Abstract
In 2006, the Canadian National Advisory Committee on Blood and Blood Products (NAC) developed a transfusion policy framework for the use of off-label recombinant factor VIIa (rFVIIa) in massive bleeding. Because the number of randomised controlled trials has doubled, the NAC undertook a review of the policy framework in 2011. On the basis of the review of 29 randomised controlled trials, there remains little evidence to support the routine use of rFVIIa in massive bleeding. Mortality benefits have not been demonstrated. Contrarily, an increase in arterial thromboembolic events has been observed with the use of off-label rFVIIa. Given the absence of evidence of benefit and with evidence of the risk of harm, the NAC recommends that recombinant VIIa no longer be used for the off-label indications of prevention and treatment of bleeding in patients without haemophilia.
Keywords: massive transfusion, off-label use, recombinant factor VIIa
In 2008, the Canadian National Advisory Committee on Blood and Blood Products (NAC) published a policy framework for the use of recombinant factor VIIa (rFVIIa) in massive bleeding (Moltzan et al., 2008). Its purpose was to provide Canadian hospitals with recommendations on the medical and prerequisite conditions for appropriate use of rFVIIa based on the existing medical literature. At that time, increasing use of off-label rFVIIa was being reported (Cameron et al., 2007; Isbister et al., 2008; Karkouti et al., 2008) and at a cost of over CDN$1000 per mg, concerns about its costs, benefits and risks were justified. Since that time, the number of available controlled clinical trials has doubled and more data have been published on the potential risks of rFVIIa. The objective of this article is to update the policy framework for the Canadian setting by reviewing the updated literature available on the benefits and risks of rFVIIa in patients without haemophilia. Particular questions of interest are as follows: What is the current use of rFVIIa in Canada? What is the evidence for the use of off-label rFVIIa in the most commonly used clinical settings? What are the benefits of rFVIIa? What are the risks? On the basis of these updated findings, what are the NAC recommendations on the use of off-label rFVIIa in patients without haemophilia?
BACKGROUND
The NAC is an interprovincial medical and technical advisory body to the provincial and territorial health ministries that fund the blood system in Canada. In 2006, the NAC assembled a panel of 11 experts to review the evidence from randomised clinical trials to reach consensus on the use of rFVIIa in a variety of off-label settings. The recommendations and conclusions were based on interpretation of the available evidence and where evidence was lacking, on consensus expert clinical opinion (Moltzan et al., 2008).
In 2011, an update of the policy framework was planned. A review of the literature was conducted based on a search strategy published in the most recent Cochrane systematic review (Simpson et al., 2012). Briefly, the search strategy included MEDLINE, EMBASE, CINAHL and CENTRAL (Cochrane Central Register of Controlled Trials, The Cochrane Library 2011, Issue 1) databases and was conducted up to March 2011. The literature search focused on controlled clinical trials on rFVIIa in patients without haemophilia. Data were summarised and presented to a panel of transfusion medicine experts on the NAC. The policy framework was revised taking into account the new data available and presented to the NAC members for feedback in November 2011. Modifications arising from the consultative process were incorporated into this document.
TRENDS IN rFVIIa USE
Although rFVIIa was licensed in 1999 in Canada (Health Canada, 2005) and the United States (Center for Biologics Evaluation and Research, 1999) for the treatment of bleeding in haemophilia A/B patients with inhibitors, it was recognised early on that rFVIIa could be exploited for its haemostatic effect (Kenet et al., 1999) and used off-label in a variety of complex clinical situations to prevent or treat significant bleeding. In fact, its use continued to grow at least until 2008 when reports showed that only 1–3% of patients being treated with rFVIIa had haemophilia (Logan et al., 2011; Phillips et al., 2011). In the report of American hospitals, use of off-label rFVIIa increased by 143-fold from 2000 to 2008. In 2008, the top indications for in-hospital use of rFVIIa were cardiac surgery (27%), trauma (18%) and intracranial haemorrhage (ICH; 11%) (Logan et al., 2011). In the Haemostasis Registry final report of rFVIIa use in Australia and New Zealand between 2000 and 2009, rFVIIa use reached a plateau in 2006 to 2008 with a slight decline in 2009 (Phillips et al., 2011). Similarly, the largest users in 2009 were cardiac surgery patients (45%), patients who had undergone ‘other surgery’ (18%) and trauma (13%).
In Canada, the trends in rFVIIa issues from Canadian Blood Services (the nation's sole blood supplier with the exception of the province of Québec) showed a rise until 2008 and then a slight decline in 2009 and 2010 (Fig. 1; David Howe, Executive Director, Product & Hospital Services, Canadian Blood Services, 23 September 2011, personal communication). In fact, data from a Canadian registry of off-label rFVIIa use confirms this trend (Keyvan Karkouti, Department of Anesthesia, University of Toronto, 7 November 2011, personal communication). The registry included 16 Canadian hospitals and captured data on off-label use at each site between 2007 and 2010. These 16 hospitals accounted for approximately 80% of cardiac surgery cases performed in Canada. The main indications in the off-label registry were comparable to our international counterparts with cardiac surgery (71%) being the predominant indication followed by trauma (7%), ICH (7%) and liver/abdominal surgery (4%).
Fig. 1.
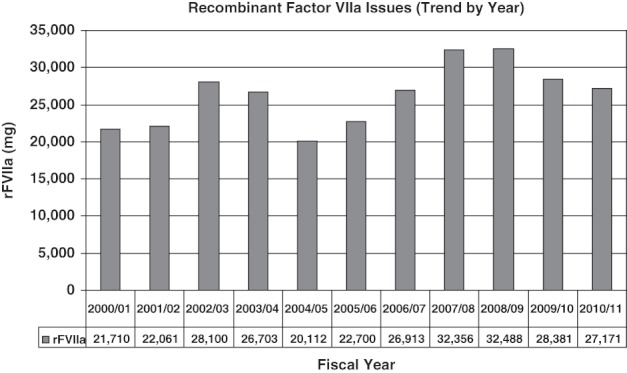
Trend in recombinant factor VIIa issues by Canadian Blood Services (courtesy of Canadian Blood Services).
RANDOMISED TRIALS OF USE OF rFVIIa IN PATIENTS WITHOUT HAEMOPHILIA
To date, there have been 27 published randomised controlled clinical trials (RCTs) on the use of off-label rFVIIa. The studies have been conducted in a variety of settings. The following sections focus on situations where off-label rFVIIa is most commonly used in Canada and where there is more than one RCT to guide our decisions.
