Abstract
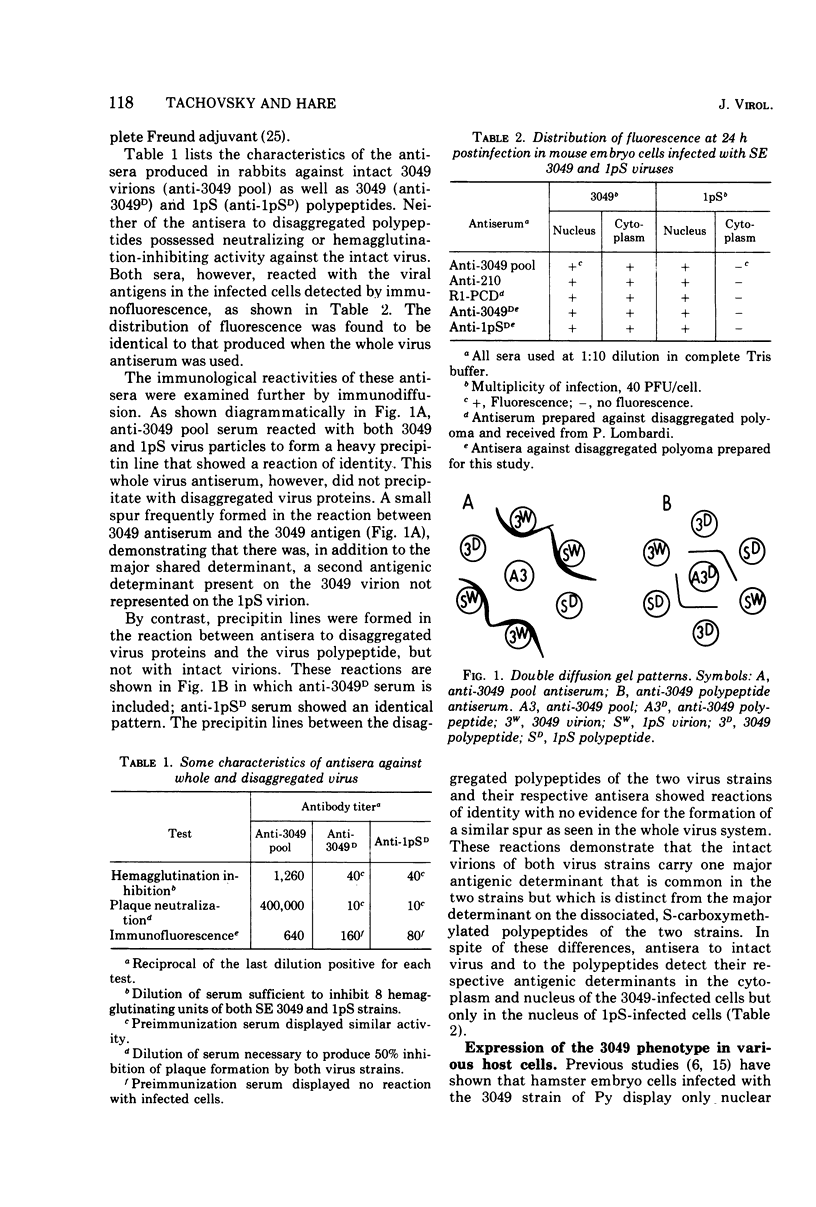
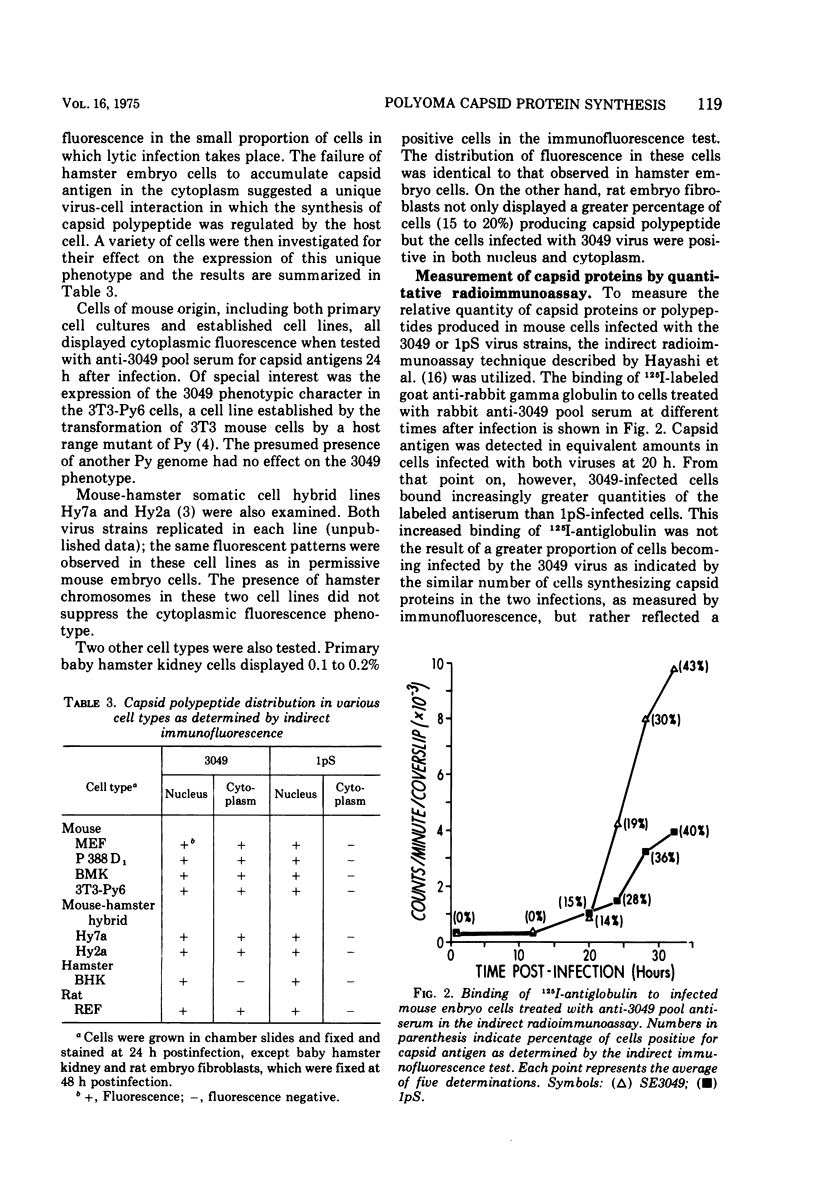
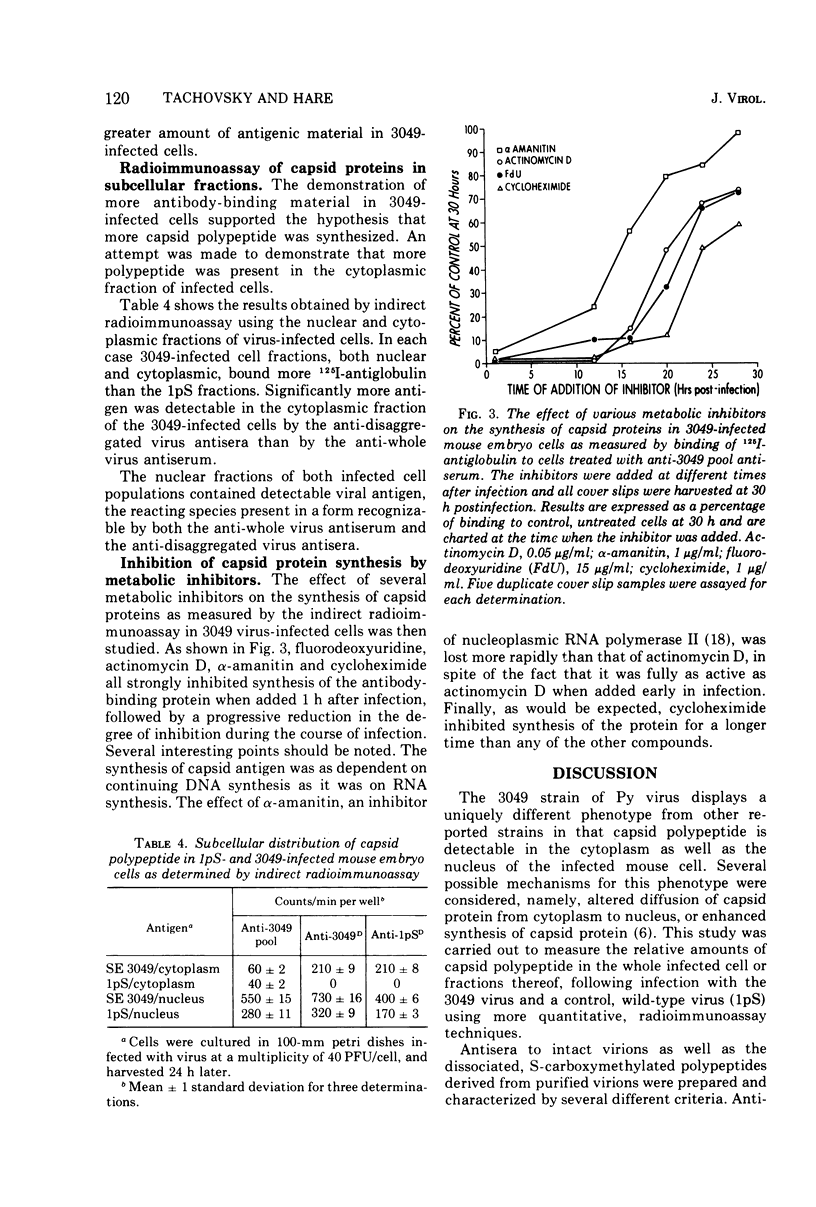
A study of the immunochemical characteristics and the synthesis of the capsid proteins of two polyoma virus strains (3049 and 1pS) was carried out to determine the mechanism responsible for the unique accumulation of those structural polypeptides in the cytoplasm of cells infected with the 3049 strain. Antisera prepared against disaggregated virus peptides and whole virus were used to measure the quantity of virus-specific antigens in cells infected by the two strains by using an indirect radioimmunoassay technique. The 3049-infected mouse embryo cells were found to contain several-fold more antibody-binding material than those infected with the 1pS strain. Furthermore, the cytoplasmic fraction of 3049-infected cells also contained more antibody-binding activity, supporting the hypothesis that the phenotype of the 3049 virus (cytoplasmic capsid protein) was a reflection of the increased synthesis of the capsid polypeptides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P. A working model for oncogenic DNA virus replication. J Theor Biol. 1974 Jul;46(1):221–227. doi: 10.1016/0022-5193(74)90148-9. [DOI] [PubMed] [Google Scholar]
- BERECZKY E., HUGHES R., BOWEN J. M., MUNYON W., DMOCHOWSKI L. STUDY OF DNA SYNTHESIS AND ANTIGEN FORMATION IN POLYOMA VIRUS-INFECTED MOUSE EMBRYO CELLS BY AUTORADIOGRAPHY AND IMMUNOFLUORESCENCE. Tex Rep Biol Med. 1965;23:3–15. [PubMed] [Google Scholar]
