Abstract
Objectives
In ancient yoga texts there are two meditative states described. One is dharana, which requires focusing, the second is dhyana, during which there is no focusing, but an expansive mental state is reached. While an earlier study did show improved performance in an attention task after dharana, the autonomic changes during these two states have not been studied.
Methods
Autonomic and respiratory variables were assessed in 30 healthy male volunteers (group mean age±SD, 29.1±5.1 years) during four mental states described in traditional yoga texts. These four mental states are random thinking (cancalata), nonmeditative focusing (ekagrata), meditative focusing (dharana), and effortless meditation (dhyana). Assessments were made before (5 minutes), during (20 minutes), and after (5 minutes), each of the four states, on four separate days.
Results
During dhyana there was a significant increase in the skin resistance level (p<0.001; post hoc analysis following ANOVA, during compared to pre) and photo-plethysmogram amplitude (p<0.05), whereas there was a significant decrease in the heart rate (p<0.001) and breath rate (p<0.001). There was a significant decrease in the low frequency (LF) power (p<0.001) and increase in the high frequency (HF) power (p<0.001) in the frequency domain analysis of the heart rate variability (HRV) spectrum, on which HF power is associated with parasympathetic activity. There was also a significant increase in the NN50 count (the number of interval differences of successive NN intervals greater than 50 ms; p<0.001) and the pNN50 (the proportion derived by dividing NN50 by the total number of NN intervals; p<0.001) in time domain analysis of HRV, both indicative of parasympathetic activity.
Conclusions
Maximum changes were seen in autonomic variables and breath rate during the state of effortless meditation (dhyana). The changes were all suggestive of reduced sympathetic activity and/or increased vagal modulation. During dharana there was an increase in skin resistance. The changes in HRV during ekagrata and cancalata were inconclusive.
Introduction
Meditation is recognized as a specific consciousness state in which deep relaxation and increased internalized attention co-exist.1
There are widely differing methods, involved in different meditations, though the practices are intended to have a common end result (viz., a calm, yet alert mind). This is supported by research from the late 1960s, since when there have been investigations on the effects of meditation in experienced as well as inexperienced meditators.2,3 In certain cases meditators practicing the same technique showed opposite trends of results, particularly for recordings of the electroencephalogram (EEG) and autonomic variables. Some studies showed that meditation practice is associated with reduced sympathetic activity, whereas other studies reported increased sympathetic activity. For three meditation techniques in particular, the results appeared suggestive of both increased arousal (in some cases) and reduced arousal (in others). These are Transcendental Meditation (TM), Zazen meditation, and Ananda Marga meditation. These are described in detail in following text.
In a previous study practitioners of TM showed a decrease in oxygen consumption, reduced heart and breath rates, lower blood lactate levels, and an increase in slow alpha and occasional theta in the EEG after 20 minutes of practice, suggestive of a quietening effect.2 In fact most of the studies on TM reported changes suggestive of increased autonomic stability and sympathetic withdrawal.4 In addition, Dillbeck and Orme-Johnson,5 carried out a meta-analysis of 31 studies evaluating the effect of meditation on reducing somatic arousal. The studies showed reduced somatic arousal with some physiological changes suggestive of increased alertness. The findings of increased alertness was supported by a study by Lang et al.6 In this study meditators who had 2 to 3 years of experience practicing TM had lower 24-hour urinary catecholamines compared to meditators with an average experience of 4.1 years. The findings contradict the idea that meditation is simply a state of reduced sympathetic activity but supports the idea of it being a “calm yet alert” state.
Similar findings (increased as well as decreased arousal) were also reported for the eyes open, Zazen meditation. In 1960, Hirai reported an increase in heart rate during Zazen meditation,7 whereas Sugi and Akatsu8 found a decrease in oxygen consumption in Zazen meditators. Hence the first report was suggestive of activation while the second report was suggestive of relaxation.
Similarly, two reports were also found for Ananda Marga meditation, which involves intense concentration. In one report during the meditation, the expert meditators showed an increase in skin conductance and absence of a deceleratory heart rate orienting response.9 These findings challenged a relaxation model for Ananda Marga meditation, which showed an increase in galvanic skin resistance, a decrease in breath rate, and a more stable EEG in another study.10
Hence, these early studies on different meditation techniques did not support a single model of meditation as either activating or relaxing. Findings like these gave rise to meditation being described as a state of “alertful rest,” a description first used by researchers studying TM, and later used for other meditation techniques as well.
Relatively recently there was a report which described three broad categories of meditation techniques and their EEG patterns.11 The three categories were (1) focused attention, which involves voluntary and sustained attention on the chosen object, (2) open monitoring meditation in which there is nonreactive monitoring of the moment-to-moment content of experience, and (3) automatic self-transcending, which includes techniques intended to transcend their own activity. Overall the report suggests that there exist differences in objective assessments in meditation techniques which differ in their methods and principles.
The concept of meditation described in ancient yoga texts fits in with the categories of meditation experience mentioned above. In Patanjali's Yoga Sutras (circa 900 bc), there are two meditative states described, one leading to the other.12 The first stage is dharana (or focusing with effort), confining the mind within a limited mental area (“desha-bandhashchittasya dharana”; Patanjali's Yoga Sutras, Chapter III, Verse 1).12a The next stage is dhyana or effortless expansion (“tatra pratyayaikatanata dhyanam”; Patanjali's Yoga Sutras, Chapter III, Verse 2).12b This state is characterized by the uninterrupted flow of the mind towards the object chosen for meditation. The practice of dharana is supposed to precede dhyana.
Dharana and dhyana may be considered as the last two of four stages which form a continuum in the process and practice of meditation. The first two stages are described in another ancient text (the Bhagavad Gita, compiled circa 500 bc). The first stage is cancalata, which is a stage of random thinking.13a The second stage is ekagrata, during which the attention is directed to a series of associated thoughts.13b If a person chooses to think thoughts related to meditation, the person would then be able to progress to the next two stages, dharana and dhyana.
