Abstract
Background: Exposure to air pollution has been linked to the exacerbation of respiratory diseases. The Air Quality Health Index (AQHI), developed in Canada, is a new health risk scale for reporting air quality and advising risk reduction actions.
Objective: We used the AQHI to estimate the impact of air quality on asthma morbidity, adjusting for potential confounders.
Methods: Daily air pollutant measures were obtained from 14 regional monitoring stations in Ontario. Daily counts of asthma-attributed hospitalizations, emergency department (ED) visits, and outpatient visits were obtained from a provincial registry of 1.5 million patients with asthma. Poisson regression was used to estimate health services rate ratios (RRs) as a measure of association between the AQHI or individual pollutants and health services use. We adjusted for age, sex, season, year, and region of residence.
Results: The AQHI values were significantly associated with increased use of asthma health services on the same day and on the 2 following days, depending on the specific outcome assessed. A 1-unit increase in the AQHI was associated with a 5.6% increase in asthma outpatient visits (RR = 1.056; 95% CI: 1.053, 1.058) and a 2.1% increase in the rate of hospitalization (RR = 1.021; 95% CI: 1.014, 1.028) on the same day and with a 1.3% increase in the rate of ED visits (RR = 1.013; 95% CI: 1.010, 1.017) after a 2-day lag.
Conclusions: The AQHI values were significantly associated with the use of asthma-related health services. Timely AQHI health risk advisories with integrated risk reduction messages may reduce morbidity associated with air pollution in patients with asthma.
Keywords: air pollution, air quality health index, asthma, health services utilization
Asthma is a common chronic respiratory disease with a worldwide prevalence ranging from 5 to 18% (Bousquet et al. 2007; Farrar 2005; Masoli et al. 2004) marked by inflammation, bronchial hyperresponsiveness, and airflow limitation. Acute asthma attacks that result in health services use are common (Carlton et al. 2005; Chapman et al. 2001; FitzGerald et al. 2006; Lai et al. 2003; Rabe et al. 2004; Sekerel et al. 2006) and have been associated with a variety of air pollutants (Gilliland 2009; Lin et al. 2005; Stieb et al. 2002, 2009; Weinmayr et al. 2010). Six pollutants are considered in the reporting of air quality in North America using the Air Quality Index (AQI): ground-level ozone (O3), fine particulate matter (PM ≤ 2.5 µm in aerodynamic diameter; PM2.5), nitrogen dioxide (NO2), sulfur dioxide (SO2), carbon monoxide (CO), and total reduced sulfur (TRS) compounds. Since 1988, AQI values in Ontario have been established by the Ministry of the Environment to reflect air quality management objectives to protect human health. The AQI is based on the six pollutants noted above and is reported as the value for the single pollutant with the highest AQI (Balluz et al. 2007; Ontario Ministry of the Environment 2012; Shenfeld and Yap 1989). Health Canada and Environment Canada began a collaboration in 2001 to develop a new index named the Air Quality Health Index (AQHI), which was derived based on the combined impact of three pollutants (NO2, O3, and PM2.5) (Environment Canada 2012a). AQHI values are linked to specific risk-reduction health messages designed to educate individuals on the impact of air quality on health, and to advise specific risk reduction actions (Table 1) (Environics Research Group Ltd 2005; Environment Canada 2012b).
Table 1.
Risk levels and health messages according to AQHI levels (Environment Canada 2012b).
| Health risk | AQHI | Health messages | ||||
|---|---|---|---|---|---|---|
| At-risk populationa | General population | |||||
| Low | 1–3 | Enjoy your usual outdoor activities. | Ideal air quality for outdoor activities. | |||
| Moderate | 4–6 | Consider reducing or rescheduling strenuous activities outdoors if you are experiencing symptoms. | No need to modify your usual outdoor activities unless you experience symptoms such as coughing and throat irritation. | |||
| High | 7–10 | Reduce or reschedule strenuous activities outdoors. Children and the elderly should also take it easy. | Consider reducing or rescheduling strenuous activities outdoors if you experience symptoms such as coughing and throat irritation. | |||
| Very high | > 10 | Avoid strenuous activities outdoors. Children and the elderly should also avoid outdoor physical exertion. | Reduce or reschedule strenuous activities outdoors, especially if you experience symptoms such as coughing and throat irritation. | |||
| aPeople with heart or breathing problems are at greater risk. | ||||||
The AQHI has been shown to predict all-cause mortality data in Canada (Stieb et al. 2008), but the AQHI has not been evaluated as a predictor of morbidity, which may be particularly important for conditions such as asthma where mortality is low. In this study, we examined associations between daily values of the AQHI and health services use for asthma, as an indication of the relationship between air quality and asthma morbidity, in the province of Ontario, Canada, from 2003 to 2006.
Methods
Data source. Our study was based in Ontario, Canada’s largest province, which has a multicultural population of > 12 million residents (more than one-third of Canada’s total population). The provincial health system is organized into 14 local health integration networks (LHINs). Ontario has a universal, single-payer health-care system that covers all physician and hospital services, and the personal health information collected for the administration of this system is available in three large databases maintained by the Institute for Clinical Evaluative Sciences (Toronto, Canada). The Ontario Health Insurance Plan Database contains information (including diagnoses) on all fee-for-service billings for physician services rendered in Ontario since 1 July 1991. The Canadian Institute for Health Information Database records the primary and secondary diagnoses for all patients discharged from acute-care hospitals. The Ontario Registered Persons Database includes information on sex, birth date, and residence postal code. We linked these databases together on an individual patient level using an encrypted version of the unique Ontario health insurance number given to all Ontario residents. Such linkage allows for protection of the identities of individual patients while examining their health services use across health administrative databases.