Cardiac surgery
Five RCTs have been conducted in patients undergoing cardiac surgery: four RCTs where rFVIIa was given after heparin reversal post-cardiopulmonary bypass (CPB; Diprose et al., 2005; Ekert et al., 2006; Ma et al., 2006; Essam, 2007) and one RCT in the immediate post-operative setting (Gill et al., 2009). Diprose et al. evaluated rFVIIa in reducing transfusion and found a reduction in the need for transfusion in 20 patients undergoing complex non-coronary cardiac surgery requiring CPB. Ma et al. evaluated rFVIIa in 22 patients undergoing cardiac valve surgery with CPB. No specific primary outcome was stated but a reduction in transfusion was observed with rFVIIa. Essam evaluated 30 patients undergoing elective coronary revascularisation. No specific primary outcome was defined but a decrease in chest tube drainage favouring rFVIIa was noted. All three studies were small and did not show a mortality benefit. Ekert et al. studied 82 infants with congenital heart disease requiring CPB. The primary outcome was time to chest closure. They found prolonged time to chest closure with rFVIIa and no difference in blood loss or transfusion requirements. Finally, Gill et al. studied 179 patients who had undergone surgery requiring CPB but had been in the post-operative care environment for at least 30 min and had met a defined bleeding rate for rFVIIa administration. The primary outcome was serious adverse events. There were more serious adverse events in the rFVIIa group, although this was not statistically significant. Secondary outcomes including need for reoperation for bleeding and allogeneic blood transfusion favoured the rFVIIa group.
A recent systematic review did explore the role of rFVIIa in the cardiac surgery setting (Yank et al., 2011). This included two RCTs (Diprose et al., 2005; Gill et al., 2009) as well as four observational studies. The review found that there was no mortality benefit and an increase in thromboembolic (TE) events with rFVIIa [risk difference (RD) 0·05; 95% CI: 0·01 to 0·10]. On the basis of the current available data, rFVIIa is not recommended in the treatment of patients undergoing cardiac surgery requiring CPB.
Trauma
There have been two publications on the use of rFVIIa in trauma (Boffard et al., 2005; Hauser et al., 2010). Both publications reported simultaneous RCTs in a blunt and penetrating trauma population. The first publication (Boffard et al., 2005) was a multicentre study on patients with severe trauma randomised after the sixth RBC unit had been administered. The primary endpoint was the number of RBC units transfused during the 48-h period after the first dose of study drug. There was no difference between the control group and rFVIIa groups for either blunt or penetrating trauma. However, when only blunt trauma patients alive at 48 h were considered, there was a statistically significant reduction of 2·6 units with the use of rFVIIa (P = 0·02). There were no differences in the secondary outcomes of 48-h or 30-day mortality. The second publication (Hauser et al., 2010) was a phase 3 multicentre RCT in bleeding blunt and/or penetrating trauma patients. Patients were treated with study drug after receiving four units of RBCs. There was no difference in the primary outcome of 30-day mortality between the rFVIIa and placebo groups. This study was terminated early because of a lower than expected mortality rate and a high likelihood of futility in demonstrating the primary endpoint favouring rFVIIa in the blunt trauma population.
A systematic review of these two trials did not show a benefit in mortality, nor did it show an increase in TE complications (Yank et al., 2011). It did, however, show a benefit favouring rFVIIa in reducing the risk of acute respiratory distress syndrome (RD −0·05; 95% confidence interval (CI): −0·02 to −0·08). Given the lack of mortality benefit, rFVIIa is not recommended in the treatment of patients with blunt or penetrating trauma.
Spontaneous ICH
There have been four RCTs evaluating rFVIIa in spontaneous ICH administered within 3 h of symptom onset (Mayer et al., 2005a, b, 2006, 2008). Two studies had primary outcomes of adverse events and found no significant differences between placebo and rFVIIa (Mayer et al., 2005b, 2006). The phase II trial evaluated the percent change in ICH volume at 24 h and showed that ICH volume increased more in the placebo group than the rFVIIa group (Mayer et al., 2005a). Secondary outcomes suggested that severe disability and mortality were improved in the rFVIIa group; however, the rFVIIa group also had a trend to increased risk of adverse thrombotic events. The phase 3 study on 841 patients was designed with a primary combined outcome of severe disability or death at 90 days after ICH (Mayer et al., 2008). There was no difference in the combined outcome between the rFVIIa treated groups compared with placebo and in fact, a statistically significant increase in arterial events was observed in the group receiving 80 mcg kg−1 rFVIIa compared with placebo. It should be noted that there are two ongoing RCTs (initiated in 2010 and 2011) examining the role of a single dose of 80 mcg kg−1 rFVIIa in a subgroup of patients with acute ICH who have a ‘spot sign’ on computed tomography (CT) angiography indicating contrast extravasation and active bleeding (Flaherty & Jauch, 2011; Gladstone et al., 2011). The estimated dates of completion for these studies are 2013 and 2015, respectively.
A systematic review of the ICH RCTs and one observational trial did not show benefit in mortality or poor functional outcome with the use of rFVIIa (Yank et al., 2011). However, an increase in arterial thromboembolism was seen with rFVIIa for medium (>40 but <120 mcg kg−1) and high (≥120 mcg kg−1) doses (medium RD 0·03, 95% CI: 0·01 to 0·06; high RD 0·06, 95% CI: 0·01 to 0·11). As a mortality benefit has not been shown and there is increased risk of arterial thromboembolism, rFVIIa is not recommended for the treatment of ICH.
Liver transplantation
Three RCTs evaluated the effect of rFVIIa in orthotopic liver transplantation (OLT). The two larger studies (209 and 87 patients, respectively) had a primary outcome of transfusion requirements and neither study showed a difference between placebo and rFVIIa groups (Lodge et al., 2005a; Planinsic et al., 2005). The third study consisting of 20 patients did not state a primary outcome but found a decrease in INR, blood loss and transfusion requirements (Pugliese et al., 2007). No mortality benefit was seen in any of the studies. rFVIIa use in the treatment of patients undergoing OLT is not recommended.
Liver resection
Two RCTs evaluated the effect and safety of rFVIIa in partial hepatectomy (Lodge et al., 2005b; Shao et al., 2006). Both studies had a primary outcome of transfusion requirements and neither showed a difference in the required RBC units between placebo and rFVIIa groups. rFVIIa use in the treatment of patients undergoing surgery for liver resection is not recommended.