The performance in a cancellation task was compared in 70 normal healthy male volunteers at the beginning and end of the four types of sessions (viz., cancalata, ekagrata, dharana, and dhyana).14 The performance in this task improved significantly after dharana (which can be considered a state of meditative focusing) and was worse after cancalata (or random thinking), suggesting better attention after dharana.
There has been no study comparing the four mental states using autonomic and respiratory variables. Hence, the present study was planned to assess the changes in autonomic and respiratory variables in normal healthy volunteers before, during, and after the four types of sessions (cancalata, ekagrata, dharana, dhyana) on separate days. These mental states are descriptions from the ancient yoga texts and studying them was hoped to increase the understanding about meditation including differences seen in earlier studies.
Materials and Methods
Participants
There were 30 male volunteers with ages ranging from 20 to 45 years (group mean age±SD, 29.1±5.1 years) who were residing at a yoga center in south India. All of them had normal health based on a routine case history and clinical examination. An electrocardiogram (EKG) recording showed that none of them had extra systoles or any abnormality in the EKG. They were not on any medication or using any other wellness strategy. The other predetermined conditions to exclude participants from the trial were any chronic illness, particularly psychiatric or neurological disorders. Male volunteers alone were selected as autonomic and respiratory variables are known to vary with the phases of the menstrual cycle.15 All the meditators had been practicing meditation on the Sanskrit syllable Om for 30 minutes each day, 4 days a week. They had a minimum of 6 months of experience in meditation on the syllable Om (group average experience±SD, 20.95±14.21 months). Apart from their prior experience of meditation on Om, they were given a 3-month orientation program under the guidance of an experienced meditation teacher.
All participants expressed their willingness to take part in the experiment. The study was approved by the institution's ethics committee. The study protocol was explained to the subjects, and their signed consent was obtained.
Design of the study
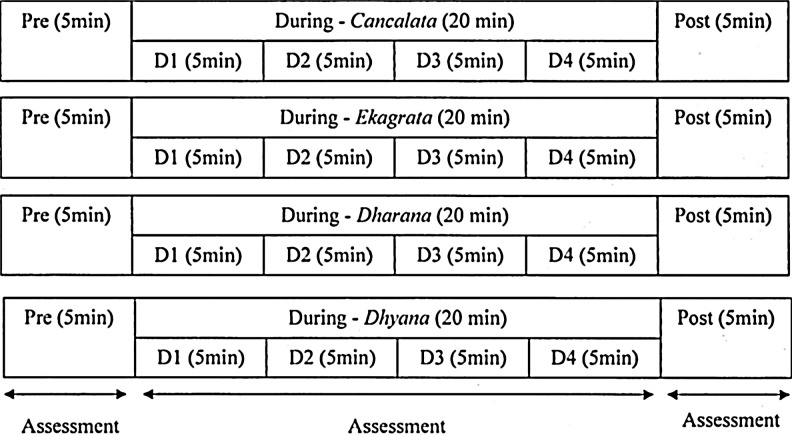
Each participant was assessed in four sessions. Two of them were meditation sessions (dharana [meditative focusing] and dhyana [meditative defocusing or effortless meditation]) and two of them were control sessions (ekagrata [nonmeditative focused thinking] and cancalata [random thinking]). All four sessions consisted of three states: pre (5 minutes), during (20 minutes), and post (5 minutes). Assessments were made on four different days, which were not necessarily on consecutive days, but at the same time of the day. The allocation of participants to the four sessions was random using a standard random number table. This was done so as to prevent the influence of being exposed to the laboratory for the first time, from influencing the results. In the cancalata session participants were asked to allow their thoughts to wander freely. This was facilitated as they were listening to a compiled audio CD consisting of brief periods of conversation on multiple subjects recorded from a local radio station. In the ekagrata session, participants were asked to focus on a single topic (i.e., listening to a lecture on meditation). In the dharana session participants were asked to focus on the Sanskrit syllable Om, whereas in the dhyana session participants moved effortlessly from thinking about Om, to quiet absorption in the single thought of Om (i.e., dhyana). Instructions for dharana and dhyana were played from compiled audio CD. The duration of all the four sessions was 20 minutes. The study design is schematically presented in Figure 1.
FIG. 1.
Schematic representation of the study.
Assessments
Autonomic variables and respiration were assessed in the four sessions using a four-channel polygraph (Polyrite D, Recorders and Medicare Systems, Chandigarh, India).
Respiration
Respiration was recorded using a volumetric pressure transducer fixed around the trunk about 8 cm below the lower costal margin as the participants sat erect.
Heart rate and heart rate variability
The EKG was recorded using a standard bipolar limb lead II configuration and an AC amplifier with 100-Hz high cut filter and 1.5-Hz low cut filter settings. The EKG was digitized using a 12-bit analog-to-digital converter (ADC) at a sampling rate of 1024 Hz and was analyzed off-line to obtain the heart rate variability (HRV) spectrum.
Photo-plethysmogram amplitude
The photo-electric transducer was placed on the volar surface of the distal phalanx of the left thumb with the light emitting diode facing the volar surface. The digit pulse volume was recorded and presented as microvolts. The amplitude of the pulse wave was used to record digit pulse volume which was presented as microvolts.
Skin resistance
Skin resistance was recorded using Ag/AgCl electrodes with electrode gel placed in contact with the volar surfaces of the distal phalanges of the index and middle fingers of the left hand. A low level DC preamplifier was used and a constant current of 10 μA was passed between the electrodes.
