SUMMARY
The purpose of this study was to evaluate the correlation between severity of obstructive sleep apnoea syndrome (OSAS), cardiovascular disease and metabolic syndrome. We recruited 1185 patients with OSAS who underwent a complete ENT examination, including nasolaryngeal fibre optic endoscopy with Müller's manoeuvre, overnight cardio-respiratory monitoring, Epworth Sleepiness Scale (ESS) to measure daytime sleepiness, body mass index (BMI), measurement of blood pressure and blood tests.
Subsequently, subjects were divided into three subgroups according to the Apnoea Hypopnoea Index (AHI): mild OSAS (AHI 5-15), moderate OSAS (AHI 15-30) and severe OSAS (AHI > 30). In the sample collected, 347 (262 males and 85 females) of 1185 patients suffered from mild OSAS, 363 (269 males and 94 females) from moderate OSAS and 475 (330 males and 145 females) from severe OSAS. In the group suffering from mild OSAS, we found: 127 patients affected by hypertension, 48 with diabetes, 11 with dyslipidaemia and 32 with metabolic syndrome. In the group with moderate OSAS there were 157 patients with hypertension, 63 with diabetes, 72 with dyslipidaemia and 47 with metabolic syndrome. In the group suffering from severe OSAS there were 244 patients with hypertension, 138 with diabetes, 47 with dyslipidaemia and 90 with metabolic syndrome. For data analysis, we used the Spearman correlation test adjusted according to Sidak between the dependent variable AHI and the independent variables BMI, ESS, average SO2 (SO2med), hypertension, diabetes mellitus, dyslipidaemia and metabolic syndrome. The results show different patterns of correlation in terms of statistical significance: BMI ρs = 0.26, SO2med ρs = -0:51, hypertension ρs = -0.05, dyslipidaemia ρs = 0.22 for women, and BMI ρs = 0.53, ESS ρs = 0.28, SO2med ρs = -0.50, hypertension ρs = 0.17, diabetes mellitus ρs = 0.28 and metabolic syndrome ρs = 0.26 for men. The results of the study confirm the existence of a statistically significant correlation between the severity of OSAS and BMI, ESS, average SO2, hypertension, diabetes mellitus, dyslipidaemia and metabolic syndrome.
KEY WORDS: Obstructive sleep apnoea syndrome, Metabolic syndrome, Arterial hypertension, Diabetes mellitus, Insulin resistance
RIASSUNTO
Lo scopo dello studio è stato valutare la correlazione tra gravità della sindrome delle apnee ostruttive del sonno (OSAS), patologia cardiovascolare e sindrome metabolica. Sono stati reclutati 1185 pazienti affetti da OSAS e sottoposti a visita otorinolaringoiatrica, scala di Epworth per la sonnolenza diurna (ESS), nasolaringofibroscopia con manovra di Mueller, monitoraggio cardio-respiratorio notturno, calcolo dell'indice di massa corporeo (BMI), misurazione della pressione arteriosa ed esami ematochimici. Successivamente la casistica è stata suddivisa in tre sottogruppi in base all' Apnea Hypopnea Index (AHI): OSAS lieve (AHI 5-15),OSAS moderato (AHI 15-30),OSAS severo (AHI > 30). Dei 1185 pazienti, 347 sono risultati affetti da OSAS di grado lieve, di cui 262 maschi e 85 femmine, 363 da OSAS di grado moderato, di cui 269 maschi e 94 femmine e 475 da OSAS di grado severo, di cui 330 maschi e 145 femmine. Nel gruppo affetto da OSAS lieve abbiamo riscontrato:127 pazienti ipertesi, 48 pazienti diabetici, 11 pazienti con dislipidemia e 32 pazienti affetti da sindrome metabolica. In quello con OSAS moderato: 157 pazienti ipertesi, 63 pazienti diabetici, 72 pazienti dislipidemici, 47 pazienti affetti da sindrome metabolica. Infine nel gruppo affetto da OSAS severo: 244 pazienti ipertesi, 138 pazienti diabetici, 47 pazienti dislipidemici e 90 pazienti affetti da sindrome metabolica. Per l'analisi dei dati è stato utilizzato il test di correlazione di Spearman adattato secondo Sidak tra la variabile dipendente AHI e le variabili indipendenti BMI, ESS, SO2 media, ipertensione arteriosa, diabete mellito, dislipidemia e sindrome metabolica. I risultati mostrano differenti pattern di correlazione in termini di significatività statistica: BMI ρs = 0,26, SO2med ρs = -0,51, ipertensione arteriosa ρs = -0,05, dislipidemia ρs = 0,22 per le donne, e BMI ρs = 0,53, ESS ρs = 0,28, SO2med ρs = -0,50, ipertensione arteriosa ρs = 0,17, diabete mellito ρs = 0,28 e sindrome metabolica ρs = 0,26 per gli uomini. I risultati dello studio confermano l'esistenza di una correlazione statisticamente significativa tra la gravità dell'OSAS ed i parametri BMI, ESS, SO2 media, ipertensione arteriosa, diabete mellito, dislipidemia e sindrome metabolica.
