Abstract
Safranines hold great promise as artificial flavin-like electron transfer cofactors with tunable properties. We report the design and chemical synthesis of the p-methoxy derivative of safranine O using a new synthetic route based on the Ulmann condensation. Spectroelectrochemical comparison of the purified parent safranine and this derivative demonstrates that the modification increases its two-electron reduction potential by 125 mV, or 5.75 kcal/mol. This modification also causes redshifts in the absorbance and fluorescence spectra of the cofactor, suggesting that it may find future utility in arrayed sensor applications.
Keywords: Safranine, Enzyme design, Reduction potential, Ulmann condensation
The combination of natural and designed proteins with artificial cofactors is a rapidly expanding focus of modern enzyme design efforts [1–4].The anticipated benefit of this combination is that a cofactor energetically and structurally optimized to perform the catalytic task at hand will aid the enzyme design or re-engineering effort by removing the necessity for the enzyme to “tune” the cofactor reactivity for the desired task[5–7]. The properties to be optimized may include reduction potentials, substrate or ligand affinity, hydrophobicity, chemical reactivity, or photophysical properties[8].
Progress to date has primarily been in the area of metalloproteins, especially the replacement of heme residues with synthetic porphyrins[9–12]. There are few reports of artificial proteins which incorporate artificial organic cofactors [13, 14]. Despite the fact that flavoenzymes form more than a tenth of known cofactor-containing enzymes [15], there have been no reports thus far of artificial flavin enzymes which utilize artificial flavin-like cofactors, and likewise no reports of the synthesis of flavin-like cofactors created for use in such a manner. We have reported the synthesis and characterization of riboflavin derivatives with differing hydrophobicity [16, 17], but these lack desirable properties such as changes in reduction potential.
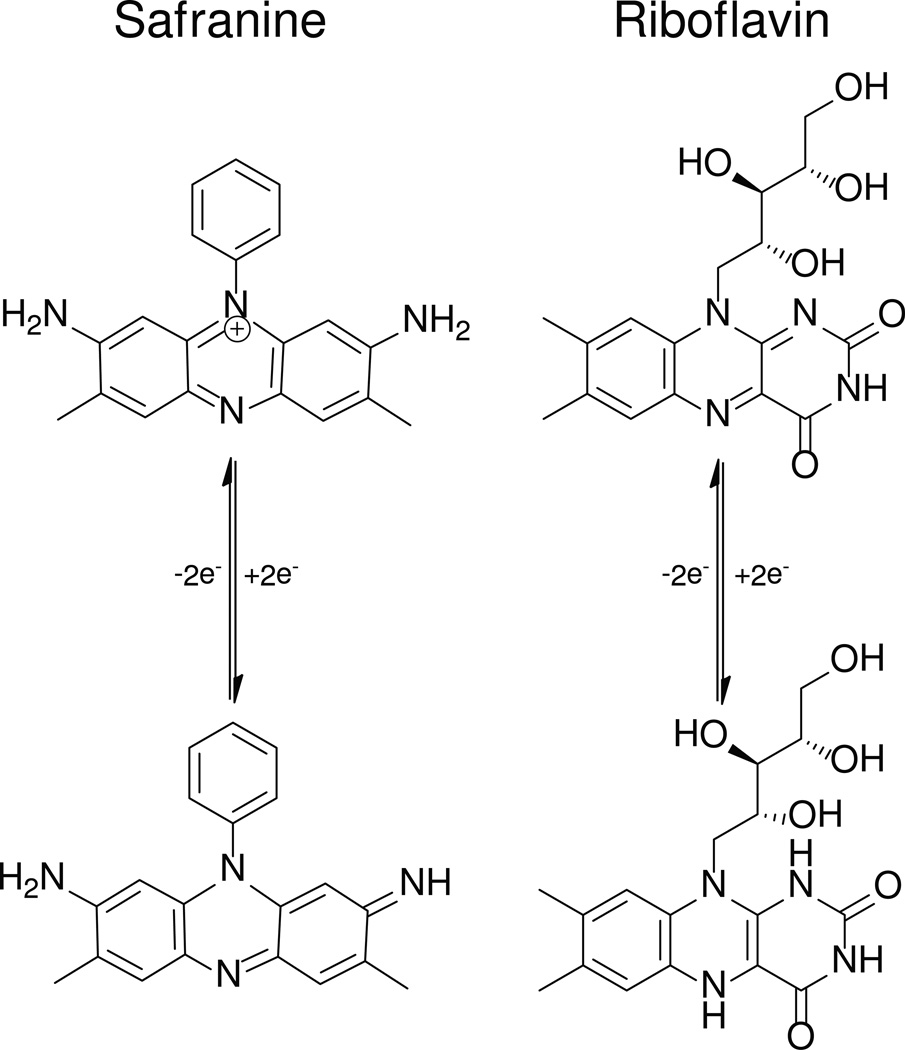
Reduction midpoint potentials, both one- and two-electron, play a large role in determining the chemical reactivity of flavin cofactors [18–21], and flavoproteins observed in nature have been shown to modulate the reduction potentials of their bound cofactors by more than half a Volt [15]. For this reason, we have set out to create a series of flavin-like cofactors in which small changes in the cofactor structure engender large changes in its reduction potentials. Safranines are ideal candidates for this as the phenazine