Interventions
Throughout all sessions participants sat cross legged and kept their eyes closed following prerecorded instructions. An emphasis was placed on carrying out the practices slowly, with awareness of physical and mental sensations, and relaxation. Participants were given a 3-month meditation orientation program under the guidance of an experienced meditation teacher. The purpose of this orientation was for all participants to practice the two different states of meditation, viz., dharana and dhyana based on specific instructions. The evaluation of the participants' practice of dharana and dhyana was based on their self-report as well as consultations with the meditation teacher. A brief description of each session is given in the following sections.
Random thinking (cancalata)
Participants were asked to allow their thoughts to wander freely as they listened to a compiled audio CD consisting of brief periods of conversation, announcements, advertisements, and talks on multiple topic recorded from a local radio station transmission. All these conversations were unconnected and were believed to induce a state of random thinking.
Nonmeditative focused thinking (ekagrata)
Participants listened to a prerecorded lecture on meditation. This was not about meditation, on the Sanskrit syllable Om, but about meditation, in general. It was speculated that listening to a lecture on a particular topics could induce the state of nonmeditative focused thinking.
Meditative focusing (dharana)
Participants were asked to follow the audio instructions for the practice of dharana. The meditative focusing on the Sanskrit syllable Om consisted of mental visualization of the symbol Om. Dharana involves conscious effort to keep the thoughts restricted to those given in the instructions.
Meditative defocusing or effortless meditation (dhyana)
Participants were asked to follow the audio instruction for the practice of dhyana. They were supposed to absorb with the object of meditation without any effort. Dhyana involves effortless defocusing induced by mental chanting of Om.
After each session participants were asked to rate their ability to comply with instructions on a scale from 0 to 10. Only those who achieved 7.5 (75%) and more were included in the study. None of the sessions had to be excluded for this reason.
Data extraction
The following data were extracted from the polygraph. The respiratory rate in cycles per minute (cpm) was calculated by counting the breath cycles in 60-second epochs, continuously. The heart rate in beats per minute (bpm) was calculated by counting the R waves of the QRS complex in the EKG in 60-second epochs, continuously. The skin resistance was obtained at 20-second intervals, continuously and expressed in kilohms (kΩ). The amplitude of the digit pulse volume was sampled from the peak of the pulse wave at 30-second intervals and presented in microvolts.
Frequency domain and time domain analysis of HRV data was carried out for 5-minute recordings for each of the following sessions (cancalata, ekagrata, dharana, dhyana). These 5-minute epochs were recorded for pre, during, and post sessions. Pre and post sessions had one epoch of 5 minutes, whereas during had four similar epochs (viz. D1, D2, D3, D4). The data recorded were visually inspected off-line and only noise-free data were included for analysis. The data were analyzed with an HRV analysis program developed by the Biomedical Signal Analysis Group (University of Kuopio, Finland).16 The energy in the HRV series in the following specific frequency bands was studied viz., the very low frequency band (0.0–.05 Hz), low frequency (LF) band (0.05–0.15 Hz), and high frequency (HF) band (0.15–0.5 Hz). The LF and HF band values were expressed as normalized units.17 The following components of time domain HRV were analyzed: (1) mean RR interval (the mean of the intervals between adjacent QRS complexes or the instantaneous heart rate), (2) RMSSD (the square root of the mean of the sum of the squares of differences between adjacent NN intervals), (3) NN50 (the number of interval differences of successive NN intervals greater than 50 milliseconds), and (4) pNN50 (the proportion derived by dividing NN50 by the total number of NN intervals).
Data analysis
Statistical analysis was done using SPSS Inc. (Chicago, USA) (Version 16.0). Repeated measures analysis of variance (ANOVA) were performed with two “within subjects” factors (i.e., Factor 1: Sessions; cancalata, ekagrata, dharana, and dhyana, and Factor 2: States; Pre, During [D1, D2, D3, D4], and Post). This was followed by a post hoc analysis with Bonferroni adjustment for multiple comparisons between the mean values of different states (Pre, During and Post) and all comparisons were made with the respective Pre state.
Results
The group mean values±SD for breath rate, heart rate, photo-plethysmogram amplitude, and skin resistance are given in Table 1. Frequency domain and time domain measures of HRV are given in Table 2 and Table 3, respectively.
Table 1.