- Bale W. F., Helmkamp R. W., Davis T. P., Izzo M. J., Goodland R. L., Contreras M. A., Spar I. L. High specific activity labeling of protein with I-131 by the iodine monochloride method. Proc Soc Exp Biol Med. 1966 Jun;122(2):407–414. doi: 10.3181/00379727-122-31148. [DOI] [PubMed] [Google Scholar]
- Basilico C., Matsuya Y., Green H. The interaction of polyoma virus with mouse-hamster somatic hybrid cells. Virology. 1970 Jun;41(2):295–305. doi: 10.1016/0042-6822(70)90082-6. [DOI] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts R. F., Tachovsky T. G., Hare J. D. Studies on the mechanism of cytoplasmic antigen accumulation following infection with a new variant of polyoma virus. J Gen Virol. 1972 Jul;16(1):29–38. doi: 10.1099/0022-1317-16-1-29. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V. The adsorption of polyoma virus. Virology. 1962 Oct;18:177–181. doi: 10.1016/0042-6822(62)90003-x. [DOI] [PubMed] [Google Scholar]
- DIAMOND L., CRAWFORD L. V. SOME CHARACTERISTICS OF LARGE-PLAQUE AND SMALL-PLAQUE LINES OF POLYOMA VIRUS. Virology. 1964 Feb;22:235–244. doi: 10.1016/0042-6822(64)90008-x. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Glover D. M. Coupling of polyoma DNA and RNA synthesis. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1137–1143. doi: 10.1016/0006-291x(74)90815-8. [DOI] [PubMed] [Google Scholar]
- HARE J. D., BALDUZZI P., MORGAN H. R. Polyoma virus and L cell relationship. I. Some characteristics of a cell line persistently infected with polyoma virus. J Natl Cancer Inst. 1963 Jan;30:45–56. [PubMed] [Google Scholar]
