among endogenous systems, not by exogenously provided compounds like antioxidant vitamins because they would be depleted or not reach intracellular locations (Wassmann, 2004). This point of view has been supported by results from clinical trials of bardoxolone methyl (BARD) (Pergola, 2011). A main mechanism of action for this drug is to activate nuclear factor (erythroid-derived 2)-like 2 (Nrf2). This nuclear factor upregulates a gene program of molecules with antioxidant activity called phase 2 genes, including HO-1 and enzymes in the glutathione biosynthesis pathway. Nrf2 translocation to the nucleus is inhibited by kelch-like ECH-associated protein 1 (Keap1), a repressor which binds Nrf2 in the cytoplasm and promotes Nrf2 proteasomal degradation. BARD interacts with cysteine residues on Keap1, making it unable to repress Nrf2, which then activates transcription of phase 2 genes.
Results from a trial of BARD in patients with advanced chronic kidney disease showed an improvement in GFR up to one year after start of treatment (Pergola, 2011) This is a remarkable result and a trial is ongoing to evaluate the efficacy of BARD on progression to end-stage renal disease. An increase in the rate of albuminuria during BARD treatment and the loss of most of the effect on GFR four weeks after withdrawal of BARD was observed, so additional studies are needed to understand kidney function during BARD treatment, in particular how renal hemodynamics are affected.
PDGF
PDGF expressed by retinal endothelial cells plays a role both in vascular cell survival and proliferative retinopathy (Lei, 2009). During sprouting angiogenesis, PDGF is produced by endothelial tip cells and acts through PDGF receptor-β expressed by pericytes. This signal recruits pericytes to developing blood vessels. Pericytes, in turn, can support endothelial cell survival as well as suppress endothelial cell proliferation. This is demonstrated by findings in PDGF knockout mouse embryos which show pericyte loss and endothelial cell proliferation (Lindahl, 1997). Mice with heterozygous deletion of the PDGF gene have increased frequency of acellular capillaries, in particular after induction of diabetes, but also an increased tendency for retinal neovascularization during ischemic retinopathy (Hammes, 2002). PDGF necessary for supporting pericytes are likely released from endothelial cell because deletion of the PDGF gene targeted to endothelium show similar pathology as whole-body deletion, whereas deletion of PDGF-B in neurons, another source of PDGF-B, does not alter pericyte coverage in the brain. As described above, we have reported that hyperglycemia can inhibit survival effects of PDGF by upregulation of SHP-1 which causes dephosphorylation of the PDGF receptor in pericytes and possibly also in podocytes (Geraldes, 2009).
TGF-β
Expression of TGF-β is increased in blood vessels in many vascular beds in diabetes and has been viewed as a causative factor for development of fibrosis in the kidney, myocardium, and other tissues. Among many preclinical studies, one showed that in db/db mice, treatment with a neutralizing antibody towards TGF-β prevents glomerulosclerosis and impairment of creatinine clearance in diabetic animals (Ziyadeh, 2000). However, a profibrotic effect can be beneficial in the context of certain vascular complications, as in atherosclerotic plaques where thinning of the fibrous plaque is a central event in precipitation of thrombosis. In addition, TGF-β has an anti-inflammatory effect on macrophages and is a negative regulator of T-cells and B-cells and, therefore, may have protective actions due to an anti-inflammatory effect.
Consistent with such beneficial roles of TGF-β, intraperitoneal injection of recombinant soluble TGF-β receptor II in apoE null mice increased inflammatory cells in the aorta and decreased collagen content (Lutgens, 2002). Similar results was obtained in a separate study by treatment of apoE null mice with antibodies toward either of 3 different TGF-β isoforms. Conversely, an inducible TGF-β1 transgene expressed in cardiomyocytes of apoE null mice, through elevations in plasma concentrations of TGF-β1, decreased signs of inflammation in the aorta and increased aortic collagen deposition (Frutkin, 2009). These diverse roles of TGF-β are a challenge for using it as a drug target. Development of targeted nanoparticles or other means of tissue-specific drug delivery may allow inhibition of TGF-β signaling in the kidney and augmentation of TGF-β signaling in arteries affected by atherosclerosis.