Changes in Autonomic and Respiratory Variables Recorded Pre, During, and Post Four Sessions. Values Are Group Mean±SD
| |
|
|
During |
|
|
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Sessions | Variables | Pre | D1 | D2 | D3 | D4 | Post | Direction of change | Interpretation of change |
| Cancalata | RR (cpm) | 14.67±2.59 | 15.83±2.73* | 16.77±6.58 | 16.10±2.50* | 15.90±2.48* | 14.87±2.40 | ↑ | ↓ SNS |
| HR (bpm) | 68.93±10.87 | 69.57±11.38 | 69.17±11.46 | 70.63±11.73 | 70.57±11.58 | 68.33±10.92 | — | ||
| PPA (μV) | 2496±1636 | 2182±1399 | 2275±1503 | 2124±1609 | 2181±1867 | 2355±1827 | — | ||
| SR (kΩ) | 429.9±127.7 | 461.4±137.2* | 462.8±113.7* | 461.5±111.8 | 474.1±116.1 | 468.3±109.7 | ↑ | ↓ SNS | |
| Ekagrata | RR (cpm) | 15.57±2.43 | 16.00±2.72 | 15.67±2.38 | 15.80±2.19 | 15.70±2.44 | 14.90±2.86 | — | |
| HR (bpm) | 70.40±10.55 | 70.20±10.32 | 68.37±10.97 | 68.00±10.68 | 69.27±11.33 | 68.63±11.18 | — | ||
| PPA (μV) | 2623±1303 | 2543±1290 | 2424±1348 | 2277±1323 | 2280±1377 | 2280±1263 | — | ||
| SR (kΩ) | 439.7±141.3 | 472.3±139.1* | 486.6±134.4** | 489.1±40.5* | 491.0±31.4* | 489.2±29.1* | ↑ | ↓ SNS | |
| Dharana | RR (cpm) | 14.97±2.53 | 14.77±2.88 | 14.40±2.70 | 13.97±3.22 | 14.37±5.01 | 13.93±2.96* | ↓ | ↑ PNS |
| HR (bpm) | 70.37±11.35 | 68.50±11.95 | 67.60±12.07 | 67.57±12.21 | 67.67±11.88 | 68.00±11.66 | — | ||
| PPA (μV) | 2350±1542 | 2586±1852 | 2422±1699 | 2235±1317 | 2231±1175 | 2184±1299 | — | ||
| SR (kΩ) | 429.1±129.1 | 451.5±125.5* | 460.1±31.4* | 465.7±127.9* | 477.9±148.3* | 463.1±125.9 | ↑ | ↓ SNS | |
| Dhyana | RR (cpm) | 15.77±2.70 | 14.27±2.74** | 13.37±2.46*** | 12.40±2.87*** | 12.73±3.12*** | 13.53±2.76*** | ↓ | ↑ PNS |
| HR (bpm) | 69.40±9.88 | 65.57±8.98* | 64.57±9.97*** | 63.73±9.27** | 64.40±9.17** | 65.90±8.59* | ↓ | NK | |
| PPA (μV) | 2548±1301 | 2860±1490* | 2887±1402 | 2766±1362 | 2738±1395 | 2605±1333 | ↑ | ↓ SNS | |
| SR (kΩ) | 434.9±132.0 | 472.3±122.5** | 495.0±116.6*** | 505.8±122.4*** | 509.0±137.6*** | 497.3±113.4*** | ↑ | ↓ SNS | |
p<0.05.
p<0.01.
p<0.001.
Repeated measures ANOVA with Bonferroni adjustment comparing During and Post values with Pre values.
RR, respiratory rate; HR, heart rate; PPA, photo plethysmogram amplitude; SR, skin resistance; cpm, cycles per minute; bpm, beats per minute; ↑, increase; ↓, decrease; —, no change; SNS, sympathetic nervous system activity; PNS, parasympathetic nervous system activity; NK, not known.
Table 2.
Changes in Frequency Domain Analysis of the Heart Rate Variability Components; Values Are Group Mean±SD
| |
|
During |
|
|
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Sessions | Variables | Pre | D1 | D2 | D3 | D4 | Post | Direction of change | Interpretation of change |
| Cancalata | LF (n.u.) (Hz) | 52.83±18.64 | 61.24±16.05* | 62.33±17.55* | 64.81±17.38** | 62.53±17.66 | 62.11±18.82* | ↑ | NK |
| HF (n.u.) (Hz) | 46.43±19.45 | 38.75±16.05 | 37.67±17.55 | 35.21±17.35 | 37.40±17.57 | 37.89±18.82 | — | ||
| LF/HF ratio | 1.85±2.03 | 2.81±4.66 | 3.19±4.79 | 3.18±3.34 | 2.91±3.98 | 2.98±3.52 | — | ||
| Ekagrata | LF (n.u.) Hz | 54.90±15.98 | 63.56±18.00* | 62.16±16.57* | 62.52±16.52 | 63.22±17.06* | 63.15±17.26* | ↑ | NK |
| HF (n.u.) Hz | 45.11±15.99 | 36.44±18.00* | 37.82±16.58* | 37.48±16.52 | 36.76±17.03* | 36.85±17.26* | ↓ | ↓ PNS | |
| LF/HF ratio | 1.65±1.45 | 3.04±3.37* | 2.36±2.03* | 2.34±1.78 | 2.51±1.92* | 2.79±2.73* | ↑ | NK | |
| Dharana | LF (n.u.) Hz | 59.45±15.56 | 55.97±19.79 | 59.22±17.69 | 59.64±17.24 | 53.74±17.23 | 58.54±15.43 | — | |
| HF (n.u.) Hz | 40.55±15.56 | 45.03±19.79 | 40.77±17.69 | 40.36±17.24 | 47.25±17.43 | 41.46±15.43 | — | ||
| LF/HF ratio | 1.86±1.19 | 1.96±2.03 | 2.05±1.75 | 2.15±1.87 | 1.66±1.65 | 1.96±1.75 | — | ||
| Dhyana | LF (n.u.) Hz | 60.42±15.57 | 46.20±20.16*** | 51.10±19.55* | 50.46±16.39* | 44.86±13.07*** | 49.50±17.07* | ↓ | NK |
| HF (n.u.) Hz | 39.58±15.57 | 53.80±20.15*** | 48.90±19.54* | 49.53±16.40* | 54.53±13.57*** | 50.50±17.07* | ↑ | ↑ PNS | |
| LF/HF ratio | 2.30±2.52 | 1.62±3.03 | 1.53±1.42 | 1.35±1.17 | 0.98±0.73 | 1.31±1.11 | — | ||
p<0.05.
p<0.01.
p<0.001.
Repeated measures ANOVA with Bonferroni adjustment comparing During and Post values with Pre values.
LF, low frequency band of the HRV; HF, high frequency band of the HRV; LF/HF, ratio of low frequency to high frequency; SNS, sympathetic nervous system activity; PNS, parasympathetic nervous system activity; NK, not known; n.u., normalized units.
Table 3.