moiety is similar in size and shape to that of isoalloxazine (see Figure 1.) The reported two-electron reduction midpoint potential of safranine O, −290 mV vs SHE [22], is 100 mV, or 2.3 kcal/mol more negative than that of riboflavin [23]. Furthermore the N(10) phenyl substituent of Safranine O is conjugated to the phenazine, raising the possibility that the reduction potential of the molecule may be simply altered by modifications of this ring.
Figure 1.
Oxidized and two electron-reduced forms of Safranine O and Riboflavin.
The safraninemauveine was the first synthetic dye, created by Perkin in 1856 [1]. It was synthesized by the oxidation of crude aniline, itself derived from the nitration and reduction of a benzene-toluene mixture, and purified in a 5% yield from a mixture of products containing a variety of oligoanilines[24]. Soon after dozens of safranine derivatives were created using similarly uncontrolled oxidative condensations of various arylamines and diamines[2, 25]. Modern industrial safranine dyes are still synthesized in this manner [24].
In this report we describe our initial effort to create a general synthetic route towards safranine O analogues modified at the para position of the N(10) phenyl ring using a stepwise synthesis in which the phenyl substituent is incorporated as aniline. Given the large number of commercially available aniline derivatives, we hoped such a synthetic route may lead to a similarly large scope of safranine products. Furthermore, given our recent demonstration that the chemical shift tensor of the isoalloxazine N(5) nitrogen in flavin compounds is very informative as to the chemical reactivity imparted upon flavins by their environments[1, 26], we desired to created a route which enables the ready and inexpensive incorporation of isotopically labeled nitrogen at the equivalent N(5) position in the phenazine ring of these analogues.
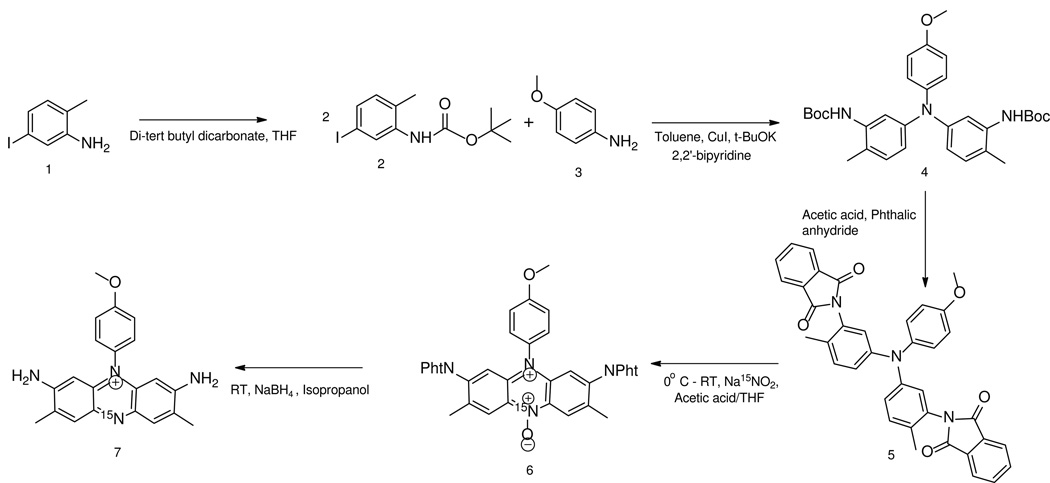
Our approach is outlined in Scheme 1 (synthetic details, including product characterization, are included in the supplementary material). 5-iodo-2-methylaniline 1 is quantitatively protected with tert-butyldicarbonate[2] and then condensed with p-anisidine 3 using copper iodide, 2,2’-bipyridine and three equivalents of potassium tert-butoxide in dry toluene forming triarylamine 4 25]. Initial attempts using 1,10-phenanthroline as a copper ligand and potassium hydroxide as a base resulted in low yields, so the rigid phenanthroline ligand was replaced with the more flexible bipyridine ligand [5] and the stronger, more hindered, tert-butoxide base was used to replace potassium hydroxide[3].