VEGF
Neutralization of VEGF is already in off-label use for treatment of proliferative diabetic retinopathy and macular edema and has been suggested as a therapy for diabetic nephropathy (Chen, 2008). However, the increased levels of VEGF in both tissues are likely an appropriate response to hypoxia. It has been a long-standing concern that neutralization of VEGF could counteract survival signaling in retinal neurons. Interestingly, injection of low doses of VEGF accelerated restoration of the physiological capillary bed and prevented preretinal neovascularization in a mouse model of proliferative retinopathy (Dorrell, 2009).
The highest expression level of VEGF in the kidney is in renal podocytes, and some of the most comprehensive work describing a role for VEGF as a survival factor in any organ susceptible to diabetes complications has been done in renal podocytes. Conditional deletion of VEGF in podocytes resulted in a complete lack of endothelial and mesangial cells in mature glomeruli and death within the first day of life (Eremina, 2003). This finding strongly supports a role for VEGF in maintenance of glomerular endothelial cells. Heterozygous knockout of VEGF in podocytes of mice resulted in proteinuria and end-stage renal failure in young adults (Eremina, 2003) and was preceded by disappearance of endothelial cell fenestrations, endothelial cell necrosis, effacement of podocyte foot processes, and a dramatic loss of mesangial cells (Eremina, 2003). When diabetes was induced with streptozotocin in mice with podocyte-specific knockout of VEGF, glomerular cell apoptosis, glomerulosclerosis, and proteinuria were all exacerbated compared with nondiabetic controls (Sivaskandarajah, 2012). As mentioned in an earlier section, established nephropathy in mouse models can be improved by treatment with anti-VEGF compounds. Future research should be able to reveal whether promoting VEGF action on glomerular cells before onset of nephropathy can prevent renal disease.
APC
Protein C has been known as an anticoagulant factor, but more recently was identified as a survival factor for renal glomerular cells (Isermann, 2007). Thrombomodulin, a pro-coagulant factor which activates protein C, was found to be highly expressed in glomeruli of mice, but was downregulated in diabetes (Isermann, 2007). Diabetic mice with a loss-of-function thrombomodulin gene mutation had more albuminuria and more severe glomerular pathology than diabetic wild-type mice, whereas diabetic mice with a gain-of-function mutation of the protein C gene had less albuminuria and glomerular pathology (Isermann, 2007). The anticoagulant effects of APC did not account for its protective actions. Rather, APC was shown to counteract apoptosis of endothelial cells and podocytes through activation of two of its receptors (Isermann, 2007). Therefore, endothelial-derived APC appears to be a protective factor with local survival effects for both podocytes and endothelial cells in the glomerulus.
Efferocytosis and autophagy
Apoptosis of plaque macrophages and foam cells is a prominent feature of advanced atherosclerotic plaques is. Susceptibility to apoptosis is increased in insulin resistant macrophages because of impaired signaling through Akt or other anti-apoptotic pathways or due to activation of ER stress (Han, 2006; Liang, 2004). Phagocytic clearance, or efferocytosis, prevents post-apoptotic necrosis. Efferocytosis is defective in advanced plaques and this contributes to formation of a necrotic core. This can amplify the proinflammatory condition and lead to disruption of the fibrous cap of the atherosclerotic plaque through release of matrix metalloproteases. Such events promote thrombosis through expression of tissue factor and release of plasma membrane microparticles from necrotic cells (Tabas, 2010).
Macrophage function in atherosclerotic plaques is also affected by autophagy, the recycling of organelles and other cytoplasmic components through lysosomal degradation (Yamada, 2012). A specialized form of autophagy, termed lipophagy, is also involved in the mobilization and degradation of intracellular lipid and is impaired in obesity. Activation of autophagy in plaque macrophages can prevent formation of foam cells and limit atherosclerosis. This is seen in mice with Wip1 knockout (Le Guezennec, 2012). Wip1 is a phosphatase which negatively regulates the ataxia telangiectasia mutated (ATM) kinase. Wip1 knockout activates ATM in macrophages and initiates autophagy, which in turn increases cholesterol efflux and prevents formation of macrophage foam cells (Le Guezennec, 2012). Macrophage autophagy also prevents apoptosis and promotes efferocytosis of apoptotic macrophages (Liao, 2012). Although not yet investigated, autophagy of plaque macrophages is likely impaired in obesity and type 2 diabetes like lipophagy in other locations such as liver (Yamada, 2012). Autophagy may also be an important protective mechanism for other diabetic complications, such as nephropathy (Kume, 2012).