Time Domain Analysis of the Heart Rate Variability Components; Values Are Group Mean±SD
| |
|
|
During |
|
|
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Sessions | Variables | Pre | D1 | D2 | D3 | D4 | Post | Direction of change | Interpretation of change |
| Cancalata | Mean RR (ms) | 0.90±0.13 | 0.89±0.13 | 0.89±0.13 | 0.89±0.13 | 0.87±0.12 | 0.90±0.13 | — | |
| RMSSD (ms) | 49.35±16.34 | 46.71±21.39 | 45.78±23.08 | 49.87±24.53 | 48.73±18.21 | 49.88±22.28 | — | ||
| NN50 count | 72.53±49.68 | 67.70±54.22 | 73.10±54.56 | 67.80±48.09 | 69.90±49.38 | 76.63±54.70 | — | ||
| pNN50 (%) | 29.90±16.84 | 21.67±19.30 | 23.13±18.83 | 21.10±16.66 | 21.46±16.74 | 24.59±17.59 | — | ||
| Ekagrata | Mean RR (ms) | 0.88±0.11 | 0.85±0.19 | 0.91±0.12* | 0.92±0.12** | 0.91±0.12* | 0.90±0.12 | ↑ | ↑ PNS |
| RMSSD (ms) | 54.43±25.10 | 50.24±23.06 | 51.73±25.95 | 55.15±25.88 | 61.04±28.57 | 53.79±23.89 | — | ||
| NN50 count | 74.73±53.28 | 74.77±51.97 | 82.47±54.60 | 85.27±54.07 | 84.67±53.03 | 77.60±49.34 | — | ||
| pNN50 (%) | 22.53±17.97 | 23.40±17.96 | 26.39±19.09 | 27.56±18.98 | 27.10±18.63 | 24.54±17.18 | — | ||
| Dharana | Mean RR (ms) | 0.87±0.12 | 0.89±0.13 | 0.90±0.15 | 0.89±0.13 | 0.90±0.12 | 0.88±0.12 | — | |
| RMSSD (ms) | 52.00±22.01 | 54.95±27.07 | 50.69±24.29 | 54.70±27.14 | 57.50±23.78 | 53.90±22.77 | — | ||
| NN50 count | 75.97±51.02 | 76.60±51.38 | 79.13±57.74 | 78.60±53.38 | 79.17±54.45 | 73.73±47.34 | — | ||
| pNN50 (%) | 23.98±18.15 | 24.29±18.12 | 25.68±20.38 | 25.60±18.70 | 25.77±19.17 | 24.28±17.14 | — | ||
| Dhyana | Mean RR (ms) | 0.89±0.10 | 0.91±0.12 | 0.92±0.13 | 0.93±0.12* | 0.88±0.16 | 0.89±0.17 | ↑ | ↑ PNS |
| RMSSD (ms) | 54.17±26.78 | 62.28±31.76 | 61.87±32.65 | 63.49±33.32 | 65.61±31.26 | 64.34±36.38 | — | ||
| NN50 count | 75.97±51.44 | 85.10±56.95 | 99.80±57.14*** | 100.63±58.06* | 86.06±61.93 | 85.87±58.82 | ↑ | ↑ PNS | |
| pNN50 (%) | 25.74±19.17 | 28.55±20.62 | 32.79±20.25** | 32.54±20.87* | 29.78±20.85 | 28.50±18.90 | ↑ | ↑ PNS | |
p<0.05.
p<0.01.
p<0.001.
Repeated measures ANOVA with Bonferroni adjustment comparing During and Post values with Pre values.
Mean RR, the average of time intervals between consecutive R-waves; RMSSD, the square root of the mean of the sum of the squares of differences between adjacent NN intervals; NN50, number of pairs of adjacent NN intervals differing by more than 50 ms in the entire recording; pNN50, NN50 count divided by the total number of all NN intervals; SNS, sympathetic nervous system activity; PNS, parasympathetic nervous system activity; NK, not known.
Repeated measures ANOVA
The significant changes in breath rate, heart rate, photo-plethysmogram amplitude, skin resistance, LF power, HF power, LF/HF ratio, the mean RR, the RMSSD, the NN50, and the pNN50 are given in Table 4.
Table 4.
Summary of the Repeated Measures Analysis of Variance (ANOVA) Showing Statically Significant Results
| Variables | Factor | F value | df | Huynh-Feldt epsilon | p value |
|---|---|---|---|---|---|
| Breath rate (cpm) | Sessions | 15.32 | 2.96, 94.67 | 0.986 | 0.001 |
| States | 4.12 | 3.80, 121.45 | 0.759 | 0.01 | |
| Sessions×states | 5.75 | 4.85, 155.17 | 0.323 | 0.001 | |
| Heart rate (bpm) | Sessions | 4.05 | 2.79, 89.25 | 0.930 | 0.05 |
| States | 8.54 | 3.15, 100.94 | 0.63 | 0.001 | |
| Sessions×states | 5.16 | 10.28, 328.96 | 0.685 | 0.001 | |
| Photo-plethysmogram amplitude (μV) | States | 4.22 | 2.14, 68.34 | 0.427 | 0.05 |
| Skin resistance (kΩ) | States | 18.56 | 2.68, 85.67 | 0.535 | 0.001 |
| Low frequency (LF) power (Hz) | Sessions | 11.39 | 2.93, 85.05 | 0.978 | 0.001 |
| States | 2.52 | 4.48, 129.82 | 0.895 | 0.05 | |
| Sessions×states | 5.73 | 13.47, 390.69 | 0.898 | 0.001 | |
| High frequency (HF) power (Hz) | Sessions | 8.03 | 2.73,79.02 | 0.908 | 0.001 |
| Sessions×states | 3.79 | 4.26, 123.56 | 0.284 | 0.01 | |
| LF/HF ratio | Sessions | 3.33 | 2.64, 76.50 | 0.879 | 0.05 |
| Sessions×states | 3.25 | 4.63, 134.27 | 0.309 | 0.05 | |
| Mean RR (ms) | States | 2.74 | 3.48, 100.82 | 0.319 | 0.05 |
| RMSSD (ms) | Sessions | 4.53 | 2.85, 82.63 | 0.95 | 0.01 |
| States | 3.18 | 4.7, 136.41 | 0.941 | 0.05 | |
| NN50 count | States | 3.52 | 4.47, 129.47 | 0.893 | 0.01 |
| pNN50 (%) | Sessions | 3.50 | 2.62, 75.96 | 0.873 | 0.05 |
| States | 4.64 | 4.14, 119.91 | 0.827 | 0.01 | |
| Sessions×states | 2.07 | 9.26, 268.51 | 0.617 | 0.05 |
Mean RR, the average of time intervals between consecutive R-waves; RMSSD, the square root of the mean of the sum of the squares of differences between adjacent NN intervals; NN50, number of pairs of adjacent NN intervals differing by more than 50 ms in the entire recording; pNN50, NN50 count divided by the total number of all NN intervals; cpm, cycles per minute; bpm, beats per minute.