Scheme 1.
The next step is nitrosative cyclization [27]. Direct cyclization of 4 proved unsuccessful as the acidic conditions required deprotected the amines, formingazo compounds from the free amines and the nitrite. We thus replaced the Boc protecting groups with acid-stable phthalate protecting groups in a single step reaction [7] forming 5 in an 86% isolated yield. 5 is cyclized with 15N-sodium nitrite in a 17/3 mixture of acetic acid/tetrahydrofuran at 0°C forming 6 in a 55% yield. Reductive deprotection of 6 results in the purple final product 7 in a 40% isolated yield. The low yield in the final two steps is compensated for in part by the fact that the isotopic label is introduced in the second to last step. This greatly reduces label loss in comparison to other possible approaches in which either the label is introduced at the beginning, which would entail a geometrically growing loss of expensive labeled material, or employing the extremely low yield uncontrolled oxidative condensation methods currently in use.
Similar syntheses with other aniline starting materials demonstrate that this reaction pathway is limited in scope: anilines with methyl, cyano or hydrogen groups at the position para to the amine had reduced yields of 52, 24 and 38% respectively in the condensation step, and all had negligible yields (<5%)of cyclization. The latter is likely due to the fact that these are not as activating as the methoxy for electrophilic substitution at the position meta to the amine.
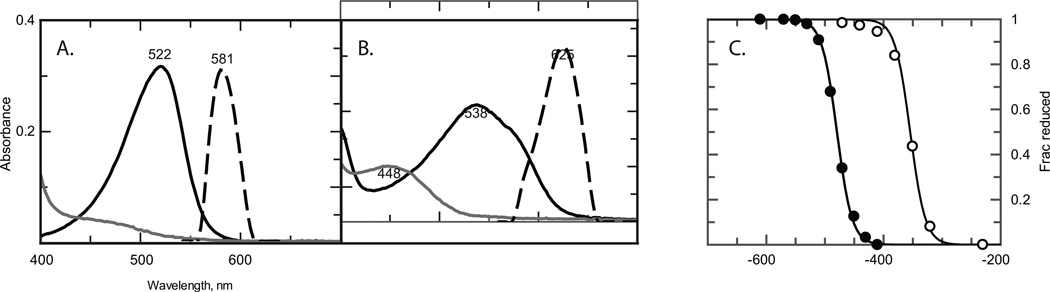
7 was compared to a commercial sample of safranine O, the latter of which was purified using both normal- and reversed-phase chromatography in succession[9]. Safranines were dissolved in 0.2M sodium phosphate, 0.2M NaCl, pH 7.0. Solutions were degassed using several cycles of applied vacuum followed by flushing with argon. Oxidized spectra were collected and then the safranines were reduced using sodium hydrosulfite. The oxidized and two-electron reduced spectra of 7 are both significantly red-shifted in comparison to the base compound safranine O, and the fluorescence emission maximum is likewise shifted by 44 nm (See Figure 2 parts A and B). Neither compound is fluorescent in the reduced state. These spectral differences are advantageous, as this means they can be separately monitored in mixed solutions of the two species, or in mixed complexes of the two cofactorss with one or more proteins, as has been proposed for biochemical sensor arrays[21].
Figure 2.
Comparative characterization of safranine O and p-methoxysafranine 7. Absorbance spectra of oxidized (black lines), reduced (grey lines) and fluorescence emission (dotted lines) spectra of oxidized safranine O (panel A) and p-methoxysafranine 7 (panel B). Emission spectra were produced by exciting at 522 and 538 nm, respectively. (C) Potentiometric comparison of safranine O (filled circles) and p-methoxysafranine 7 (open circles) in 0.2M sodium phosphate, 0.2M NaCl, pH 7.0 buffer. Lines are fits with the Nernst equation with n = 2.0 electrons. Potentials are referenced to aAg/AgCl2 electrode.