Study population. The Ontario Asthma Surveillance Information System (OASIS) Database [maintained by the Institute for Clinical Evaluative Sciences (Toronto, Canada)] is a validated registry of all Ontario residents with asthma and was generated by using the Ontario Health Insurance Plan and Canadian Institute for Health Information health administrative databases described above. To compile the OASIS database, patients with asthma were identified using a previously validated asthma case definition, as described in detail elsewhere and used in previous studies (Gershon et al. 2009; To et al. 2004b, 2006a, 2010). This case definition, which requires at least two physician visits for asthma within 2 consecutive years, or at least one asthma hospitalization ever, yielded 89% sensitivity and 72% specificity in children (0–17 years of age), and 84% sensitivity and 76% specificity in adults (≥ 18 years of age), compared with physician diagnosis documented in medical charts (Gershon et al. 2009; To et al. 2004b, 2006a, 2010). Patients remain in the OASIS database as part of the asthma population until they move out of the province or die, which is consistent with previous evidence indicating that asthma, once diagnosed, may remit but does not resolve (Stern et al. 2008; van Den Toorn et al. 2000). The present study included data from all patients in the OASIS database who had case-defined asthma from 1 January 2003 to 31 December 2006 (To et al. 2004a, 2006b).
This study was approved by the research ethics boards at The Hospital for Sick Children Research Institute and the Institute for Clinical Evaluative Sciences, Toronto, Canada.
Air quality measures. Hourly AQHI calculations and air pollutant measures (NO2, O3, and PM2.5) from 1 January 2003 to 31 December 2006 were obtained from the Ontario Ministry of the Environment for 22 monitoring stations across the 14 Ontario LHINs. Air pollutants were measured hourly, 24 hr/day. For LHINs with more than one monitoring station, a mean daily maximum AQHI was calculated using the maximum daily AQHI measured by the monitors within the LHIN, that is, a LHIN-specific daily maximum AQHI was calculated. All patients living within a given LHIN were assigned the same exposure. The same method of exposure assignment was used to determine exposures to the individual pollutants on which the AQHI is based. For descriptive purposes the LHINs were grouped into North, South, Central, East, and West Ontario regions.
Asthma-related outcomes. Daily counts of asthma incidence, prevalence, asthma-attributed hospital admissions, emergency department (ED) visits, and outpatient physician claims were identified from OASIS using International Classification of Diseases, 10th Revision (ICD-10; World Health Organization 1992) codes (J45, J46). Each day, new asthma cases not previously identified were included (as incidence) and added to the existing asthma cases (prevalence) from that day forward. Count data were arranged by the 14 LHINs of residence and five age groups (0–4, 5–9, 10–19, 20–59, and ≥ 60 years of age). Asthma incidence and prevalence rates were calculated per 1,000 Ontario residents, whereas rates of hospitalizations, ED visits, and outpatient visits were calculated per 1,000 residents with asthma (i.e., patients who were included in the OASIS database).
Statistical analysis. For descriptive analysis, we calculated annual mean daily maximum values of air quality measures and annual rates of asthma incidence, prevalence, and health services use for each year and for the study period as a whole (2003–2006). Poisson regression was used to estimate associations between daily AQHI values or individual pollutant measures and daily health service use, including exposures on the same day (D0) and exposures lagged 1 and 2 days (D1 and D2, respectively). All regression models included offset terms for asthma prevalence and included indicator terms to adjust for age (five groups), season, LHIN, and year. Rate ratios (RRs) from the Poisson regression models were used to estimate associations between asthma-attributed health service and a 1-unit increase in the AQHI or an incremental increase in individual air pollutants (10 ppb for NO2 and O3; 10 μg/m3 for PM2.5) (Frome 1983). All tests were performed at a 5% significance level. Associations with the individual pollutant components of the AQHI (NO2, O3, and PM2.5) were estimated using Poisson regression models that included all three pollutants. In addition, all models were stratified by age group and by season. Finally, we derived predicted average daily rates of asthma health services use for each level of AQHI with all model covariates at their mean values. Analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Air quality measures. The overall mean daily maximum AQHI was 3.66 ± 1.29, indicating low-to-moderate health risk (Table 2). The highest mean daily maximum AQHI was 3.87 in 2003, and the lowest was 3.34 in 2006. The mean daily maximum AQHI showed yearly fluctuations. Of the five regions, the Central Ontario region, which includes Toronto, had the highest mean daily maximum AQHI [3.94 ± 1.19 (average over all years of the study)], and the North region had the lowest (3.30 ± 1.17; Table 2). Daily maximum AQHI was highest in the summer (4.07 ± 1.43) and lowest in the fall (3.18 ± 1.19).
Table 2.