Vascular progenitor cells
Bone-marrow derived cells, including endothelial progenitor cells (EPC) and myeloid progenitors contribute to postnatal angiogenesis (Bautch, 2011). The mechanism for this is currently not well-characterized despite intensive interest. It appears that structural contribution by incorporation of EPC into newly formed blood vessels plays a minor role. Release of proangiogenic factors from EPC temporarily associating with neovascular structures may play a more important role. In patients with diabetes, both the number and function of EPC have been found to be impaired (Loomans, 2004; Tepper, 2002). Differentiation of EPC from bone-marrow derived cells may also play a role (Tamarat, 2004). eNOS is necessary for mobilization of EPC from the bone marrow as this phenomenon is impaired in eNOS knockout mice (Aicher, 2003). Uncoupling of eNOS, with synthesis of superoxide rather than NO by eNOS, could be one mechanism for impaired EPC function. In fact, EPC function is improved after inhibition of eNOS ex vivo in EPC isolated from patients with diabetes (Thum, 2007). Interestingly, bone marrow neuropathy may cause reduced mobilization of EPC. Thus, rats with streptozotocin-induced diabetes had a reduction in nerve terminals in bone marrow and denervation resulted in increased number of EPC in the bone marrow, but decreased release of EPC to the circulation and an increase in retinal acellular capillaries (Busik, 2009).
Transplantation of non-diabetic EPC to diabetic animals has been shown to improve angiogenesis in peripheral ischemia. Similarly, injection of a subpopulation of myeloid progenitor cells differentiated into retinal macrophages known as microglia, improved intraretinal angiogenesis, and partly prevented pathological, preretinal angiogenesis in ischemic retinopathy (Ritter, 2006). Studies like these suggest that it may be possible to promote repair of ischemic tissue in diabetes by therapy aimed at improving mobilization, differentiation and function of EPC or other progenitors.
Conclusion
The most effective prevention of diabetic complications would likely be to achieve perfect metabolic control and normalize insulin resistance, but this goal is unrealistic in the foreseeable future. A wealth of mechanistic information has accumulated regarding the adverse effects of hyperglycemia and insulin resistance on the vascular wall of arteries and the microvasculature. Recognition of the importance of endogenous protective factors in determining the course of diabetic vascular complications is providing a new perspective for understanding the development of diabetic complications. Characterizing pathways controlled by protective factors should also provide new opportunities for development of therapeutics effective at preventing or delaying the vascular complications of diabetes.
Acknowledgements
Dr. Madsen is supported by NIH grant K08 EY018677 and Dr. King by NIH grant R01 DK053105 and EY016150. This work was also supported by NIDDK Diabetes Research Center grant P30DK036836. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Neither of the authors declare any conflict of interest.