Introduction
Obstructive sleep apnoea syndrome (OSAS) is an underestimated disorder affecting 2-4% of men and 1-2% of women in the general adult population 1. The syndrome is characterized by partial or complete collapse of upper airways during sleep that is secondary to functional and/or anatomical factors 2. The most common symptoms of OSAS include chronic snoring, breathing pauses followed by loud snoring, excessive daytime sleepiness, headache at awakening and progressive deterioration of attention and memory 3.
In recent years, OSAS has been defined as a cardio-metabolic disorder 4. It is considered an important risk factor for serious systemic diseases like hypertension, obesity and diabetes 5 7. Indeed, intermittent hypoxia and sleep deprivation or defragmentation typical of OSAS, in the long term, can entail pathophysiological changes that induce the onset of these diseases 8 9.
A study of more than 6000 patients showed that individuals with OSAS had a risk of hypertension that was increased two-fold, while the risks of ischaemic heart disease and cerebrovascular disease were increased by 3- and 4-fold, respectively compared to the control population 10. An increased prevalence of OSAS and sleep-disorder breathing (SDB) has been reported in type 2 diabetes mellitus, and up to 70% of obese patients with diabetes have moderate to severe OSAS. In addition, the length and quality of sleep is indicative of glycaemic control, as OSAS affects glucose tolerance due to an increase in insulin resistance 11.
From a study on 281 severe snorers with OSAS, strong reciprocal association between the OSAS and the metabolic syndrome emerged 12. Metabolic syndrome is a cluster of metabolic abnormalities including hyperglycaemia, dyslipidaemia [involving elevated triglycerides (TG) and low high-density lipoprotein cholesterol (HDL-C)], hypertension and abdominal obesity. The definition of metabolic syndrome includes 3 or more of the following parameters: waist circumference (WC) > 102 cm in men and > 88 cm in women, HDL-C < 40 mg/dl in men and < 50 mg/dl in women, TG ≥ 150 mg/dl, blood pressure (BP) ≥ 130/85 mmHg and fasting plasma glucose ≥110 mg/dl. These conditions predispose the subject to increased risk of developing diabetes mellitus (DM), pro-inflammatory and pro-thrombotic conditions and cardiovascular disease 13 14.
Some authors have shown that the prevalence of OSAS in patients with BMI >30 varies from 40 to 60%. In addition, the high degree of obesity, especially if visceral, is considered a crucial pathogenetic factor for the onset and exacerbation of OSAS. In fact, if OSAS is not treated, the patient gradually increases in weight, while, conversely, weight loss reduces the OSAS severity 15.
However, the common association of OSAS with hypertension and obesity in general population makes it difficult to separate their respective independent role in the long-term cardiovascular and metabolic consequences associated with OSAS.
This justifies the importance of better understanding the possible relationship between severity of OSAS and metabolic syndrome.
Materials and methods
Our study was performed on 1185 patients observed from January 2000 to June 2011 (324 women and 861 men), suffering from OSAS with mean age 13.7 ± 55.1 years (range: 15-83) and mean BMI of 29.6 ± 5.8 (range: 19-52).