Mean measures of AQHI and asthma outcomes by year, age, season, and region.
| Covariate | AQHI (mean ± SD) | Annual asthma incidence and prevalence ratea | Annual asthma health services use rateb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outpatient visits | ED visits | Hospital admissions | ||||||||||
| Incidencec | Prevalenced | |||||||||||
| Year | ||||||||||||
| 2003 | 3.87 ± 1.37 | 7.11 | 122.60 | 622.90 | 41.71 | 5.41 | ||||||
| 2004 | 3.64 ± 1.18 | 6.84 | 124.80 | 577.20 | 38.95 | 5.11 | ||||||
| 2005 | 3.83 ± 1.40 | 7.03 | 128.20 | 563.80 | 39.10 | 5.38 | ||||||
| 2006 | 3.34 ± 1.12 | 6.65 | 131.20 | 524.00 | 35.22 | 4.14 | ||||||
| 2003–2006 | 3.66 ± 1.29 | 6.91 | 126.70 | 572.00 | 38.75 | 5.01 | ||||||
| Age group | ||||||||||||
| 0–4 | NA | 41.72 | 114.86 | 1759.06 | 174.96 | 42.08 | ||||||
| 5–9 | NA | 13.54 | 217.56 | 622.64 | 44.34 | 6.63 | ||||||
| 10–19 | NA | 5.67 | 224.10 | 315.00 | 23.37 | 1.67 | ||||||
| 20–59 | NA | 3.90 | 100.42 | 526.68 | 34.56 | 2.74 | ||||||
| ≥ 60 | NA | 4.88 | 110.17 | 693.54 | 27.28 | 3.84 | ||||||
| Season | ||||||||||||
| Spring (Mar–May) | 3.95 ± 1.17 | 7.20 | 126.08 | 591.08 | 40.42 | 5.20 | ||||||
| Summer (Jun–Aug) | 4.07 ± 1.43 | 5.47 | 126.88 | 486.52 | 29.71 | 3.30 | ||||||
| Fall (Sep–Nov) | 3.18 ± 1.19 | 7.59 | 127.38 | 628.57 | 45.92 | 6.67 | ||||||
| Winter (Dec–Feb) | 3.45 ± 1.14 | 7.38 | 126.51 | 581.20 | 38.80 | 4.85 | ||||||
| Region | ||||||||||||
| North | 3.30 ± 1.17 | 5.81 | 121.69 | 534.71 | 56.16 | 6.92 | ||||||
| South | 3.74 ± 1.43 | 5.70 | 113.82 | 514.78 | 37.43 | 6.10 | ||||||
| Central | 3.94 ± 1.19 | 7.91 | 130.10 | 622.76 | 29.70 | 4.59 | ||||||
| East | 3.47 ± 1.14 | 7.21 | 137.80 | 561.24 | 43.75 | 4.13 | ||||||
| West | 3.77 ± 1.14 | 5.74 | 114.45 | 529.24 | 45.73 | 5.77 | ||||||
| NA, not applicable. Data stratified by age group, season, and region are based on data averaged from 2003–2006. aPer 1,000 individuals. bPer 1,000 residents with asthma (population includes all Ontario residents in the OASIS database). cNumber of new cases identified each day not known prior to that day. dSum of current and new cases. | ||||||||||||
Health services use. The mean annual asthma incidence and prevalence rates per 1,000 Ontario residents during 2003–2006 were 6.9 and 126.7, respectively (Table 2). The annual incidence of asthma fluctuated between a low of 6.7 in 2006 and a high of 7.1/1,000 Ontario residents in 2003. The overall prevalence of asthma increased by 7.0% from 2003 to 2006 (Table 2). The annual mean rates of asthma outpatient visits, ED visits, and hospitalizations over the entire study period per 1,000 residents with asthma were 572.0, 38.8, and 5.0, respectively. All asthma health services outcomes were higher in 2003 than in 2006, although outpatient visits were the only outcome that decreased monotonically over time.
The Central Ontario region had the highest annual mean rate of outpatient visits per 1,000 residents with asthma (622.8), and the North region had the highest mean rate of ED visits (56.2) (Table 2). Annual rates for use of all three asthma health services were highest among 0–4 year olds and lowest among 10–19 year olds, and were highest in the fall and lowest in the summer.
Adjusted asthma health services RRs. Daily maximum AQHI was associated with a positive, significant increase in the use of each asthma health service evaluated during at least one lag period (Table 3). The adjusted asthma outpatient visit rate ratio was highest for AQHI on the same day (D0 RR = 1.056; 95% CI: 1.053, 1.058) indicating that a unit increase in the AQHI was associated with an estimated 5.6% increase in asthma outpatient visits. However, there was a significant negative association between asthma outpatient visits and the AQHI 2 days before the visit (D2 RR = 0.983; 95% CI: 0.981, 0.986). The asthma hospitalization rate ratio also was highest for AQHI on the same day and the previous day (both D0 and D1 RR = 1.021; 95% CI: 1.014, 1.028), suggesting a 2.1% increase in hospital admissions attributed to asthma for each unit increase in the AQHI. The RR for asthma ED visits was highest for AQHI 2 days before the visit (D2 RR = 1.013; 95% CI: 1.010, 1.017), suggesting a 1.3% increase in asthma ED visits per unit increase in AQHI.
Table 3.