References
- Abdouh M, Talbot S, Couture R, Hassessian HM. Retinal plasma extravasation in streptozotocin-diabetic rats mediated by kinin B(1) and B(2) receptors. Br J Pharmacol. 2008;154:136–43. doi: 10.1038/bjp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–6. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Bursell SE, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla TA, Ways K, Jirousek M, Smith LE, King GL. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Vignati L, Sheetz MJ, Zhi X, Girach A, Davis MD, Wolka AM, Shahri N, Milton RC. Oral protein kinase C beta inhibition using ruboxistaurin: efficacy, safety, and causes of vision loss among 813 patients (1,392 eyes) with diabetic retinopathy in the Protein Kinase C beta Inhibitor-Diabetic Retinopathy Study and the Protein Kinase C beta Inhibitor-Diabetic Retinopathy Study 2. Retina. 2011;31:2084–94. doi: 10.1097/IAE.0b013e3182111669. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366:1227–39. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Baumgartl J, Baudler S, Scherner M, Babaev V, Makowski L, Suttles J, McDuffie M, Tobe K, Kadowaki T, Fazio S, Kahn CR, Hotamisligil GS, Krone W, Linton M, Bruning JC. Myeloid lineage cell-restricted insulin resistance protects apolipoproteinE-deficient mice against atherosclerosis. Cell Metab. 2006;3:247–56. doi: 10.1016/j.cmet.2006.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautch VL. Stem cells and the vasculature. Nat Med. 2011;17:1437–43. doi: 10.1038/nm.2539. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Burnier M, Zanchi A. Blockade of the renin-angiotensin-aldosterone system: a key therapeutic strategy to reduce renal and cardiovascular events in patients with diabetes. J Hypertens. 2006;24:11–25. doi: 10.1097/01.hjh.0000191244.91314.9d. [DOI] [PubMed] [Google Scholar]
- Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S, Player D, Nakagawa T, Afzal A, Kielczewski J, Sochacki A, Hasty S, Li Calzi S, Kim S, Duclas SK, Segal MS, Guberski DL, Esselman WJ, Boulton ME, Grant MB. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206:2897–906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ziyadeh FN. Vascular endothelial growth factor and diabetic nephropathy. Curr Diab Rep. 2008;8:470–6. doi: 10.1007/s11892-008-0081-3. [DOI] [PubMed] [Google Scholar]
- Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–9. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr., Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–61. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clermont A, Chilcote TJ, Kita T, Liu J, Riva P, Sinha S, Feener EP. Plasma kallikrein mediates retinal vascular dysfunction and induces retinal thickening in diabetic rats. Diabetes. 2011;60:1590–8. doi: 10.2337/db10-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell MI, Aguilar E, Jacobson R, Trauger SA, Friedlander J, Siuzdak G, Friedlander M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia. 2009 doi: 10.1002/glia.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–52. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- Du X, Matsumura T, Edelstein D, Rossetti L, Zsengeller Z, Szabo C, Brownlee M. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–57. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbay E, Babaev VR, Mayers JR, Makowski L, Charles KN, Snitow ME, Fazio S, Wiest MM, Watkins SM, Linton MF, Hotamisligil GS. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–91. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eremina V, Sood M, Haigh J, Nagy A, Lajoie G, Ferrara N, Gerber HP, Kikkawa Y, Miner JH, Quaggin SE. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–16. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T, Cuny J, Nawroth G, Djuric Z, Humpert PM, Zeier M, Bierhaus A, Nawroth PP. Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia. 2012;55:1151–5. doi: 10.1007/s00125-012-2452-1. [DOI] [PubMed] [Google Scholar]
- Frutkin AD, Otsuka G, Stempien-Otero A, Sesti C, Du L, Jaffe M, Dichek HL, Pennington CJ, Edwards DR, Nieves-Cintron M, Minter D, Preusch M, Hu JH, Marie JC, Dichek DA. TGF-beta1 limits plaque growth, stabilizes plaque structure, and prevents aortic dilation in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2009;29:1251–7. doi: 10.1161/ATVBAHA.109.186593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina EV, Butcher M, Keller SR, Goff M, Bruce A, Pei H, Sarembock IJ, Sanders JM, Nagelin MH, Srinivasan S, Kulkarni RN, Hedrick CC, Lattanzio FA, Dobrian AD, Nadler JL, Ley K. Accelerated atherosclerosis in Apoe−/− mice heterozygous for the insulin receptor and the insulin receptor substrate-1. Arterioscler Thromb Vasc Biol. 2012;32:247–56. doi: 10.1161/ATVBAHA.111.240358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BB, Clermont A, Rook S, Fonda SJ, Srinivasan VJ, Wojtkowski M, Fujimoto JG, Avery RL, Arrigg PG, Bursell SE, Aiello LP, Feener EP. Extracellular carbonic anhydrase mediates hemorrhagic retinal and cerebral vascular permeability through prekallikrein activation. Nat Med. 2007;13:181–8. doi: 10.1038/nm1534. [DOI] [PubMed] [Google Scholar]
- Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–13. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. Activation of PKC-delta and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–306. doi: 10.1038/nm.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106:1319–31. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58:2238–45. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Feng Y, Pfister F, Brownlee M. Diabetic retinopathy: targeting vasoregression. Diabetes. 2011;60:9–16. doi: 10.2337/db10-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51:3107–12. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- Han S, Liang CP, DeVries-Seimon T, Ranalletta M, Welch CL, Collins-Fletcher K, Accili D, Tabas I, Tall AR. Macrophage insulin receptor deficiency increases ER stress-induced apoptosis and necrotic core formation in advanced atherosclerotic lesions. Cell Metab. 2006;3:257–66. doi: 10.1016/j.cmet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, Yan SF. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–91. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997;81:363–71. doi: 10.1161/01.res.81.3.363. [DOI] [PubMed] [Google Scholar]
- Hermann C, Assmus B, Urbich C, Zeiher AM, Dimmeler S. Insulin-mediated stimulation of protein kinase Akt: A potent survival signaling cascade for endothelial cells. Arterioscler.Thromb.Vasc.Biol. 2000;20:402–9. doi: 10.1161/01.atv.20.2.402. [DOI] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med. 2007;13:1349–58. doi: 10.1038/nm1667. [DOI] [PubMed] [Google Scholar]
- Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–31. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–57. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Ratnam K, Chuang PY, Fan Y, Zhong Y, Dai Y, Mazloom AR, Chen EY, D’Agati V, Xiong H, Ross MJ, Chen N, Ma’ayan A, He JC. A systems approach identifies HIPK2 as a key regulator of kidney fibrosis. Nat Med. 2012;18:580–8. doi: 10.1038/nm.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–7. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Do YS, Kawano Y, Starnes V, Barr M, Law RE, Hsueh WA. Angiotensin II has multiple profibrotic effects in human cardiac fibroblasts. Circulation. 2000;101:1130–7. doi: 10.1161/01.cir.101.10.1130. [DOI] [PubMed] [Google Scholar]
- Keenan HA, Costacou T, Sun JK, Doria A, Cavellerano J, Coney J, Orchard TJ, Aiello LP, King GL. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care. 2007;30:1995–7. doi: 10.2337/dc06-2222. [DOI] [PubMed] [Google Scholar]
- Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW, Kim CD. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes. 2002;51:522–7. doi: 10.2337/diabetes.51.2.522. [DOI] [PubMed] [Google Scholar]
- Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–47. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, Feener EP, Herbert TP, Rhodes CJ, King GL. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo: a specific vascular action of insulin. Circulation. 2000;101:676–81. doi: 10.1161/01.cir.101.6.676. [DOI] [PubMed] [Google Scholar]
- Kume S, Thomas MC, Koya D. Nutrient sensing, autophagy, and diabetic nephropathy. Diabetes. 2012;61:23–9. doi: 10.2337/db11-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–90. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–8. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- Le Guezennec X, Brichkina A, Huang YF, Kostromina E, Han W, Bulavin DV. Wip1-dependent regulation of autophagy, obesity, and atherosclerosis. Cell Metab. 2012;16:68–80. doi: 10.1016/j.cmet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Lei H, Rheaume MA, Kazlauskas A. Recent developments in our understanding of how platelet-derived growth factor (PDGF) and its receptors contribute to proliferative vitreoretinopathy. Exp Eye Res. 2009;90:376–81. doi: 10.1016/j.exer.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CP, Han S, Okamoto H, Carnemolla R, Tabas I, Accili D, Tall AR. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–73. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Sluimer JC, Wang Y, Subramanian M, Brown K, Pattison JS, Robbins J, Martinez J, Tabas I. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–53. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–9. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- Lutgens E, Gijbels M, Smook M, Heeringa P, Gotwals P, Koteliansky VE, Daemen MJ. Transforming growth factor-beta mediates balance between inflammation and fibrosis during plaque progression. Arterioscler Thromb Vasc Biol. 2002;22:975–82. doi: 10.1161/01.atv.0000019729.39500.2f. [DOI] [PubMed] [Google Scholar]