Post hoc analyses with Bonferroni adjustment
Post hoc analyses with Bonferroni adjustment were performed and all comparisons were made with respective pre states. These have been summarized in Table 5.
Table 5.
Summary of the Level of Significance and Direction of Change for Post-hoc Analyses with Bonferroni Adjustment Comparing During and Post with the Respective Pre Values
| |
Cancalata |
Ekagrata |
Dharana |
Dhyana |
||||
|---|---|---|---|---|---|---|---|---|
| Variables | During | Post | During | Post | During | Post | During | Post |
| Breath rate (cpm) | p < 0.05 ↑ | NS | NS | NS | NS | p < 0.05 ↓ | p < 0.001 ↓ | p < 0.001 ↓ |
| Heart rate (bpm) | NS | NS | NS | NS | NS | NS | p < 0.001 ↓ | p < 0.05 ↓ |
| Photo-plethysmogram amplitude (μv) | NS | NS | NS | NS | NS | NS | p < 0.05 ↑ | NS |
| Skin resistance (kΩ) | p < 0.05 ↑ | NS | p < 0.05 ↑ | p < 0.01 ↑ | <0.05 ↑ | NS | p < 0.001 ↑ | p < 0.001 ↑ |
| Low frequency power (Hz) | p < 0.001 ↑ | p < 0.05 ↑ | p < 0.05 ↑ | p < 0.05 ↑ | NS | NS | p < 0.001 ↓ | p < 0.05 ↓ |
| High frequency power (Hz) | NS | NS | p < 0.05 ↓ | p < 0.05 ↓ | NS | NS | p < 0.001 ↑ | p < 0.05 ↑ |
| LF/HF ratio | NS | NS | p < 0.05 ↑ | p < 0.05 ↑ | NS | NS | NS | NS |
| Mean RR (ms) | NS | NS | p < 0.01 ↑ | NS | NS | NS | p < 0.05 ↑ | NS |
| RMSSD (ms) | NS | NS | NS | NS | NS | NS | NS | NS |
| NN50 count | NS | NS | NS | NS | NS | NS | p < 0.001 ↑ | NS |
| pNN50 (%) | NS | NS | NS | NS | NS | NS | p < 0.01 ↑ | NS |
NS, not significant; ↑ , Increase; ↓ , Decrease.
Discussion
Autonomic variables and the breath rate were recorded during random thinking (cancalata), nonmeditative focusing (ekagrata), meditative focusing (dharana), and meditative defocusing or effortless meditation (dhyana).
Maximum changes in autonomic variables and the breath rate occurred during the stage of effortless meditation (dhyana). The changes were all suggestive of reduced sympathetic activity and/or increased vagal modulation. These were a decrease in heart rate, an increase in digit pulse volume (based on the photo-plethysmogram amplitude), an increase in skin resistance, a decrease in the LF power of HRV, and an increase in the HF power, also an increase in NN50 and pNN50, with a reduction in breath rate.
The main difference between dharana and dhyana sessions was apparent in the autonomic variables and breath rate. As described above, most of the changes during dhyana were suggestive of reduced activity in the different subdivisions of sympathetic nervous system activity, though some variables are regulated by several factors. The heart rate for example, is regulated by dual innervation (sympathetic and vagal), as well as humoral factors.18 This makes the decrease in heart rate less easy to interpret (i.e., it could be due to increased vagal tone or due to sympathetic withdrawal). This also applies to HRV components.
There was a general understanding that the LF band of the HRV is an index of cardiac sympathetic activity.17 However, this has been debated. Neither the LF band (<0.15 Hz) nor the HF band (>0.15 Hz) are considered exclusive markers of sympathetic or parasympathetic tone, respectively.19 The HRV represents the integrated end-organ response to the complex nonlinear interaction between the two divisions of the autonomic nervous system as well as other factors. This particularly applies to the relationship between the LF power and cardiac sympathetic tone. It was found that the LF power was reduced by selective cardiac parasympathectomy and was not totally removed when β-adrenoceptor blockade was combined with denervation.20 Also activities that were expected to increase sympathetic activity failed to increase the LF power and actually significantly reduced the LF power. In fact sympathetic activity can also modulate the HF component of HRV, though to a lesser extent than the parasympathetic influence on the LF power. The association between HF power and cardiac parasympathetic activity is stronger. However the association is qualitative rather than quantitative. Hence the HRV provides a qualitative marker of cardiac parasympathetic regulation and changes in the LF power and LF/HF ratio have to be viewed with caution.