RRs (95% CIs) for asthma health outcomes in association with a 1-unit increase in the AQHI.
| Lag | Outpatient visits | ED visits | Hospital admissions | |||
|---|---|---|---|---|---|---|
| D0 | ||||||
| AQHI | 1.056 | (1.053, 1.058) | 1.003 | (0.999, 1.007) | 1.021 | (1.014, 1.028) |
| NO2 | 1.117 | (1.114, 1.120) | 0.976 | (0.971, 0.980) | 1.025 | (1.017, 1.034) |
| O3 | 0.979 | (0.976, 0.981) | 1.008 | (1.004, 1.012) | 1.017 | (1.009, 1.024) |
| PM2.5 | 0.982 | (0.978, 0.985) | 1.028 | (1.022, 1.035) | 0.997 | (0.986, 1.007) |
| D1 | ||||||
| AQHI | 1.019 | (1.016, 1.021) | 1.005 | (1.001, 1.009) | 1.021 | (1.014, 1.028) |
| NO2 | 1.022 | (1.020, 1.025) | 0.976 | (0.972, 0.981) | 1.011 | (1.003, 1.018) |
| O3 | 1.018 | (1.015, 1.020) | 1.014 | (1.009, 1.018) | 1.031 | (1.023, 1.039) |
| PM2.5 | 0.990 | (0.986, 0.993) | 1.022 | (1.016, 1.028) | 1.002 | (0.991, 1.012) |
| D2 | ||||||
| AQHI | 0.983 | (0.981, 0.986) | 1.013 | (1.010, 1.017) | 1.008 | (1.001, 1.015) |
| NO2 | 0.959 | (0.956, 0.962) | 0.994 | (0.990, 0.999) | 0.991 | (0.983, 0.999) |
| O3 | 1.016 | (1.014, 1.019) | 1.010 | (1.006, 1.014) | 1.043 | (1.036, 1.051) |
| PM2.5 | 1.006 | (1.002, 1.009) | 1.017 | (1.011, 1.023) | 0.992 | (0.981, 1.002) |
Results from the multipollutant Poisson regression model adjusted for covariates are also shown in Table 3. The highest NO2-specific RR was found on D0 for asthma outpatient visits (RR = 1.117; 95% CI: 1.114, 1.120), suggesting a nearly 12% increase in outpatient claims per 10 unit increase in NO2. The highest O3-specific RR was found on D2 for hospitalizations (RR = 1.043; 95% CI: 1.036, 1.051). The highest PM2.5-specific RR was observed on D0 for ED visits (RR = 1.028; 95% CI: 1.022, 1.035).
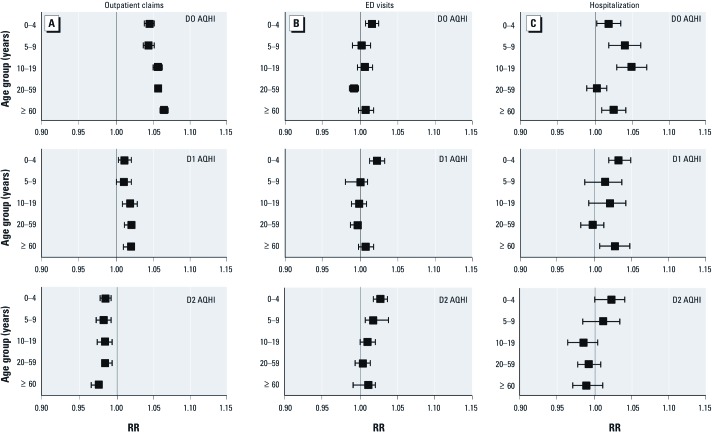
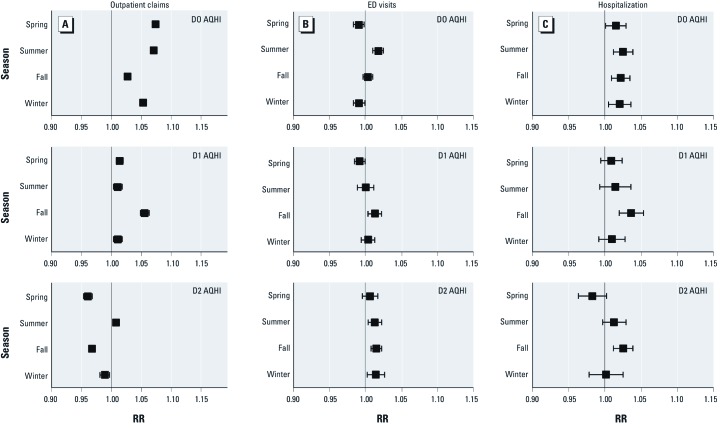
Figure 1 shows the results of the Poisson regression models stratified by age group. The youngest (0–4 years of age) and the oldest age groups (≥ 60 years of age) had the highest RRs for asthma ED visits on D2 and hospitalization on D1. The oldest age group had the highest RR for asthma outpatient claims on D0. Figure 2 shows results stratified by season. Although the RRs showed no difference in asthma ED visits or hospitalization by seasons, RRs for D0 were higher in the spring and summer for asthma outpatient claims.
Figure 1.
RRs (95% CIs) for asthma health services by AQHI and lags stratified by age group. Outpatient claims (A), ED visits (B), and hospitalization (C) for AQHI on D0 (top), D1 (center), and D2 (bottom). All health services RRs were derived from multivariable poisson regression models adjusted for season, region, and year. The AQHI-specific RRs were per unit increase in AQHI.
Figure 2.
RRs (95% CIs) for asthma health services by AQHI and lags stratified by season. Outpatient claims (A), ED visits (B), and hospitalization (C) for AQHI on D0 (top), D1 (center), and D2 (bottom). All health services RRs were derived from multivariable poisson regression models adjusted for age, region, and year. The AQHI-specific RRs were per unit increase in AQHI.