Cirrhosis with gastrointestinal bleeding
Two RCTs evaluated rFVIIa in controlling upper gastrointestinal bleeding (UGIB) in patients with cirrhosis when administered as an add-on to standard therapy (Bosch et al., 2004, 2008). The primary outcome in both studies was failure to control bleeding. In the initial RCT, there was no difference observed (Bosch et al., 2004). However, a subgroup analysis suggested that there might be benefit of using rFVIIa in patients with more advanced liver disease (Child Pugh class B and C). The second RCT specifically included Child Pugh class B and C patients with cirrhosis and variceal bleeding (Bosch et al., 2008). Again, there was no difference in the primary outcome of failure to control bleeding. rFVIIa use in the treatment of UGIB in patients with cirrhosis is not recommended.
Other settings
In addition to the RCTs outlined in the previous sections, off-label rFVIIa has also been studied in liver biopsy (Jeffers et al., 2002), prostatectomy (Friederich et al., 2003), pelvic fracture (Raobaikady et al., 2005), haematopoietic stem cell transplant (Pihusch et al., 2005), dengue haemorrhagic fever (Chuansumrit et al., 2005), burn patients requiring surgery (Johansson et al., 2007), spinal fusion surgery (Sachs et al., 2007), traumatic ICH (Narayan et al., 2008) and craniofacial reconstruction (Hanna et al., 2010). All these studies were small with 100 patients or less and only four had more than 50 patients (Jeffers et al., 2002; Pihusch et al., 2005; Sachs et al., 2007; Narayan et al., 2008). No mortality benefit was seen in any of these studies. The effect of rFVIIa on the primary outcome of these RCTs is summarised in Tables 1 (prophylactic RCTs where rFVIIa was used to prevent bleeding) and 2 (therapeutic RCTs where rVIIa was used to treat bleeding). Given the limited data, these studies do not demonstrate sufficient evidence to recommend the use of rFVIIa in any of these indications.
Table 1.
Summary of prophylactic randomised controlled trials using off-label rFVIIa
| Category | Study | Participants | Number of patients (rFVIIa/C) | Dosing | Primary outcome | Primary results |
|---|---|---|---|---|---|---|
| Cardiac surgery | Diprose et al. (2005) | Complex non-coronary cardiac surgery requiring CPB | 20 (10/10) | 1 dose 90 mcg kg−1 or placebo after CPB | Number of patients receiving allogeneic transfusion (total); total units of RBC and coagulation products; occurrence of AE | Number of patients transfused: rFVIIa 2 vs C 8 patients (P = 0·037) |
| Units transfused: rFVIIa 13 vs C 105 units (P = 0·011) | ||||||
| No difference in AE | ||||||
| Ekert et al. (2006) | Infants <1 year with congenital heart disease undergoing cardiac surgery requiring CPB | 82 (36/40) | 40 mcg kg−1 or placebo after CPB; repeated up to two times if ongoing bleeding | Time to chest closure after reversal of heparin | rFVIIa 99 min vs C 55 min (P = 0·0263) | |
| Ma et al. (2006) | Cardiac valve replacement surgery requiring CPB | 22 (11/11) | 1 dose 40 mcg kg−1 or placebo after CPB | No stated primary outcome | No stated primary outcome but reported decreased blood loss and transfusion requirements in rFVIIa group | |
| Essam (2007) | Elective cardiac revascularisation requiring CPB | 30 (15/15) | 1 dose 90 mcg kg−1 after CPB (control: no rFVIIa) | No stated primary outcome | No stated primary outcome but reported decreased blood loss and transfusion requirements in rFVIIa group | |
| Gill et al. (2009) | Cardiac surgery requiring CPB and admitted to post-operative care environment for at least 30 min | 179 (104/68) | A single dose 40, 80 mcg kg−1 or placebo on reaching pre-defined bleeding trigger | Critical serious AE at 30 days | No difference: rFVIIa 80 mcg kg−1, 12% vs 40 mcg kg−1, 14% vs placebo 7% | |
| Hepatic procedures | Lodge et al. (2005a, b) | Liver transplantation | 209 (121/61) | First dose 60, 120 mcg kg−1 or placebo; repeated every 2 h until end of surgery | Units of RBC transfused during the perioperative period (24 h) | No difference: rFVIIa 60 mcg kg−1, 7·0 vs 120 mcg kg−1, 6·3 vs C 8·2 units |
| Planinsic et al. (2005) | Liver transplantation | 87 (54/19) | A single dose 20, 40, 80 mcg kg−1 or placebo | Units of RBC transfused during the perioperative period (24 h) | No difference: rFVIIa 20 mcg kg−1, 10·0 vs 40 mcg kg−1, 13·0 vs 80 mcg kg−1, 10·0 vs C 11·1 units | |
| Pugliese et al. (2007) | Liver transplantation | 20 (10/10) | 1 dose 40 mcg kg−1 or placebo | No stated primary outcome | No stated primary outcome but reported decreased blood loss and transfusion requirements in rFVIIa group | |
| Lodge et al. (2005a, b) | Non-cirrhotic patients undergoing partial hepatectomy | 204 (112/63) | First dose 20, 80 mcg kg−1 or placebo; second dose at 5 h if operation expected to be longer than 6 h | Number of patients receiving allogeneic transfusion during perioperative period (48 h) | No difference: rFVIIa 20 mcg kg−1, 41% vs 80 mcg kg−1, 25% vs C 37% (P = 0·09) | |
| Shao et al. (2006) | Cirrhotic patients undergoing partial hepatectomy | 235 (145/76) | First dose of 50, 100 mcg kg−1 or placebo; repeated every 2 h until end of surgery (maximum four doses) | Number of patients receiving allogeneic transfusion and units of RBC transfused during perioperative period (48 h) | No difference in either outcome. Patients transfused: rFVIIa 50 mcg kg−1, 51% vs 100 mcg kg−1, 36% vs C 38% (P = 0·59) | |
| Units transfused: rFVIIa 50 mcg kg−1, 0·9 vs 100 mcg kg−1, 0 vs C 0 units (P = 0·68) | ||||||
| Jeffers et al. (2002) | Cirrhosis and coagulopathy undergoing laparoscopic liver biopsy | 66 (66/0) | A single dose 5, 20, 80 or 120 mcg kg−1 (no control group) | Duration of normal PT | Longer duration of normal PT with higher doses of rFVIIa (80, 120 mcg kg−1) vs lower doses (5, 20 mcg kg−1) | |