All patients underwent a general physical examination, complete overnight cardio-respiratory monitoring (8 channels), blood tests (glucose, cholesterol, triglycerides), monitoring of blood pressure (BP), complete ENT visit, nasolaryngeal fibre optic endoscopy with Muller manoeuvre, Epworth sleepiness scale (ESS) to measure daytime sleepiness and calculation of body mass index (BMI). Complete overnight cardio-respiratory monitoring included finger pulse oximeter, strain gauges for thoracic and abdominal efforts, one electrocardiography (ECG) lead, nasal airflow (pressure cannula), sensor for body position and a digital microphone for snoring detection. Based on the results of cardio-respiratory monitoring, patients were divided in three subgroups according to the severity of OSAS: mild OSAS (AHI 5-15), moderate OSAS (AHI 15-30) and severe OSAS (AHI > 30). Patients with BP ≥ 130/85 mmHg or already on medical therapy for hypertension were considered hypertensive. Patients with blood glucose ≥110 mg/dl or already on medication for diabetes were considered diabetic. Patients with triglycerides ≥150 mg/dl and HDL cholesterol <40 mg/dl in men and < 50 mg/dl in women were classified as dyslipidaemic. A BMI > 28 was considered as obese. Diagnosis of metabolic syndrome was made in the presence of at least 3 of the 5 significant clinical signs (obesity, hypertension, diabetes mellitus, hypertriglyceridaemia, hypercholesterolaemia). The study was conducted according to the guidelines on biomedical research involving human subjects (Declaration of Helsinki). Informed consent was obtained from each patient.
Descriptive statistics stratified by the variables OSAS and SEX are in Tables I and II. Given the ordinal nature of the variable OSAS, a Sidak adjusted (α = 0.05) Spearman correlation with BMI, ESS, average SO2 (SO2med), hypertension, diabetes mellitus (DM), dyslipidaemia and metabolic syndrome was calculated for both sexes. The correlation structure gave us the rationale to set a linear regression model in which AHI occurs as a dependent variable with BMI, SO2med, hypertension, dyslipidaemia, ESS, DM and metabolic syndrome as explicative variables that were statistically significant at P < 0.05. The coefficients of the regression model (Table III) estimated the impact of each predictor on AHI response. Moreover, we analyzed variance inflation factors to determine possible collinearities among the explanatory variables used. We achieved a mean VIF of 1.82 less than 10 so that the choice of explanatory variables was not a cause of concern 16. Statistical analysis was carried out using the statistical software STATA ver. 11.
Table I.
Sample distribution.
| OSAS | |||||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | Total | ||
| Sex | Female | 85 | 94 | 145 | 324 |
| Male | 262 | 269 | 330 | 861 | |
| Total | 347 | 363 | 475 | 1185 | |
| Hypertension | Female | 46 (54.12%) | 76 (80.85%) | 79 (54.48%) | 201 |
| Male | 81 (30.92%) | 81 (30.11%) | 165 (50.00%) | 327 | |
| Total | 127 | 157 | 244 | 528 | |
| Diabetes Mellitus | Female | 32 (38.65%) | 30 (31.91%) | 36 (24.83%) | 98 |
| Male | 16 (6.11%) | 33 (12.27%) | 102 (30.91%) | 151 | |
| Total | 48 | 63 | 138 | 249 | |
| Dyslipidaemia | Female | 0 (0.00%) | 38 (40.43%) | 43 (22.66%) | 81 |
| Male | 11 (4.20%) | 34 (12.64%) | 4 (1.21%) | 49 | |
| Total | 11 | 72 | 47 | 130 | |
| Metabolic Syndrome | Female | 28 (32.94%) | 30 (31.91%) | 23 (15.86%) | 81 |
| Male | 4 (1.53%) | 17 (6.32%) | 67 (20.30%) | 88 | |
| Total | 32 | 47 | 90 | 169 | |
Sample distribution of patients stratified by gender and degree of OSAS in relation to hypertension, diabetes mellitus, dyslipidaemia and metabolic syndrome.
Table II.