NO2 was associated with higher asthma outpatient visits and hospitalizations, particularly in the summer; O3 had the highest association with outpatient claims in the spring and summer, whereas PM2.5 had the highest associations with ED visits and outpatient claims in the winter (Table 3). [For mean air pollutant measures by year, season, and region in Ontario, see Supplemental Material, Table S1 (http://dx.doi.org/10.1289/ehp.1104816).] In general, higher associations were observed in the younger age groups.
Predicted average daily rate of asthma health services use. Predicted average daily rates of asthma health services use per unit increase in AQHI at D0 in total and by age group were calculated from the adjusted Poisson regression models. The increase in predicted daily rates of asthma health services use per unit increase in AQHI was highest in the very young and the oldest populations. Table 4 shows predicted daily rates and the expected counts of asthma health services use by AQHI values as applied to an asthma-prevalent population with average values of model covariates. About 1.5 million persons living with asthma in Ontario during the study period based on the provincial population of 12 million and asthma prevalence of 12.6%. The predicted daily rates per 1,000 residents with asthma on days when the AQHI = 3 (indicating low health risk) were 1.498 for outpatient asthma claims, 0.106 for asthma ED visits, and 0.013 for asthma hospitalizations, which we estimate would result in nearly 2,278 outpatient visits, 160 ED visits, and 20 hospital admissions attributed to asthma (Table 4). If the AQHI = 10 (high health risk), these daily expected counts would increase to 3,330, 164, and 24, representing increases of 46%, 2%, and 16% relative to counts on days when AQHI = 3, respectively. As these are daily expected counts calculated from daily rates, the absolute increase in health care burden could be large if more days in a year have higher AQHI measures.
Table 4.
Predicted daily average rates and daily counts for use of asthma health services according to AQHI levels.
| AQHI = 3 (low health risk) | AQHI = 6 (moderate health risk) | AQHI = 10 (high health risk) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asthma Morbidity measures | Predicted ratea | Expected numberb | Predicted rate | Expected number | Percent differencec | Predicted rate | Expected number | Percent difference | ||||||||
| Outpatient visits | 1.498 | 2,278 | 1.763 | 2,681 | 17.7 | 2.190 | 3,330 | 46.2 | ||||||||
| ED visits | 0.106 | 160 | 0.106 | 162 | 0.8 | 0.108 | 164 | 2.0 | ||||||||
| Hospital admissions | 0.013 | 20 | 0.014 | 22 | 6.4 | 0.016 | 24 | 15.7 | ||||||||
| aPredicted daily average rates were obtained from the adjusted Poisson regression models with age, season, region, and year held at their mean values. bExpected counts were calculated by multiplying the predicted rates to the average asthma prevalence (in the example above, we used the Ontario 1.5 million asthma prevalence population for illustration). cPercent difference compared to AQHI = 3. | ||||||||||||||||
Discussion
This study extends our understanding of the deleterious health effects of air pollutants by associating asthma morbidity directly with a simple population-based air quality health risk scale. Our results suggest that an increase in the daily maximum AQHI is associated with an increase in asthma health services use. Associations are evident on the day of exposure and for exposure 1 and 2 days before the outcome. The AQHI, as well as individual pollutants, demonstrated associations with health services use.
Our findings are supported by previous studies of individual pollutants and the multivariable AQHI scale (Table 5). According to a study of 12 Canadian cities that included data for nearly two decades, each unit increase in the AQHI was associated with a 1.2% increase in mortality (Stieb et al. 2008). A comprehensive, systematic synthesis of 109 daily time–series studies suggested that acute exposures to air pollutants such as NO2, O3, and PM10 (PM ≤ 10 µm in aerodynamic diameter; thoracic PM) contribute to all-cause mortality, with NO2 and PM10 showing stronger associations with respiratory mortality (Stieb et al. 2002). Furthermore, a study of 11 Canadian cities from 1980 to 1991 found significant associations between NO2 and O3 and non-accidental mortality (Burnett et al. 1998).
Table 5.
Summary of studies examining the association between air quality measures and asthma.