- Ma RC, Tam CH, Wang Y, Luk AO, Hu C, Yang X, Lam V, Chan AW, Ho JS, Chow CC, Tong PC, Jia W, Ng MC, So WY, Chan JC. Genetic variants of the protein kinase C-beta 1 gene and development of end-stage renal disease in patients with type 2 diabetes. JAMA. 2010;304:881–9. doi: 10.1001/jama.2010.1191. [DOI] [PubMed] [Google Scholar]
- Maeno Y, Li Q, Park K, Rask-Madsen C, Gao B, Matsumoto M, Liu Y, Wu IH, White MF, Feener EP, King GL. Inhibition of insulin signaling in endothelial cells by protein kinase C-induced phosphorylation of p85 subunit of phosphatidylinositol 3-kinase (PI3K) J Biol Chem. 2012;287:4518–30. doi: 10.1074/jbc.M111.286591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier M, Menne J, Park JK, Holtz M, Gueler F, Kirsch T, Schiffer M, Mengel M, Lindschau C, Leitges M, Haller H. Deletion of protein kinase C-epsilon signaling pathway induces glomerulosclerosis and tubulointerstitial fibrosis in vivo. J Am Soc Nephrol. 2007;18:1190–8. doi: 10.1681/ASN.2005070694. [DOI] [PubMed] [Google Scholar]
- Menne J, Park JK, Boehne M, Elger M, Lindschau C, Kirsch T, Meier M, Gueler F, Fiebeler A, Bahlmann FH, Leitges M, Haller H. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-alpha-deficient diabetic mice. Diabetes. 2004;53:2101–9. doi: 10.2337/diabetes.53.8.2101. [DOI] [PubMed] [Google Scholar]
- Mima A, Kitada M, Geraldes P, Li Q, Matsumoto M, Mizutani K, Qi W, Li C, Leitges M, Rask-Madsen C, King GL. Glomerular VEGF resistance induced by PKCdelta/SHP-1 activation and contribution to diabetic nephropathy. FASEB J. 2012;26:2963–74. doi: 10.1096/fj.11-202994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Frey T, Lin C, Antonetti DA. Protein kinase cbeta phosphorylates occludin regulating tight junction trafficking in vascular endothelial growth factor-induced permeability in vivo. Diabetes. 2012;61:1573–83. doi: 10.2337/db11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruse K, Rask-Madsen C, Takahara N, Ha SW, Suzuma K, Way KJ, Jacobs JR, Clermont AC, Ueki K, Ohshiro Y, Zhang J, Goldfine AB, King GL. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–8. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, Yagi K, Arikawa E, Kern TS, King GL. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006;55:3112–20. doi: 10.2337/db06-0895. [DOI] [PubMed] [Google Scholar]
- Oliver FJ, de la Rubia G, Feener EP, Lee ME, Loeken MR, Shiba T, Quertermous T, King GL. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J Biol Chem. 1991;266:23251–6. [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Jr., Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–31. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med. 2010a;16:429–37. doi: 10.1038/nm.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Zhou Y, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010b;107:19320–5. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365:327–36. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- Perkins BA, Ficociello LH, Silva KH, Finkelstein DM, Warram JH, Krolewski AS. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–93. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- Rask-Madsen C, Buonomo E, Li Q, Park K, Clermont AC, Yerokun O, Rekhter M, King GL. Hyperinsulinemia does not change atherosclerosis development in apolipoprotein E null mice. Arterioscler Thromb Vasc Biol. 2012;32:1124–31. doi: 10.1161/ATVBAHA.111.239558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask-Madsen C, Li Q, Freund B, Feather D, Abramov R, Wu IH, Chen K, Yamamoto-Hiraoka J, Goldenbogen J, Sotiropoulos KB, Clermont A, Geraldes P, Dall’Osso C, Wagers AJ, Huang PL, Rekhter M, Scalia R, Kahn CR, King GL. Loss of insulin signaling in vascular endothelial cells accelerates atherosclerosis in apolipoprotein E null mice. Cell Metab. 2010;11:379–89. doi: 10.1016/j.cmet.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter MR, Banin E, Moreno SK, Aguilar E, Dorrell MI, Friedlander M. Myeloid progenitors differentiate into microglia and promote vascular repair in a model of ischemic retinopathy. J Clin Invest. 2006;116:3266–76. doi: 10.1172/JCI29683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senokuchi T, Liang CP, Seimon TA, Han S, Matsumoto M, Banks AS, Paik JH, DePinho RA, Accili D, Tabas I, Tall AR. Forkhead transcription factors (FoxOs) promote apoptosis of insulin-resistant macrophages during cholesterol-induced endoplasmic reticulum stress. Diabetes. 