The LF power significantly increased during random thinking (cancalata) and nonmeditative focusing (ekagrata), while there was a significant decrease during meditation (dhyana). Conversely, the HF power increased during meditation (dhyana), while it was decreased during nonmeditative focusing (ekagrata). The increase in LF during ekagrata and cancalata could reflect either a change in sympathetic or parasympathetic activity as described above. Given the complexity in interpreting these changes, at this stage it may be said that the change in LF in ekagrata and cancalata reflects a change in autonomic activity that would need further investigation. The frequency domain analysis indicated a possible increase in parasympathetic activity based on the increase in HF power in dhyana alone. This is supported by the changes in the HRV with time domain analysis. The pNN50 and the NN50 are both indicative of vagal tone.21 Both values increased during dhyana, which was also suggestive of parasympathetic dominance. Hence during dhyana there was a shift in the autonomic balance towards vagal dominance.
The skin resistance level is an indicator of the level of activity in the cholinergic sudomotor sympathetic nerves supplying the eccrine sweat glands.22 This is believed to be the main contributor to changes in the spontaneous electrodermal activity.23 The increase in the skin resistance level in all four sessions suggests relaxation during all of them.
An increase in photo-plethysmogram amplitude correlates with decreased noradrenergic vasomotor sympathetic control of the cutaneous blood vessels.24 Hence during the dhyana session there was decreased activity in the sympathetic nerves supplying the cutaneous blood vessels.
Unlike these variables the breath rate depends upon numerous factors ranging from the level of physical activity to psychological stress.25 A decrease in breath rate is generally associated with relaxation, which can explain the decrease seen during dhyana. The increase in breath rate during cancalata could suggest that participants found the diverse auditory inputs (taken from a local radio station and put together at random) stressful.
Taken together the results suggest that effortless meditation or dhyana is associated with changes in the autonomic nervous system suggesting vagal dominance. Hence earlier studies that gave contrasting results (i.e., of sympathetic withdrawal in some studies, while other studies showed sympathetic activation), when meditators practiced the same technique may have been due to some meditators being in the dharana phase, while others were in the dhyana phase. Examples for this are Ananda Marga Meditation, for which one study reported sympathetic withdrawal,10 while another study reported increased sympathetic activity in meditators.9 Similarly there were conflicting reports for Zazen meditation.7,26
A TM session has been shown to consist of phenomenologically and physiologically distinct substates.27 The three qualitatively different substates that have been described are (1) the inward stroke in which there is progressive reduction of all activity, (2) transcendental consciousness in which thoughts are absent yet consciousness is maintained, and (3) the outward stroke in which mental and physical activity progressively increase.28 These three substates or phases have easily measured markers.29 It was observed that meditators went through the three phases several times in a session. However, it was possible to note that the inward stroke was characterized by less heart rate deceleration and lowered skin conductance compared to the state of transcendental consciousness. Hence, in different phases of meditation, sympathetic activity may differ. This is similar to the present results. These differences during a meditation session could also be the reason for the apparently contradictory results seen in Ananda Marga and Zazen meditators. Also the differences could be due to the fact that different techniques are often given the same name.
In summary, the changes were hence suggestive of reduced activation in dhyana. However dhyana is not the ultimate stage described in the ancient texts. Following dhyana, the eighth step in the astanga (eight limbs) yoga of Patanjali is samadhi which means, the state of ultimate realization. Samadhi has two stages, sabija samadhi (bija=seed, in Sanskrit), which means realization in its seed or unmanifest form (Patanjali's Yoga Sutras, Chapter I, Verse 50). With continued practice this leads to nirbija samadhi or the manifest state of ultimate realization (Patanjali's Yoga Sutras, Chapter I, Verse 51).
There have been studies that have attempted to find objective physiological correlates for the experience of pure awareness as in samadhi. In 40 meditators practicing the TM technique, 11 participants were chosen to press a button after an episode of pure consciousness experience. There was a significant relationship between button presses and breath suspension.30
Breath suspension periods when experiencing pure consciousness in TM were correlated with increased total EEG coherence with implications for functional integration and better mind–body health, along with reduced heart rate and phasic skin conductance responses.31
In summary, the present results show that when meditation is divided as two traditionally described stages, meditative focusing (dharana) and meditative defocusing (dhyana), the changes in the autonomic nervous system are distinct and different. The present findings make it apparent that studying yoga practices using present-day scientific methods may be made more meaningful if the techniques are understood based on the descriptions in the traditional texts.
Conclusions
Maximum changes were seen in autonomic variables and breath rate during the state of effortless meditation (dhyana). The changes were all suggestive of reduced sympathetic activity and/or increased vagal modulation. During dharana there was an increase in skin resistance. The changes with HRV during ekagrata and cancalata were inconclusive.
Acknowledgment
The authors gratefully acknowledge the funding from the Indian Council of Medical Research (ICMR), Government of India, as part of a grant (Project No. 2001-05010) towards the Center for Advanced Research in Yoga and Neurophysiology (CAR-Y&N).
Author Disclosure Statement
The authors declare that they have no competing financial interests.