| Reference | Data collection period | Location | Study population | Sample size (n or no. studies) | Outcomes | Air quality measures | Findings | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stieb et al. 2008 | 1981–2000 | 12 Canadian cities | All ages | NA | Overall mortality | AQHI, SO2, NO2, O3, CO, PM10, PM2.5 | Each unit increase in AQHI was associated with an increase of 1.2% in mortality | ||||
| Stieb et al. 2002 | 1985–2000 | Worldwide | All ages | 109 studies | All-cause, respiratory mortality | SO2, NO2, O3, CO, PM10 | Acute air pollution exposure was significantly associated with mortality; stronger associations with respiratory mortality for all pollutants except O3 | ||||
| Burnett et al. 1998 | 1980–1991 | 11 Canadian cities | All ages | 816,991 | Mortality of nonaccidental causes | SO2, NO2, O3, CO | All pollutants were significantly associated with mortality; NO2 had the strongest association | ||||
| Weinmayr et al. 2010 | 1990–2008 | Europe, USA, other | ≤ 18 years | 36 studies | LRS, cough, PEF of children with asthma | NO2, PM10 | PM10 was significantly associated with asthma symptom episode; NO2 was significantly associated with asthma symptoms in overall analysis only considering all possible lags | ||||
| Stieb et al. 2009 | 1992–2003 | 7 Canadian cities | All ages | 83,563 (asthma); 125,145 (respiratory) | ED visits for asthma and respiratory infection | SO2, NO2, O3, CO, PM10, PM2.5 | Ozone was associated with visits for respiratory conditions; PM2.5 and PM10 were associated with asthma visits in warm season | ||||
| Lin et al. 2005 | 1998–2001 | Toronto, Canada | ≤ 14 years | 6,782 | Hospitalization for respiratory infection | SO2, NO2, O3, CO, PM10, PM2.5, PM10–2.5 | All PM fractions and NO2 were significantly associated with hospital admissions for respiratory infections | ||||
| Current study 2012 | 2003–2006 | Province of Ontario, Canada | All ages | 1.5 million (asthma) | Outpatient, ED visits | AQHI, NO2, O3, PM2.5 | AQHI was significantly associated with asthma morbidity on the current day and 1–2 days prior | ||||
| Abbreviations: LRS, lower respiratory symptoms; NA, not available; PEF, peak expiratory flow; PM10–2.5, PM, with an aerodynamic diameter between 2.5 and 10 µm, coarse PM . | |||||||||||
While AQHI has been associated with mortality, its association with morbidity outcomes has not been fully assessed. Several recent studies have reported associations between individual air pollutants and adverse health outcomes. According to a systematic review of 36 studies, PM10 and potentially NO2 were significantly associated with the occurrence of asthma symptom episodes among patients ≤ 18 years of age (Weinmayr et al. 2010). A time–series analysis based on nearly 400,000 ED visits at 14 hospitals in seven Canadian cities during the 1990s through the early 2000s concluded that daily average concentrations of O3 exhibited the most consistent associations with ED visits for respiratory conditions, and that PM10 and PM2.5 were strongly associated with visits for asthma during the warm season (Stieb et al. 2009). Furthermore, a 4-year study found associations between hospitalization for respiratory infections in children ≤ 14 years of age in Toronto and relatively low levels of ambient particulate matter and gaseous pollutants, especially PM10–2.5 (PM with an aerodynamic diameter between 2.5 and 10 µm) and NO2 (Lin et al. 2005).
Although our study is not a formal validation study of AQHI morbidity outcomes, it is the first to use a large body of population-based data to evaluate associations between AQHI and asthma-related morbidity. We used asthma as an index disease because it is very common and is the fastest-growing chronic disease in North America, and because air pollutants have been associated with asthma symptoms and exacerbations. Recent studies have suggested that other chronic diseases may also be aggravated by air pollution, including chronic obstructive pulmonary disease, heart disease (including heart attack and stroke), and diabetes (Andersen et al. 2012; Hoffmann et al. 2012; Ko and Hui 2012; Lavigne et al. 2012; Wellenius et al. 2012). Our study supports the utility of AQHI as an exposure metric for studies of the impact of ambient air pollution on health outcomes, and our approach may serve as a prototype for studies of the impact of air quality on other chronic diseases.
The use of large health administrative and environmental databases helped ensure the comprehensiveness, representativeness, and generalizability of our findings while minimizing selection bias, but there are some limitations. We used a large population-based database from Canada, potentially limiting the generalizability of our findings to other populations. The AQHI, a recent Canadian innovation, is an index of air quality that is focused on health risk and on the communication of that risk to the general public; however, at this time the AQHI is not used outside of Canada. Although our estimates were adjusted for several confounding factors, we could not account for other potential confounders such as smoking, housing conditions, indoor air quality, and ethnicity. Because all persons residing within a given region were assigned the same level of exposure without formally accounting for variations within the region, there is the potential for misclassifying exposure. In addition, health administrative data may underestimate morbidities associated with asthma and misdiagnosis was possible. However, we attempted to reduce the misclassification of outcomes by using a validated and highly specific case definition of asthma.
The multivariable analyses in our study were conducted using fixed-effect Poisson regression models that adjusted for confounders including region and year. Because our study used data from 2003 to 2006 obtained for various regions in Ontario, there may be some degree of spatial autocorrelation as well as time dependency in the data for which we have not fully accounted. Methods used by others that take into account spatial autocorrelation include complex regression approaches such as Poisson regressions with distance-based agglomeration-specific spatial random effects and Poisson regressions with neighborhood-based agglomeration-specific spatial random effects (Mohebbi et al. 2011). According to simulation results reported by Mohebbi et al. (2011), ignoring spatial autocorrelation may potentially overstate the degrees of freedom in the data and consequently underestimate standard errors. Even though this error would not affect rate ratio estimates, it is likely that we have overstated their statistical significance. Although it would be desirable to account for residual spatial correlation in analyses, it is challenging to specify the correct correlation structure and apply appropriate spatial smoothing. However, a more sophisticated temporal and spatial analysis could be considered in the future to account for potential autocorrelation and time dependency of the data.
The AQHI is designed to help persons make decisions to protect their health by limiting short-term exposure to air pollution and adjusting their activity when air pollution levels are high. In our study, rate ratios were estimated assuming constant linear associations per unit increase of AQHI. Future studies should examine specific AQHI cut points in relation to the levels of severity of health risks.
The National Illness Cost of Air Pollution (ICAP) study conducted by the Canadian Medical Association in 2008 suggested that respiratory illness associated with exposure to air pollution accounted for a significant burden to the health care system and productivity loss (Canadian Medical Association 2008). Our study suggests a statistically significant increase in asthma health services use per unit increase in AQHI also exists. The AQHI health messages providing recommendations on how to adjust outdoor activity levels in accordance with AQHI levels may play an important role in informing persons about health risks and air pollution and may contribute to reducing unnecessary health care use due to adverse health outcomes attributable to exposure to air pollution.