| Other surgical settings | Friederich et al. (2003) | Retropubic prostatectomy | 36 (24/12) | A single dose 20, 40 mcg kg−1 or placebo | Blood loss during perioperative period (24 h) and units of RBC transfused | Blood loss: rFVIIa 20 mcg kg−1, 1235 mL vs 40 mcg kg−1, 1089 mL vs C 2688 mL (P = 0·001) |
| Units transfused: rFVIIa 20 mcg kg−1, 0·6 vs 40 mcg kg−1, 0 vs C 1·5 units (P = 0·0003) | ||||||
| Raobaikady et al. (2005) | Reconstructive surgery for traumatic pelvic fractures | 48 (24/24) | First dose 90 mcg kg−1 or placebo; second dose at 2 h if ongoing bleeding | Blood loss during perioperative period (48 h) | No difference: rFVIIa 2070 mL vs C 1535 mL (P = 0·79) | |
| Johansson et al. (2007) | Thermal burns undergoing skin excision and grafting | 18 (9/9) | First dose 40 mcg kg−1 or placebo; second dose at 90 min | Units of blood components transfused per patient and percentage full thickness wound in perioperative period (24 h) | rFVIIa 0·9 vs C 2·2 units (P = 0·0013) | |
| Sachs et al. (2007) | Spinal fusion surgery | 60 (36/13) | First dose 30, 60, 120 mcg kg−1 or placebo; given at dosing trigger and repeated at 2 and 4 h | AE at 30 days and blood loss | No difference in AE and mean surgical blood loss. However, a priori planned adjusted blood loss: rFVIIa 30 mcg kg−1, 1120 mL vs 60 mcg kg−1, 400 mL vs 120 mcg kg−1, 823 mL vs C 2536 mL (P≤ 0·001) | |
| Hanna et al. (2010) | Children undergoing craniofacial reconstruction surgery | 45 (15/15; 15 received tranexamic acid) | 100 mcg kg−1 bolus followed by 10 mcg kg−1 h−1 infusion until skin closure or placebo | No stated primary outcome | No stated primary outcome but reported decreased blood loss and transfusion requirements in rFVIIa group |
AE, adverse events; C, control; CPB, cardiopulmonary bypass.
Table 2.
Summary of therapeutic randomised controlled trials using off-label rFVIIa
| Category | Study | Participants | Number of patients (rFVIIa/C) | Dosing | Primary outcome | Primary results |
|---|---|---|---|---|---|---|
| Trauma | Boffard et al. (2005) | Blunt trauma | 158 (69/74) | 200 mcg kg−1 followed by 100 mcg kg−1 at 1 and 3 h or placebo | Units of RBCs transfused within 48 h of first dose of rFVIIa | No difference: rFVIIa 7·8 vs C 7·2 units (P = 0·07) |
| When only patients alive at 48 h included: rFVIIa 7·0 vs C 7·5 units with an estimated RBC reduction of 2·6 units favouring rFVIIa (P = 0·02) | ||||||
| Boffard et al. (2005) | Penetrating trauma | 143 (70/64) | 200 mcg kg−1 followed by 100 mcg kg−1 at 1 and 3 h or placebo | Units of RBCs transfused within 48 h of first dose of rFVIIa | No difference: rFVIIa 4·0 vs C 4·8 units (P = 0·24) | |
| No difference for only patients alive at 48 h | ||||||
| Hauser et al. (2010) | Blunt trauma | 481 (226/255) | 200 mcg kg−1 followed by 100 mcg kg−1 at 1 and 3 h or placebo | 30-day mortality | Terminated early | |
| No difference: rFVIIa 11·0% vs C 10·7% (P = 0·93) | ||||||
| Hauser et al. (2010) | Penetrating trauma | 92 (47/45) | 200 mcg kg−1 followed by 100 mcg kg−1 at 1 and 3 h or placebo | 30-day mortality | Terminated early | |
| No difference: rFVIIa 18·2% vs C 13·2% (P = 0·40) | ||||||
| ICH | Mayer et al. (2005a, b) | Spontaneous ICH | 48 (36/12) | A single dose 10, 20, 40, 80, 120, 160 mcg kg−1 or placebo | AE at 90 days | No difference in type, severity or frequency of AEs |
| Mayer et al. (2006) | Spontaneous ICH | 41 (32/8) | A single dose 5, 20, 40, 80 mcg kg−1 or placebo | AE at 90 days | No difference in type, severity or frequency of AEs | |
| Mayer et al. (2005a, b) | Spontaneous ICH | 400 (303/96) | A single dose 40, 80, 160 mcg kg−1 or placebo | Percent change in volume of ICH at 24 h | rFVIIa 40 mcg kg−1, 16% vs 80 mcg kg−1, 14% vs 160 mcg kg−1, 11% vs C 29% (P = 0·01 rFVIIa groups combined vs placebo) | |
| Secondary outcomes showed reduced mortality and improved functional outcomes at 90 days with non-significant increase in thromboembolic AE | ||||||
| Mayer et al. (2008) | Spontaneous ICH | 841 (558/263) | A single dose 20, 80 mcg kg−1 or placebo | Combined outcome of severe disability or death at 90 days | No difference: rFVIIa 20 mcg kg−1, 26% vs 80 mcg kg−1, 29% vs C 24% | |
| Increased frequency of arterial thromboembolic AE in the 80 mcg kg−1 group (9% vs C 4%, P = 0·04) | ||||||
| Narayan et al. (2008) | Traumatic ICH | 97 (61/36) | A single dose 40, 80, 120, 160, 200 mcg kg−1 or placebo | AE at 15 days | No difference in number or type of AE | |
| GI bleeding | Bosch et al. (2004) | Upper gastrointestinal haemorrhage in patients with cirrhosis | 245 (121/121) | First dose of 100 mcg kg−1 or placebo; repeated doses at 2, 4, 6, 12, 18, 24 and 30 h | Combined endpoint of failure to control bleeding or failure to prevent rebleeding or death | No difference: rFVIIa 14% vs C 16% (P = 0·72). Exploratory analyses of patients with Child Pugh class B or C showed benefit with rFVIIa |
| Bosch et al. (2008) | Upper gastrointestinal haemorrhage in patients with cirrhosis and severe liver disease (Child Pugh class B and C) | 265 (170/86) | First dose 200 mcg kg−1 or placebo; repeated at 2, 8, 14 and 20 h (600 mcg kg−1 total); or repeated only at 2 h (300 mcg kg−1) | Combined endpoint of failure to control bleeding or failure to prevent rebleeding or death | No difference: rFVIIa 600 mcg kg−1, 20% vs 300 mcg kg−1, 13% vs C 23% (P = 0·37) | |
| Other settings | Pihusch et al. (2005) | Bleeding post-haematopoietic stem cell transplantation | 100 (77/23) | First dose of 40, 80, 160 mcg kg−1 or placebo; repeated every 6 h × 6 | Change in bleeding score at 38 h compared with baseline | No difference |
| Chuansumrit et al. (2005) | Children with dengue haemorrhagic fever | 28 (18/10) | First dose 100 mcg kg−1 or placebo; second dose at 30 min if ongoing bleeding | Change in bleeding | Cessation of bleeding at 2 h: rFVIIa 75% vs C 44% (no statistics provided) |
AE, adverse events; C, control; GI, gastrointestinal; ICH, intracranial haemorrhage.