References
- 1.Murata T. Takahashi T. Hamada T, et al. Individual trait anxiety levels characterizing the properties of Zen meditation. Neuropsychobiology. 2004;50:189–194. doi: 10.1159/000079113. [DOI] [PubMed] [Google Scholar]
- 2.Wallace RK. Benson H. Wilson AF. A wakeful hypometabolic physiologic state. Am J Physiol. 1971;221:795–799. doi: 10.1152/ajplegacy.1971.221.3.795. [DOI] [PubMed] [Google Scholar]
- 3.Wallace RK. Physiological effects of Transcendental Meditation. Science. 1970;167:1751–1754. doi: 10.1126/science.167.3926.1751. [DOI] [PubMed] [Google Scholar]
- 4.Orme-Johnson DW. Autonomic stability and Transcendental Meditation. Psychosom Med. 1973;35:341–349. doi: 10.1097/00006842-197307000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Dillbeck MC. Orme-Johnson DW. Physiological differences between Transcendental Meditation and rest. American Psychologist. 1987;42:879–881. [Google Scholar]
- 6.Lang R. Dehof K. Meurer KA, et al. Sympathetic activity and Transcendental meditation. J Neural Transm. 1979;44:117–135. doi: 10.1007/BF01252706. [DOI] [PubMed] [Google Scholar]
- 7.Hirai T. Electroencephalographic study on the Zen meditation (ZAZEN)-EEG changes during concentrated relaxation. Jpn J Psychiatry Neurol. 1960;62:76–105. [Google Scholar]
- 8.Sugi Y. Akatsu K. Studies on respiration and energy metabolism during sitting in Zazen. Res J Phys Educ. 1968;12:190–206. [Google Scholar]
- 9.Corby JC. Roth WT. Zarcone VP, Jr, et al. Physiological correlates of the practice of Tantric Yoga meditation. Arch Gen Psychiatry. 1978;35:571–577. doi: 10.1001/archpsyc.1978.01770290053005. [DOI] [PubMed] [Google Scholar]
- 10.Elson BD. Hauri P. Cunis D. Physiological changes in yoga meditation. Psychophysiology. 1977;14:52–57. doi: 10.1111/j.1469-8986.1977.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 11.Travis F. Shear J. Focused attention, open monitoring and automatic self-transcending: categories to organize meditations from Vedic, Buddhist and Chinese traditions. Consciousness and Cognition. 2010;19:1110–1118. doi: 10.1016/j.concog.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Taimini IK. The science of yoga. Madras: The Theosophical Publishing House; 2005. pp. 275–278. (a) (b) 278–280. [Google Scholar]
- 13.Sarasvati M. Swami G. Bhagavad Gita. Calcutta: Advaita Ashrama; 1998. pp. 459–461. (a) (b) 393–394. [Google Scholar]
- 14.Kumar S. Telles S. Meditative states based on yoga texts and their effects on performance of a cancellation task. Percept Mot Skills. 2009;109:679–689. doi: 10.2466/pms.109.3.679-689. [DOI] [PubMed] [Google Scholar]
- 15.Yildirir A. Kabakci G. Akgul E, et al. Effects of menstrual cycle on cardiac autonomic innervation as assessed by heart rate variability. Ann Noninvasive Electrocardiol. 2002;7:60–63. doi: 10.1111/j.1542-474X.2001.tb00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niskanen JP. Tarvainen MP. Ranta-aho PO, et al. Software for advanced HRV analysis. Comput Methods Programs Biomed. 2004;76:73–81. doi: 10.1016/j.cmpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 18.Andreassi JL. Mahwah, NJ: Lawrence Earl Baum Associates; 2000. Psychophysiology: human behavior and physiological response. [Google Scholar]
- 19.Malliani A. Julien C. Billman GE, et al. Cardiovascular variability is not an index of autonomic control of circulation. J Appl Physiol. 2006;101:684–688. doi: 10.1152/japplphysiol.00562.2006. [DOI] [PubMed] [Google Scholar]
- 20.Randall DC. Brown DR. Raisch RM, et al. SA nodal parasympathectomy delineates autonomic control of heart rate power spectrum. Am J Physiol. 1991;260:H985–988. doi: 10.1152/ajpheart.1991.260.3.H985. [DOI] [PubMed] [Google Scholar]
- 21.Wennerblom B. Lurje L. Tygesen H, et al. Patients with uncomplicated coronary artery disease have reduced heart rate variability mainly affecting vagal tone. Heart. 2000;83:290–294. doi: 10.1136/heart.83.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields SA. MacDowell KA. Fairchild SB, et al. Is mediation of sweating cholinergic, adrenergic, or both? A comment on the literature. Psychophysiology. 1987;24:312–319. doi: 10.1111/j.1469-8986.1987.tb00301.x. [DOI] [PubMed] [Google Scholar]
- 23.Fowles DC. Porges SW. The eccrine system and electrodermal activity. In: Coles MGH, editor; Donchin E, editor; Psychophysiology: Systems, Processes and Applications. New York: Guilford Press; 1986. pp. 51–96. [Google Scholar]
- 24.Delius W. Kellerová E. Reactions of arterial and venous vessels in the human forearm and hand to deep breath or mental strain. Clin Sci. 1971;40:271–282. doi: 10.1042/cs0400271. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson I. Ripley HS. Variations in respiration and in respiratory symptoms during changes in emotion. Psychosom Med. 1952;14:476–490. doi: 10.1097/00006842-195211000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Kasamatsu A. Hirai T. An electroencephalographic study on the Zen meditation (Zazen) Folia Psychiatr Neurol Jpn. 1966;20:315–336. doi: 10.1111/j.1440-1819.1966.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 27.Travis FT. Autonomic and EEG patterns distinguish transcending from other experiences during Transcendental Meditation practice. Int J Psychophysiol. 2001;42:1–9. doi: 10.1016/s0167-8760(01)00143-x. [DOI] [PubMed] [Google Scholar]
- 28.Wallace RK. The neurophysiology of enlightenment. Fairfield, IA: MIU Press; 1986. [Google Scholar]
- 29.Travis F. Wallace RK. Autonomic patterns during respiratory suspensions: possible markers of Transcendental Consciousness. Psychophysiology. 1997;34:39–46. doi: 10.1111/j.1469-8986.1997.tb02414.x. [DOI] [PubMed] [Google Scholar]
- 30.Farrow JT. Hebert JR. Breath suspension during the transcendental meditation technique. Psychosom Med. 1982;44:133–153. doi: 10.1097/00006842-198205000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Badawi K. Wallace RK. Orme-Johnson D. Rouzere AM. Electrophysiologic characteristics of respiratory suspension periods occurring during the practice of the Transcendental Meditation Program. Psychosom Med. 1984;46:267–276. doi: 10.1097/00006842-198405000-00008. [DOI] [PubMed] [Google Scholar]