- HENLE G., DEINHARDT F., RODRIGUEZ J. The development of polyoma virus in mouse embryo cells as revealed by fluorescent antibody staining. Virology. 1959 Jul;8(3):388–391. doi: 10.1016/0042-6822(59)90040-6. [DOI] [PubMed] [Google Scholar]
- Hare J. D. A new type of variation among the polyoma viruses characterized by cytoplasmic accumulation of capsid antigen. Virology. 1970 Apr;40(4):978–988. doi: 10.1016/0042-6822(70)90144-3. [DOI] [PubMed] [Google Scholar]
- Hare J. D., Chan J. C. Antigenic variation among polyoma viruses from different sources demonstrated by plaque neutralization. Virology. 1966 Sep;30(1):62–73. doi: 10.1016/s0042-6822(66)81010-3. [DOI] [PubMed] [Google Scholar]
- Hare J. D. Location and characteristics of the phenylalanine transport mechanism in normal and polyoma-transformed hamster cells. Cancer Res. 1967 Dec;27(12):2357–2363. [PubMed] [Google Scholar]
- Hare J. D. Transplant immunity to polyoma virus-induced tumor cells. IV. A polyoma strain defective in transplant antigen induction. Virology. 1967 Apr;31(4):625–632. doi: 10.1016/0042-6822(67)90191-2. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Rosenthal J., Notkins A. L. Iodine-125-labeled antibody to viral antigens: binding to the surface of virus-infected cells. Science. 1972 May 5;176(4034):516–518. doi: 10.1126/science.176.4034.516. [DOI] [PubMed] [Google Scholar]