Conclusion
Our study was the first to use population data to study associations between asthma morbidity and the AQHI. Daily rates of asthma health services use predicted on the basis of our estimates may be useful for health care resource allocation and planning and may serve as a guide for the timing of asthma education and management interventions and air quality risk reduction campaigns.
Our findings support the use of the AQHI as a chronic disease morbidity index. As an air quality health risk advisory tool, the composite AQHI reflects the combined effects of ambient air pollutant exposures relevant to patients with asthma. Furthermore, the AQHI was developed as a communication tool that includes simple risk-reduction advice, permitting practical implementation as an asthma trigger avoidance management strategy. The AQHI may be useful for forecasting asthma morbidity associated with outdoor air pollution, and education about the AQHI may help reduce health services use by patients living with asthma.
Supplemental Material
Acknowledgments
The authors thank the Ministry of the Environment and the Institute for Clinical Evaluative Sciences (Toronto, Ontario, Canada), for providing the provincial data for this study.
Footnotes
This study was funded by the Ontario Lung Association through a grant from the Government of Ontario. T.T. is supported by the University of Toronto, Life Sciences Committee, Dales Award in Medical Research.
The opinions, results and conclusions reported in this article are those of the authors and do not necessarily represent those of the Ontario Lung Association, the government of Ontario, or the Ministry of the Environment.
The authors declare they have no actual or potential competing financial interests.
References
- Andersen ZJ, Raaschou-Nielsen O, Ketzel M, Jensen SS, Hvidberg M, Loft S, et al. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35(1):92–98. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balluz L, Wen XJ, Town M, Shire JD, Qualter J, Mokdad A. Ischemic heart disease and ambient air pollution of particulate matter 2.5 in 51 counties in the US. Public Health Rep. 2007;122(8):626–633. doi: 10.1177/003335490712200510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet J, Clark T, Hurd S, Khaltaev N, Lenfant C, O’Byrne P, et al. GINA guidelines on asthma and beyond. Allergy. 2007;62(2):102–112. doi: 10.1111/j.1398-9995.2006.01305.x. [DOI] [PubMed] [Google Scholar]
- Burnett RT, Cakmak S, Brook JR. The effect of the urban ambient air pollution mix on daily mortality rates in 11 Canadian cities. Can J Public Health. 1998;89(3):152–156. doi: 10.1007/BF03404464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Medical Association. No Breathing Room—National Illness Costs of Air Pollution. Technical Report. ICAP Model Version 3.0: Provincial Models and National Damage Estimates. Ottawa, Ontario, Canada:The Canadian Medical Association. 2008. Available: http://www.cma.ca/multimedia/CMA/Content_Images/Inside_cma/Office_Public_Health/ICAP/CMAICAPTec_e-29aug.pdf [accessed 2 November 2012]
- Carlton BG, Lucas DO, Ellis EF, Conboy-Ellis K, Shoheiber O, Stempel DA. The status of asthma control and asthma prescribing practices in the United States: results of a large prospective asthma control survey of primary care practices. J Asthma. 2005;42(7):529–535. doi: 10.1081/JAS-67000. [DOI] [PubMed] [Google Scholar]
- Chapman KR, Ernst P, Grenville A, Dewland P, Zimmerman S. Control of asthma in Canada: failure to achieve guideline targets. Can Respir J. 2001;8(suppl A):35A–40A. doi: 10.1155/2001/245261. [DOI] [PubMed] [Google Scholar]
- Environics Research Group Ltd 2005. Development of a Health-based Air Quality Index for Canada–Public Opinion Research 2004-05 H1011-040011/001CY. Ottawa, Ontario, Canada: Health Canada. [Google Scholar]
- Environment Canada. Air Quality Health Index. 2012a. Available: http://www.ec.gc.ca/cas-aqhi/default.asp?lang=En&n=CB0ADB16-1 [accessed 11 March 2011]
- Environment Canada. Air Quality Health Index Categories and Health Messages. 2012b. Available: http://www.ec.gc.ca/cas-aqhi/default.asp?lang=En&n=79A8041B-1 [accessed 11 March 2011]
- Farrar JR 2005. The global burden of asthma and current approaches to its management. Eur Pharmacother 126:998; Available: http://www.touchbriefings.com/pdf/1134/Farrar.pdf [accessed 5 November 2012].