We performed spectroelectrochemical analysis of the two proteins using an apparatus described previously [13]. Briefly, the cell is a 10mm quartz cuvette that contains a pair of gold slides that serve as the working electrode, a platinum wire auxiliary electrode, and a Ag-AgCl reference microelectrode (Microelectrodes Inc). The gold working electrode was coated with 1-mercaptohexanol by soaking the slides in a 1-proponal solution containing 1-mercaptohexanol 1% (v/v) for 20 hours. The applied potential is set by using a PWR-3 Power Module potentiostat (Bioanalytical Systems Inc.). Spectra were collected in a PerkinElmer Lambda 35 UV/Vis spectrometer. Safranines were dissolved in water containing 0.2M sodium phosphate, 0.2M NaCl, pH 7.0. The applied potentials are referenced against Ag/AgCl2 which is +210 mV (NHE). Solutions were equilibrated at each potential for at least ten minutes before spectra were collected. Absorbance values at the oxidized maximum were used to calculate the fractional oxidation at each potential and these data were fit with the Nernst equation.
Figure 2C depicts the data obtained for the two compounds in pH 7.0 buffer solution. p-Methoxysafranine 7 has a reduction potential 125 mV higher than that of safranine O. Each displays two-state behavior, directly transforming from the oxidized state to the two-electron reduced state during the titration. No semiquinone spectral intermediates are observed. The two-electron nature of these reductions are further evinced both by the presence of isosbestic wave lengths in each titration and by the fact that the fits to the titration data each report a 2.0 ± 0.1 electron reduction. Thus a protein containing 7 as a cofactor will have 125 mV, or 5.75 kcal/mol, more driving force for two-electron oxidative reactions such as the oxidation of nicotinamide cofactors such as NADH and NADPH.
In conclusion, we have developed a synthetic route to a flavin-like cofactor, a safranine O analogue which incorporates a single substitution at a point far removed from the phenazine head group at which reduction occurs. This route enables the inexpensive incorporation of an isotopic reporter atom at the reduction site. This analogue displays large differences in both reduction potentials and photophysical properties from its parent compound. We are currently exploring in more detail the pH-dependent oxidation-reduction potentials of both one- and two-electron reduction of both molecules in solution, and investigating their properties when complexed with artificial proteins designed to bind and activate them.
Supplementary Material
Acknowledgments
RLK gratefully acknowledges support by the following grants: S06GM008168 from the National Institutes of Health, infrastructure support from P41 GM-66354 to the New York Structural Biology Center and the NIH National Center for Research Resources to CCNY (NIH 5G12 RR03060).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Cui DT, et al. (15)N Solid-State NMR as a Probe of Flavin H-Bonding. Journal Of Physical Chemistry B. 2011;115(24):7788–7798. doi: 10.1021/jp202138d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muchowski JM, Venuti MC. Ortho functionalization of N-(tert-butoxycarbonyl)aniline. Journal of Organic Chemistry. 1980;45(23):4798–4801. [Google Scholar]
- 3.Kelkar AA, Patil NM, Chaudhari RV. Copper-catalyzed amination of aryl halides: single-step synthesis of triarylamines. Tetrahedron Letters. 2002;43(40):7143–7146. [Google Scholar]
- 4.Nanda V, Koder RL. Designing artificial enzymes by intuition and computation. Nature Chemistry. 2010;2(1):15–24. doi: 10.1038/nchem.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manifar T, et al. Copper(I)-medliated ligand-accelerated Ullmann-type coupling of anilines with aryliodides: Ligand selection and reaction kinetics for synthesis of tri-p-tolylamine. Industrial & Engineering Chemistry Research. 2005;44(4):789–798. [Google Scholar]
- 6.Koder RL, Dutton PL. Intelligent design: the de novo engineering of proteins with specified functions. Dalton Transactions. 2006;25:3045–3051. doi: 10.1039/b514972j. [DOI] [PubMed] [Google Scholar]
- 7.Osby JO, Martin MG, Ganem B. An exceptionally mild deprotection of phthalimides. Tetrahedron Letters. 1984;25(20):2093–2096. [Google Scholar]