SUMMARY OF BENEFITS OF rFVIIa
The benefits of rFVIIa can be considered across the clinical trials that have been conducted. The recently updated Cochrane systematic review (Table 3) included RCTs of off-label rFVIIa and divided RCTs into prophylactic and therapeutic studies (Simpson et al., 2012). There were 16 prophylactic RCTs where rFVIIa was used to prevent bleeding (Jeffers et al., 2002; Friederich et al., 2003; Diprose et al., 2005; Lodge et al., 2005a,b; Planinsic et al., 2005; Raobaikady et al., 2005; Ekert et al., 2006; Ma et al., 2006; Shao et al., 2006; Essam, 2007; Johansson et al., 2007; Pugliese et al., 2007; Sachs et al., 2007; Gill et al., 2009; Hanna et al., 2010). All these studies were perioperative giving rFVIIa either before the procedure or at a distinct bleeding trigger in the perioperative setting (Sachs et al., 2007; Gill et al., 2009). Thirteen RCTs were considered therapeutic where rFVIIa was administered to bleeding patients (Bosch et al., 2004, 2008; Boffard et al., 2005; Chuansumrit et al., 2005; Mayer et al., 2005a, b, 2006, 2008; Pihusch et al., 2005; Narayan et al., 2008; Hauser et al., 2010). Each of the trauma publications were considered as two RCTs reporting results of a blunt and penetrating trauma population within a single publication (Boffard et al., 2005; Hauser et al., 2010). The results are summarised in Table 2. In the prophylactic studies, there was no difference in mortality. A non-significant trend was noted towards a decreased number of patients transfused with the use of rFVIIa. However, when volumes of total blood loss and RBCs transfused were considered, only modest differences (approximating one RBC unit) favouring rFVIIa were noted. It is important to emphasise that these results likely overestimate the effect of rFVIIa because (i) smaller studies that showed larger benefit were heavily weighted because of very precise estimation of blood loss and (ii) results from larger negative RCTs were not reported in such a manner (i.e. means and standard deviations or medians and interquartile ranges) so that they could be included in the pooled estimates. For therapeutic studies, there was no difference in mortality, control of bleeding or amount of red blood cells transfused. There was a trend to decreased number of patients transfused but this was based on a pooled estimate from only three RCTs. Although no significant increase in TE events was observed in the prophylactic or therapeutic RCTs, when TE events were combined across all 29 trials, there was a significant increase in arterial TE events (relative risk 1·45, 95% CI: 1·02 to 2·05). Overall, the review concluded that based on the available RCTs, there was little evidence of benefit for the use of off-label rFVIIa in patients without haemophilia.
Table 3.
Summary estimates from 2012 Cochrane systematic review on rFVIIa in patients without haemophilia
| Outcome | Number of studies | Number of patients | Summary estimate (95% CI) | I2 value (%) |
|---|---|---|---|---|
| Prophylactic use | ||||
| Mortality, RR | 15 | 1219 | 1·04 (0·55 to 1·97) | 0 |
| Blood loss1, mL, WMD | 10 | 707 | −297 (−416 to −177) | 79 |
| Red blood cell transfusion1, mL, WMD | 12 | 774 | −261 (−367 to −154) | 62 |
| Number of patients receiving transfusion, RR | 8 | 868 | 0·85 (0·72 to 1·01) | 57 |
| Thromboembolic events, RR | 13 | 1159 | 1·35 (0·82 to 2·25) | 0 |
| Therapeutic use | ||||
| Mortality, RR | 13 | 2856 | 0·91 (0·78 to 1·06) | 0 |
| Control of bleeding, RR | 4 | 616 | 0·95 (0·88 to 1·03) | 0 |
| Red blood cell transfusion1, mL, WMD | 5 | 911 | −89 (−264 to 87) | 16 |
| Number of patients receiving transfusion, RR | 3 | 579 | 0·94 (0·89 to 1·00) | 0 |
| Thromboembolic events, RR | 13 | 2873 | 1·14 (0·89 to 1·47) | 0 |
| All studies | ||||
| Total thromboembolic events, RR | 26 | 4032 | 1·18 (0·94 to 1·48) | 0 |
| Arterial thromboembolic events, RR | 25 | 3849 | 1·45 (1·02 to 2·05) | 0 |
| Venous thromboembolic events, RR | 25 | 3849 | 0·92 (0·67 to 1·26) | 0 |
CI, confidence interval; RR, relative risk; WMD, weighted mean difference.
One unit of red blood cells was assumed to have a volume of 300 mL
From the available RCT data, there is little evidence of a mortality benefit of off-label rFVIIa. No single trial showed a mortality benefit with the exception of the phase 2 RCT in ICH where mortality was a secondary outcome (Mayer et al., 2005a); the larger phase 3 RCT in ICH powered to detect a mortality difference was negative (Mayer et al., 2008). Other signals support this conclusion. Improved outcomes with higher doses of rFVIIa were not observed in either of the recent systematic reviews described above (Yank et al., 2011; Simpson et al., 2012). Follow-up larger RCTs did not reinforce earlier promising results specifically in the setting of trauma (Hauser et al., 2010), ICH (Mayer et al., 2008) and gastrointestinal bleeding in cirrhosis (Bosch et al., 2008). Even for the outcomes of blood loss and red cell transfusion requirements, the amount saved was modest and likely overestimated.