- FitzGerald JM, Boulet LP, McIvor RA, Zimmerman S, Chapman KR. Asthma control in Canada remains suboptimal: The Reality of Asthma Control (TRAC) study. Can Respir J. 2006;13(5):253–259. doi: 10.1155/2006/753083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frome EL. The analysis of rates using Poisson regression methods. Biometrics. 1983;39:665–674. [PubMed] [Google Scholar]
- Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying patients with physician-diagnosed asthma in health administrative databases. Can Respir J. 2009;16(6):183–188. doi: 10.1155/2009/963098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland FD. Outdoor air pollution, genetic susceptibility, and asthma management: opportunities for intervention to reduce the burden of asthma. Pediatrics. 2009;123(suppl 3):S168–S173. doi: 10.1542/peds.2008-2233G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Luttmann-Gibson H, Cohen A, Zanobetti A, de Souza C, Foley CS, et al. Opposing effects of particle pollution, ozone, and ambient temperature on arterial blood pressure. Environ Health Perspect. 2012;120:241–246. doi: 10.1289/ehp.1103647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko FW, Hui DS. Air Pollution and COPD. Respirology. 2012;17(3):395–401. doi: 10.1111/j.1440-1843.2011.02112.x. [DOI] [PubMed] [Google Scholar]
- Lai CK, De Guia TS, Kim YY, Kuo SH, Mukhopadhyay A, Soriano JB, et al. Asthma control in the Asia-Pacific region: the Asthma Insights and Reality in Asia-Pacific study. J Allergy Clin Immunol. 2003;111(2):263–268. doi: 10.1067/mai.2003.30. [DOI] [PubMed] [Google Scholar]
- Lavigne E, Villeneuve PJ, Cakmak S. Air pollution and emergency department visits for asthma in Windsor, Canada. Can J Public Health. 2012;103(1):4–8. doi: 10.1007/BF03404060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Stieb DM, Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics. 2005;116(2):e235–e240. doi: 10.1542/peds.2004-2012. [DOI] [PubMed] [Google Scholar]
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- Mohebbi M, Wolfe R, Jolley D.2011A Poisson regression approach for modelling spatial autocorrelation between geographically referenced observations. BMC Med Res Methodol 11133; doi: 10.1186/1471-2288-11-133[Online 3 October 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontario Ministry of the Environment. Current Air Quality Index Readings for Ontario. 2012. Available: http://www.airqualityontario.com/reports/summary.php [accessed 22 December 2011]
- Rabe KF, Adachi M, Lai CK, Soriano JB, Vermeire PA, Weiss KB, et al. Worldwide severity and control of asthma in children and adults: the global asthma insight and reality surveys. J Allergy Clin Immunol. 2004;114(1):40–47. doi: 10.1016/j.jaci.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Sekerel BE, Gemicioglu B, Soriano JB. Asthma insights and reality in Turkey (AIRET) study. Respir Med. 2006;100(10):1850–1854. doi: 10.1016/j.rmed.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Shenfeld L, Yap D 1989. Ontario’s New Air Quality Index– Design and Operating Experience. Toronto, Ontario, Canada:Ontario Ministry of the Environment. [Google Scholar]
- Stern DA, Morgan WJ, Halonen M, Wright AL, Martinez FD. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet. 2008;372(9643):1058–1064. doi: 10.1016/S0140-6736(08)61447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Burnett RT, Smith-Doiron M, Brion O, Hyun Shin H, Economou V. A new multipollutant, no-threshold air quality health index based on short-term associations observed in daily time-series analyses. J Air Waste Manag Assoc. 2008;58:435–450. doi: 10.3155/1047-3289.58.3.435. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Judek S, Burnett RT. Meta-analysis of time-series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc. 2002;52:470–484. doi: 10.1080/10473289.2002.10470794. [DOI] [PubMed] [Google Scholar]
- Stieb DM, Szyszkowicz M, Rowe BH, Leech JA.2009Air pollution and emergency department visits for cardiac and respiratory conditions: a multi-city time-series analysis. Environ Health 825: doi: [Online 10 June 2009] 10.1186/1476-069X-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To T, Dell S, Dick PT, Cicutto L, Harris JK, MacLusky IB, et al. Case verification of children with asthma in Ontario. Pediatr Allergy Immunol. 2006a;17(1):69–76. doi: 10.1111/j.1399-3038.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- To T, Dell S, Dick P, Cicutto L, Harris J, Tassoudji M, et al. Burden of Childhood Asthma: ICES Investigative Report. Toronto:Institute for Clinical Evaluative Sciences. 2004a. Available: http://www.ices.on.ca/file/ACF77.pdf [accessed 2 November 2012]
- To T, Dell S, Dick P, Cicutto L, MacLusky I, Tassoudji M, et al. Defining asthma in children for surveillance. Am J Respir Crit Care Med. 2004b;169(7):A383. [Google Scholar]
- To T, Gershon A, Tassoudji M, Guan J, Wang C, Estrabillo E, et al. The Burden of Asthma in Ontario: ICES Investigative Report. Toronto, Ontario, Canada:Institute for Clinical Evaluative Sciences. 2006b. Available: http://www.ices.on.ca/file/Burden_of_Asthma_Aug-07.pdf [accessed 2 November 2012]
- To T, Wang C, Guan J, McLimont S, Gershon AS. What is the lifetime risk of physician-diagnosed asthma in Ontario, Canada? Am J Respir Crit Care Med. 2010;181(4):337–343. doi: 10.1164/rccm.200907-1035OC. [DOI] [PubMed] [Google Scholar]
- van Den Toorn LM, Prins JB, Overbeek SE, Hoogsteden HC, de Jongste JC. Adolescents in clinical remission of atopic asthma have elevated exhaled nitric oxide levels and bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000;162(3 Pt 1):953–957. doi: 10.1164/ajrccm.162.3.9909033. [DOI] [PubMed] [Google Scholar]
- Weinmayr G, Romeo E, De Sario M, Weiland SK, Forastiere F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: a systematic review and meta-analysis. Environ Health Perspect. 2010;118:449–457. doi: 10.1289/ehp.0900844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Burger MR, Coull BA, Schwartz J, Suh HH, Koutrakis P, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172(3):229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Geneva: WHO; 1992. International Statistical Classification of Diseases and Related Health Problems. Tenth Revision. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.