- 8.Lu Y. Design and engineering of metalloproteins containing unnatural amino acids or non-native metal-containing cofactors. Current Opinion In Chemical Biology. 2005;9(2):118–126. doi: 10.1016/j.cbpa.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Meth-Cohn O, Smith M. What did W. H. Perkin actually make when he oxidized aniline to obtain mauveine? Journal of the Chemical Society-Perkin Transactions. 1994;1(1):5–7. [Google Scholar]
- 10.Fry HC, et al. Computational Design and Elaboration of a de Novo Heterotetrameric alpha-Helical Protein That Selectively Binds an Emissive Abiological (Porphinato)zinc Chromophore. Journal of the American Chemical Society. 2010;132(11):3997–4005. doi: 10.1021/ja907407m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bender GM, et al. De novo design of a single-chain diphenylporphyrin metalloprotein. Journal Of The American Chemical Society. 2007;129(35):10732–10740. doi: 10.1021/ja071199j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhuang JY, et al. Evaluating the roles of the heme a side chains in cytochrome c oxidase using designed heme proteins. Biochemistry. 2006;45(41):12530–12538. doi: 10.1021/bi060565t. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein BR, et al. Reversible proton coupled electron transfer in a peptide-incorporated naphthoquinone amino acid. Chemical Communications. 2009;(2):168–170. doi: 10.1039/b815915g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp RE, et al. Design, synthesis, and characterization of a photoactivatable flavocytochrome molecular maquette. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(18):10465–10470. doi: 10.1073/pnas.95.18.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massey V. The chemical and biological versatility of riboflavin. Biochemical Society Transactions. 2000;28:283–296. [PubMed] [Google Scholar]
- 16.Koder RL, et al. A flavin analogue with improved solubility in organic solvents. Tetrahedron Letters. 2007;48(31):5517–5520. doi: 10.1016/j.tetlet.2007.05.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerda JF, et al. Hydrogen bond-free flavin redox properties: managing flavins in extreme aprotic solvents. Organic & Biomolecular Chemistry. 2008;6:2204–2212. doi: 10.1039/b801952e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schopfer LM, Ludwig ML, Massey V. A working proposal for the role of the apoprotein in determining the redox potential of the flavin in flavoproteins: correlations between potentials and flavin pKs. In: Curti B, Ronchi S, Zanetti G, editors. Flavins and Flavoproteins. New York: Walter de Gruyter; 1990. [Google Scholar]
- 19.Koder RL, et al. Flavin thermodynamics explain the oxygen insensitivity of enteric nitroreductases. Biochemistry. 2002;41(48):14197–14205. doi: 10.1021/bi025805t. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, et al. Manipulating cofactor binding thremodynamics in an artificial oxygen transport protein. 2011 doi: 10.1021/bi201242a. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iordanov VP, et al. Integrated sensor arrays for bioluminescence and fluorescence biochemical analysis. Proceedings of IEEE Sensors 2004. 2004;2:810–813. [Google Scholar]
- 22.Clarke WM. Oxidation Reduction Potentials of Organic Systems. Baltimore: The Williams and Wilkins Co; 1960. [Google Scholar]
- 23.Draper RD, Ingraham LL. A potentiometric study of the flavin semiquinone equilibrium. Archives of Biochemistry and Biophysics. 1968;125:802–808. doi: 10.1016/0003-9861(68)90517-1. [DOI] [PubMed] [Google Scholar]
- 24.Heichert C, Hartmann H. On the Formation of Mauvein: Mechanistic Considerations and Preparative Results. Zeitschrift Fur Naturforschung Section B-a Journal of Chemical Sciences. 2009;64(6):747–755. [Google Scholar]
- 25.Goodbrand HB, Hu NX. Ligand-accelerated catalysis of the Ullmann condensation: Application to hole conducting triarylamines. Journal of Organic Chemistry. 1999;64(2):670–674. [Google Scholar]
- 26.Koder RL, et al. 15N Solid-state NMR provides a sensitive probe of oxidized flavin reactive sites. Journal of the American Chemical Society. 2006;128:15200–15208. doi: 10.1021/ja0648817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoneda F, et al. Syntheses of Isoalloxazines and Isoalloxazine 5-Oxides - New Synthesis of Riboflavin. Journal of the American Chemical Society. 1976;98(3):830–835. doi: 10.1021/ja00419a034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.