It is important to note, however, that the use of rFVIIa in the RCTs reported may differ from situations where rFVIIa may be used as a ‘last ditch’ effort or in what could be termed as ‘refractory’ bleeding. These are situations where its efficacy has not been formally assessed such as in the setting of massive bleeding in obstetrical bleeding or in the management of life-threatening bleeding associated with new oral anticoagulants such as direct thrombin inhibitors or factor Xa inhibitors. These patients with ‘refractory’ bleeding are not the patients included in the RCTs conducted to date where typically rFVIIa was used in anticipation of bleeding or at a defined point earlier in the resuscitation of a bleeding patient. Patients with refractory bleeding are more likely to be found in observational reports and registries where no well-matched control group is available for comparison, nor a group using an alternative intervention (e.g. tranexamic acid or fibrinogen concentrates). These observational reports are prone to patient selection bias and observer bias as the treatment is not masked and lack generalisability (Dzik, 2006). Interpretation of the effect of rFVIIa in refractory bleeding is further hampered by the complex coagulopathy that exists in these situations and the multiple blood components, blood products and potentially haemostatic drugs administered concurrently with rFVIIa. On the other hand, the haemostatic effect of rFVIIa may be negated by physiologic conditions such as hypothermia and acidosis (Meng et al., 2003), which have been shown to adversely affect rFVIIa's effect. Registry data have reinforced the lack of benefit of rFVIIa under acidotic conditions (Isbister et al., 2008; Karkouti et al., 2008; Phillips et al., 2011). A discussion of the management of massive bleeding is outside the scope of this review; however, a recent consensus conference statement on massive transfusion may provide additional guidance to hospitals on this challenging topic (Dzik et al., 2011).
RISKS OF rFVIIa
Because rFVIIa is a procoagulant agent, the primary concern about its toxicity is the risk of TE events. Both the Cochrane review (Simpson et al., 2012) and the review by Yank et al. (2011) reported increased risks of arterial TE events as described above. The most comprehensive review to date specifically examining the safety of off-label rFVIIa was conducted by Levi et al. (2010) on data from 26 RCTs involving patients. The overall rate of arterial TE events was higher with the use of rFVIIa compared with placebo (5·5 vs 3·2%, P = 0·003), specifically there was an increase observed in coronary events (2·9 vs 1·1%, P = 0·002). No difference was observed in venous TE events (5·3 vs 5·7%). A striking finding was the increase in risk of arterial TE events with increasing age. Compared with patients of <18 years of age, a higher risk of TE events were observed in patients aged 65–74 years (odds ratio 2·11; 95% CI: 0·95 to 4·71) and even higher for those ≥75 years (odds ratio 3·02, 95% CI: 1·22 to 7·48). Similar to the Yank et al. review, the risk of arterial TE events increased with dose in the setting of ICH.
The risks of TE events reported in clinical trials may underestimate the true risk of TE events with rFVIIa. The RCTs included in these reviews (i) did not actively screen for TE events, (ii) excluded patients who had a recent history of TE events and (iii) may have limitations in generalisability given that only a minority of patients who were screened were enrolled into the trials. Translating these risks to the real world was raised as a concern by O'Connell et al. (2006), who reported on the serious TE events associated with rFVIIa reported to the United States Food and Drug Administration (FDA) Adverse Event Reporting System. Three important observations were made: (i) 90% of reports occurred when rFVIIa was used outside of its labelled indication, (ii) 65% of the reports occurred in patients outside of clinical trials and (iii) 38% of reports occurred in the setting of concomitant haemostatic agents, including blood products (plasma, platelets and cryoprecipitate) and antifibrinolytics. Although these findings are limited by passive surveillance, these observations do suggest that the risk of thromboembolism reported in RCTs may underestimate risks in the real world where sicker patients are treated with off-label rFVIIa in situations where other procoagulant blood products or drugs are also being used.
NAC RECOMMENDATIONS ON THE USE OF OFF-LABEL rFVIIa
Given the absence of evidence of benefit and with evidence of the risk of harm, the NAC recommends that rFVIIa no longer be used for the off-label indications of prevention and treatment of bleeding in patients without haemophilia. The NAC recognises that ongoing clinical trials are evaluating rFVIIa and that this recommendation will be reconsidered if favourable findings of clinically important benefits outweighing the risks are observed.
CONCLUSION
Current available evidence does not support the use of off-label rFVIIa for massive bleeding. Mortality benefits have not been demonstrated in randomised controlled trials. Other benefits such as reduced transfusion or blood loss are modest and may be overestimated. Contrarily, an increase in arterial TE events has been observed. Given the absence of evidence of benefit and with evidence of the risk of harm, the NAC recommends that rFVIIa no longer be used for the off-label indications of prevention and treatment of bleeding in patients without haemophilia.
Acknowledgments
The Canadian National Advisory Committee on Blood and Blood Products provided funding for the writing of this report. The authors wish to acknowledge Carolyn Doree (Systematic Review Initiative, NHS Blood and Transplant, Oxford, UK) for performing the literature search. Y. L. conducted the review and drafted the manuscript. Y. L., C. M. and D. A. interpreted the data, revised the manuscript critically and approved the submitted and final versions.
APPENDIX
The members of the National Advisory Committee on Blood and Blood Products are Lucinda Whitman (Chair, Newfoundland), David Anderson (Nova Scotia), Ted Alport (Saskatchewan), Jeannie Callum (Ontario), Karen Dallas (Saskatchewan), Dana Devine (Canadian Blood Services), Cheryl Doncaster (NAC), Jennifer Fesser (Prince Edward Island), David Howe (Canadian Blood Services), Elenore Kingsbury (Canadian Blood Services), Debra Lane (Manitoba), Vincent Laroche (Québec), Doug Morrison (British Columbia), Brian Muirhead (Manitoba), Susan Nahirniak (Alberta), Katerina Pavenski (Ontario), Tanya Petraszko (British Columbia), Lakshmi Rajappannair (New Brunswick) and Meer-Taher Shabani-Rad (Alberta).
CONFLICT OF INTEREST
Y. L. is a site investigator for a registry on the off-label use of rFVIIa that is funded by an unrestricted educational grant from Novo Nordisk. No competing interests declared by C. M. and D. A.