- Jorgensen P. E. Freund's adjuvants: their influence on the specificity of viral antisera. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):931–933. [PubMed] [Google Scholar]
- Lindell T. J., Weinberg F., Morris P. W., Roeder R. G., Rutter W. J. Specific inhibition of nuclear RNA polymerase II by alpha-amanitin. Science. 1970 Oct 23;170(3956):447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- MALMGREN R. A., RABOTTI G., RABSON A. S. Intracellular localization of polyoma virus antigen demonstrated with fluorescein-labeled antiserums. J Natl Cancer Inst. 1960 Mar;24:581–587. doi: 10.1093/jnci/24.3.581. [DOI] [PubMed] [Google Scholar]
- Roblin R., Härle E., Dulbecco R. Polyoma virus proteins. 1. Multiple virion components. Virology. 1971 Sep;45(3):555–566. doi: 10.1016/0042-6822(71)90171-1. [DOI] [PubMed] [Google Scholar]
- Rutherford R. B., Hare J. D. Evidence for a regulatory function related to the expression of the polyoma genome following the onset of virus DNA replication. Biochem Biophys Res Commun. 1974 Jun 4;58(3):839–846. doi: 10.1016/s0006-291x(74)80493-6. [DOI] [PubMed] [Google Scholar]
- SACHS L., FOGEL M. Polyoma virus synthesis in tumor cells as measured by the fluorescent antibody technique. Virology. 1960 Aug;11:722–736. doi: 10.1016/0042-6822(60)90116-1. [DOI] [PubMed] [Google Scholar]
- WILLIAMS M. G., SHEININ R. Cytological studies of mouse embryo cells infected with polyoma virus, using acridine orange and fluorescent antibody. Virology. 1961 Mar;13:368–370. doi: 10.1016/0042-6822(61)90158-1. [DOI] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]