2008;57:2967–76. doi: 10.2337/db08-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaskandarajah GA, Jeansson M, Maezawa Y, Eremina V, Baelde HJ, Quaggin SE. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61:2958–66. doi: 10.2337/db11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniderman AD, Scantlebury T, Cianflone K. Hypertriglyceridemic hyperapoB: the unappreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus. Ann Intern Med. 2001;135:447–59. doi: 10.7326/0003-4819-135-6-200109180-00014. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Vladykovskaya E, Barski OA, Spite M, Kaiserova K, Petrash JM, Chung SS, Hunt G, Dawn B, Bhatnagar A. Aldose reductase protects against early atherosclerotic lesion formation in apolipoprotein E-null mice. Circ Res. 2009;105:793–802. doi: 10.1161/CIRCRESAHA.109.200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care. 2011;34:968–74. doi: 10.2337/dc10-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuma K, Takahara N, Suzuma I, Isshiki K, Ueki K, Leitges M, Aiello LP, King GL. Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc Natl Acad Sci U S A. 2002;99:721–6. doi: 10.1073/pnas.022644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamarat R, Silvestre JS, Le Ricousse-Roussanne S, Barateau V, Lecomte-Raclet L, Clergue M, Duriez M, Tobelem G, Levy BI. Impairment in ischemia-induced neovascularization in diabetes: bone marrow mononuclear cell dysfunction and therapeutic potential of placenta growth factor treatment. Am J Pathol. 2004;164:457–66. doi: 10.1016/S0002-9440(10)63136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–6. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57:460–9. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–81. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–74. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, Depinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–81. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28:2686–90. doi: 10.2337/diacare.28.11.2686. [DOI] [PubMed] [Google Scholar]
- Vikramadithyan RK, Hu Y, Noh HL, Liang CP, Hallam K, Tall AR, Ramasamy R, Goldberg IJ. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–43. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AM, Callaghan BC, Smith AL, Feldman EL. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7:573–83. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- Wassmann S, Wassmann K, Nickenig G. Modulation of oxidant and antioxidant enzyme expression and function in vascular cells. Hypertension. 2004;44:381–6. doi: 10.1161/01.HYP.0000142232.29764.a7. [DOI] [PubMed] [Google Scholar]
- Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstadt H, Tavare JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–40. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR. A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med. 2010;16:438–45. doi: 10.1038/nm.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Zhang R, Torreggiani M, Ting A, Xiong H, Striker GE, Vlassara H, Zheng F. Induction of diabetes in aged C57B6 mice results in severe nephropathy: an association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol. 2010;176:2163–76. doi: 10.2353/ajpath.2010.090386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zhang Z, Hu J, Stillman IE, Leopold JA, Handy DE, Loscalzo J, Stanton RC. Glucose-6-phosphate dehydrogenase-deficient mice have increased renal oxidative stress and increased albuminuria. FASEB J. 2009;24:609–16. doi: 10.1096/fj.09-135731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada E, Singh R. Mapping autophagy on to your metabolic radar. Diabetes. 2012;61:272–80. doi: 10.2337/db11-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kato I, Doi T, Yonekura H, Ohashi S, Takeuchi M, Watanabe T, Yamagishi S, Sakurai S, Takasawa S, Okamoto H, Yamamoto H. Development and prevention of advanced diabetic nephropathy in RAGE-overexpressing mice. J Clin Invest. 2001;108:261–8. doi: 10.1172/JCI11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–53. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T, Ma RC, Park JY, Isshiki K, Sotiropoulos KB, Rauniyar RK, Bornfeldt KE, King GL. Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes. 2003;52:838–45. doi: 10.2337/diabetes.52.3.838. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Li J, Chen Y, Wang JJ, Ratan R, Zhang SX. Activation of endoplasmic reticulum stress by hyperglycemia is essential for Muller cell-derived inflammatory cytokine production in diabetes. Diabetes. 2012;61:492–504. doi: 10.2337/db11-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La, Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A. 2000;97:8015–20. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]