REFERENCES
- Boffard KD, Riou B, Warren B, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. The Journal of Trauma. 2005;59:8–15. doi: 10.1097/01.ta.0000171453.37949.b7. discussion 15-8. [DOI] [PubMed] [Google Scholar]
- Bosch J, Thabut D, Albillos A, et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized, controlled trial. Hepatology (Baltimore, Md.) 2008;47:1604–1614. doi: 10.1002/hep.22216. [DOI] [PubMed] [Google Scholar]
- Bosch J, Thabut D, Bendtsen F, et al. Recombinant factor VIIa for upper gastrointestinal bleeding in patients with cirrhosis: a randomized, double-blind trial. Gastroenterology. 2004;127:1123–1130. doi: 10.1053/j.gastro.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Cameron P, Phillips L, Balogh Z, Joseph A, Pearce A, Parr M, Jankelowitz G. The use of recombinant activated factor VII in trauma patients: experience from the Australian and New Zealand haemostasis registry. Injury. 2007;38:1030–1038. doi: 10.1016/j.injury.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Center for Biologics Evaluation and Research, US Food and Drug Administration. 1999. Novoseven Product Information [WWW document]. URL http://www.fda.gov/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/ucm089228.htm (Accessed 10/01/12)
- Chuansumrit A, Wangruangsatid S, Lektrakul Y, Chua MN, Zeta Capeding MR, Bech OM, Dengue Study Group Control of bleeding in children with dengue hemorrhagic fever using recombinant activated factor VII: a randomized, double-blind, placebo-controlled study. Blood Coagulation & Fibrinolysis: An International Journal in Haemostasis and Thrombosis. 2005;16:549–555. doi: 10.1097/01.mbc.0000186837.78432.2f. [DOI] [PubMed] [Google Scholar]
- Diprose P, Herbertson MJ, O'Shaughnessy D, Gill RS. Activated recombinant factor VII after cardiopulmonary bypass reduces allogeneic transfusion in complex non-coronary cardiac surgery: randomized double-blind placebo-controlled pilot study. British Journal of Anaesthesia. 2005;95:596–602. doi: 10.1093/bja/aei244. [DOI] [PubMed] [Google Scholar]
- Dzik WH. Off-label reports of new biologics: exciting new therapy or dubious research? Examples from recombinant activated factor VII. Journal of Intensive Care Medicine. 2006;21:54–59. doi: 10.1177/0885066605285223. [DOI] [PubMed] [Google Scholar]
- Dzik WH, Blajchman MA, Fergusson D, et al. Clinical review: Canadian National Advisory Committee on Blood and Blood Products – Massive Transfusion Consensus Conference 2011: Report of the panel. Critical Care. 2011;15:242. doi: 10.1186/cc10498. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekert H, Brizard C, Eyers R, Cochrane A, Henning R. Elective administration in infants of low-dose recombinant activated factor VII (rFVIIa) in cardiopulmonary bypass surgery for congenital heart disease does not shorten time to chest closure or reduce blood loss and need for transfusions: a randomized, double-blind, parallel group, placebo-controlled study of rFVIIa and standard haemostatic replacement therapy versus standard haemostatic replacement therapy. Blood Coagulation & Fibrinolysis: An International Journal in Haemostasis and Thrombosis. 2006;17:389–395. doi: 10.1097/01.mbc.0000233369.03358.c1. [DOI] [PubMed] [Google Scholar]
- Essam MA. Prophylactic administration of recombinant activated factor VII in coronary revascularization surgery. Internet Journal of Anesthesiology. 2007;13:10. doi: 10.4103/1658-354X.115364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty ML, Jauch EC. 2011. The Spot Sign for Predicting and Treating ICH Growth Study (STOP-IT) [WWW document]. URL http://www.clinicaltrials.gov/ct2/show/NCT00810888 (Accessed 10/01/12)
- Friederich PW, Henny CP, Messelink EJ, Geerdink MG, Keller T, Kurth KH, Buller HR, Levi M. Effect of recombinant activated factor VII on perioperative blood loss in patients undergoing retropubic prostatectomy: a double-blind placebo-controlled randomised trial. Lancet. 2003;361:201–205. doi: 10.1016/S0140-6736(03)12268-4. [DOI] [PubMed] [Google Scholar]
- Gill R, Herbertson M, Vuylsteke A, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–27. doi: 10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Aviv RI, Demchuk AM. 2011. “Spot sign” selection of intracerebral hemorrhage to guide hemostatic therapy (SPOTLIGHT) [WWW document]. URL http://www.clinicaltrials.gov/ct2/show/NCT01359202 (Accessed 10/01/12)
- Hanna MG, Refaie A, Gouda N, Obaya G. Reduction of peri-operative bleeding in craniofacial surgeries in pediatrics: comparison between recombinant factor VII and tranexamic acid. Egyptian Journal of Anaesthesia. 2010;26:53–61. [Google Scholar]
- Hauser CJ, Boffard K, Dutton R, et al. Results of the CONTROL trial: efficacy and safety of recombinant activated factor VII in the management of refractory traumatic hemorrhage. The Journal of Trauma. 2010;69:489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- Health Canada. 2005. Notice of Compliance with Conditions (NOC/c) [WWW document]. URL http://www.hc-sc.gc.ca/dhp-mps/prodpharma/notices-avis/conditions/index-eng.php (Accessed 10/01/12)
- Isbister J, Phillips L, Dunkley S, Jankelowitz G, McNeil J, Cameron P. Recombinant activated factor VII in critical bleeding: experience from the Australian and New Zealand haemostasis register. Internal Medicine Journal. 2008;38:156–165. doi: 10.1111/j.1445-5994.2007.01472.x. [DOI] [PubMed] [Google Scholar]
- Jeffers L, Chalasani N, Balart L, Pyrsopoulos N, Erhardtsen E. Safety and efficacy of recombinant factor VIIa in patients with liver disease undergoing laparoscopic liver biopsy. Gastroenterology. 2002;123:118–126. doi: 10.1053/gast.2002.34164. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Eriksen K, Nielsen SL, Rojkjaer R, Alsbjorn B. Recombinant FVIIa decreases perioperative blood transfusion requirement in burn patients undergoing excision and skin grafting – results of a single centre pilot study. Burns : Journal of the International Society for Burn Injuries. 2007;33:435–440. doi: 10.1016/j.burns.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Karkouti K, Beattie WS, Arellano R, et al. Comprehensive Canadian review of the off-label use of recombinant activated factor VII in cardiac surgery. Circulation. 2008;118:331–338. doi: 10.1161/CIRCULATIONAHA.108.764308. [DOI] [PubMed] [Google Scholar]
- Kenet G, Walden R, Eldad A, Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354:1879. doi: 10.1016/S0140-6736(99)05155-7. [DOI] [PubMed] [Google Scholar]
- Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. The New England Journal of Medicine. 2010;363:1791–1800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- Lodge JP, Jonas S, Jones RM, et al. Efficacy and safety of repeated perioperative doses of recombinant factor VIIa in liver transplantation. Liver Transplantation: Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005a;11:973–979. doi: 10.1002/lt.20470. [DOI] [PubMed] [Google Scholar]
- Lodge JP, Jonas S, Oussoultzoglou E, et al. Recombinant coagulation factor VIIa in major liver resection: a randomized, placebo-controlled, double-blind clinical trial. Anesthesiology. 2005b;102:269–275. doi: 10.1097/00000542-200502000-00006. [DOI] [PubMed] [Google Scholar]
- Logan AC, Yank V, Stafford RS. Off-label use of recombinant factor VIIa in U.S. hospitals: analysis of hospital records. Annals of Internal Medicine. 2011;154:516–522. doi: 10.7326/0003-4819-154-8-201104190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Wang ZN, Zhang BR, Xu ZY, Yang LX, Chen KB, Li J. Effect of recombinant activated factor VIIa on early recovery of patients undergoing cardiac valve replacement under cardiopulmonary bypass: a randomized double-blind placebo-controlled trial. Academic Journal of Second Military Medical University. 2006;27:1110–1113. [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, et al. Recombinant activated factor VII for acute intracerebral hemorrhage. The New England Journal of Medicine. 2005a;352:777–785. doi: 10.1056/NEJMoa042991. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Broderick J, Davis S, Diringer MN, Skolnick BE, Steiner T, Europe/AustralAsia NovoSeven ICH Trial Investigators Safety and feasibility of recombinant factor VIIa for acute intracerebral hemorrhage. Stroke: A Journal of Cerebral Circulation. 2005b;36:74–79. doi: 10.1161/01.STR.0000149628.80251.b8. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. The New England Journal of Medicine. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- Mayer SA, Brun NC, Broderick J, Davis SM, Diringer MN, Skolnick BE, Steiner T, United States NovoSeven ICH Trial Investigators Recombinant activated factor VII for acute intracerebral hemorrhage: US phase IIA trial. Neurocritical Care. 2006;4:206–214. doi: 10.1385/NCC:4:3:206. [DOI] [PubMed] [Google Scholar]
- Meng ZH, Wolberg AS, Monroe DM, 3rd, Hoffman M. The effect of temperature and pH on the activity of factor VIIa: implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. The Journal of Trauma. 2003;55:886–891. doi: 10.1097/01.TA.0000066184.20808.A5. [DOI] [PubMed] [Google Scholar]
- Moltzan CJ, Anderson DA, Callum J, et al. The evidence for the use of recombinant factor VIIa in massive bleeding: development of a transfusion policy framework. Transfusion Medicine (Oxford, England) 2008;18:112–120. doi: 10.1111/j.1365-3148.2008.00846.x. [DOI] [PubMed] [Google Scholar]
- Narayan RK, Maas AI, Marshall LF, Servadei F, Skolnick BE, Tillinger MN, rFVIIa Traumatic ICH Study Group Recombinant factor VIIA in traumatic intracerebral hemorrhage: results of a dose-escalation clinical trial. Neurosurgery. 2008;62:776–786. doi: 10.1227/01.neu.0000316898.78371.74. discussion 786-8. [DOI] [PubMed] [Google Scholar]
- O'Connell KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of recombinant human coagulation factor VIIa. JAMA: The Journal of the American Medical Association. 2006;295:293–298. doi: 10.1001/jama.295.3.293. [DOI] [PubMed] [Google Scholar]
- Phillips LE, Zatta A, Kandane-Rathnayake R, Aoki N, on behalf of the Haemostasis Registry Steering Committee 2011. Haemostasis registry final report: ten years of data on the use of recombinant activated factor VII in Australia and New Zealand Monash University.
- Pihusch M, Bacigalupo A, Szer J, von Depka Prondzinski M, Gaspar-Blaudschun B, Hyveled L, Brenner B, F7BMT-1360 Trial Investigators Recombinant activated factor VII in treatment of bleeding complications following hematopoietic stem cell transplantation. Journal of Thrombosis and Haemostasis: JTH. 2005;3:1935–1944. doi: 10.1111/j.1538-7836.2005.01523.x. [DOI] [PubMed] [Google Scholar]
- Planinsic RM, van der Meer J, Testa G, et al. Safety and efficacy of a single bolus administration of recombinant factor VIIa in liver transplantation due to chronic liver disease. Liver Transplantation: Official Publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2005;11:895–900. doi: 10.1002/lt.20458. [DOI] [PubMed] [Google Scholar]
- Pugliese F, Ruberto F, Summonti D, et al. Activated recombinant factor VII in orthotopic liver transplantation. Transplantation Proceedings. 2007;39:1883–1885. doi: 10.1016/j.transproceed.2007.05.062. [DOI] [PubMed] [Google Scholar]
- Raobaikady R, Redman J, Ball JA, Maloney G, Grounds RM. Use of activated recombinant coagulation factor VII in patients undergoing reconstruction surgery for traumatic fracture of pelvis or pelvis and acetabulum: a double-blind, randomized, placebo-controlled trial. British Journal of Anaesthesia. 2005;94:586–591. doi: 10.1093/bja/aei102. [DOI] [PubMed] [Google Scholar]
- Sachs B, Delacy D, Green J, et al. Recombinant activated factor VII in spinal surgery: a multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial. Spine. 2007;32:2285–2293. doi: 10.1097/BRS.0b013e3181557d45. [DOI] [PubMed] [Google Scholar]
- Shao YF, Yang JM, Chau GY, Sirivatanauksorn Y, Zhong SX, Erhardtsen E, Nivatvongs S, Lee PH. Safety and hemostatic effect of recombinant activated factor VII in cirrhotic patients undergoing partial hepatectomy: a multicenter, randomized, double-blind, placebo-controlled trial. American Journal of Surgery. 2006;191:245–249. doi: 10.1016/j.amjsurg.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Simpson E, Lin Y, Stanworth S, Birchall J, Doree C, Hyde C. 2012. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database of Systematic Reviews, Issue 3. Art. No.: CD005011. [DOI] [PubMed]
- Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Annals of Internal Medicine. 2011;154:529–540. doi: 10.7326/0003-4819-154-8-201104190-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]