Average values and relative standard deviation.
| AHI | BMI | ESS | SO2 MED | DM | DYSLIP | MetS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OSAS | Mild | Women (N=85) | MEAN | 9.02 | 29.74 | 9.71 | 93.61 | 0.38 | 0.00 | 0.33 |
| SD | 2.61 | 7.54 | 7.30 | 2.66 | 0.49 | 0.00 | 0.47 | |||
| Men (N=262) | MEAN | 10.10 | 26.38 | 9.11 | 92.18 | 0.06 | 0.04 | 0.02 | ||
| SD | 2.22 | 2.46 | 4.74 | 4.86 | 0.24 | 0.20 | 0.12 | |||
| Moderate | Women (N=94) | MEAN | 22.88 | 30.11 | 10.86 | 92.52 | 0.32 | 0.40 | 0.32 | |
| SD | 3.94 | 4.07 | 4.17 | 2.18 | 0.47 | 0.49 | 0.47 | |||
| Men (N=269) | MEAN | 22.68 | 27.60 | 8.38 | 91.42 | 0.12 | 0.13 | 0.06 | ||
| SD | 4.66 | 2.68 | 5.06 | 3.11 | 0.33 | 0.33 | 0.24 | |||
| Severe | Women (N=145) | MEAN | 51.22 | 33.19 | 12.22 | 86.86 | 0.25 | 0.30 | 0.16 | |
| SD | 27.26 | 7.36 | 7.07 | 7.43 | 0.43 | 0.46 | 0.37 | |||
| Men (N=330) | MEAN | 49.33 | 32.70 | 13.62 | 85.61 | 0.31 | 0.01 | 0.20 | ||
| SD | 17.81 | 5.99 | 6.85 | 8.59 | 0.46 | 0.11 | 0.40 |
The average values and standard deviation of AHI, BMI, ESS, average O2 saturation, diabetes mellitus, dyslipidaemia and metabolic syndrome in patients with mild, moderate and severe OSAS. AHI: apnoea-hypopnoea index, BMI: body mass index, ESS: Epworth sleepiness scale; SO2MED: average O2 saturation, DM: diabetes mellitus, DYSLIP: dyslipidaemia, MetS: metabolic syndrome.
Table III.
Linear regression model.
| AHI | Coef. | SEM | t | P > |t| | [95% CI] |
|---|---|---|---|---|---|
| SEX | -3.14 | 1.23 | -2.56 | 0.01 | [-5.54; -0.73] |
| BMI | 0.77 | 0.13 | 6.07 | 0.00 | [0.52; 1.02] |
| ESS | 0.86 | 0.09 | 9.78 | 0.00 | [0.68; 1.03] |
| SO2 MED | -1.34 | 0.09 | -14.56 | 0.00 | [-1.52; -1.16] |
| DM | 10.36 | 2.14 | 4.85 | 0.00 | [6.17; 14.56] |
| DYSLIP | 6.89 | 1.85 | 3.72 | 0.00 | [3.26; 10.53] |
| MetS | -26.75 | 2.51 | -10.64 | 0.00 | [-31.6; -21.82] |
Linear regression results reporting the covariates coefficients estimation of AHI with SEM (standard error of mean), 95% confidence interval and their respective statistical test outcomes. BMI: body mass index, ESS: Epworth sleepiness scale, SO2MED: average O2 saturation, DM: diabetes mellitus, DYSLIP: dyslipidaemia, MetS: metabolic syndrome (P > |t| statistically significant).
Results
In the sample cohort, 347 of 1185 patients were suffering from mild OSAS (262 males and 85 females), 363 from moderate OSAS (269 males and 94 females) and 475 from severe OSAS (330 males and 145 females). The average age of patients with mild OSAS was 52.77 ± 13.49 years (range: 20-76), in moderate OSAS was 56.10 ± 14.32 years (range: 20-83) and in severe OSAS was 55.95 ± 13.26 years (range: 15-75). In the group of patients with mild OSAS, 127 were hypertensive (81 males and 46 females, mean 0.36 ± 0.48), while there were 48 diabetics (16 males and 32 females, mean 0.14 ± 0.34), 11 with dyslipidaemia (11 males and 0 females, mean 0.32 ± 0.17) and 32 with metabolic syndrome (4 males and 28 females, mean 0.09 ± 0.29). In the moderate OSAS group, 157 were hypertensive (81 males, 76 females, mean 0.43 ± 0.50), while there were 63 diabetics (33 males and 30 females, mean 0.17 ± 0.38), 72 with dyslipidaemia (34 males, 38 females, mean 0.20 ± 0.40) and 47 with metabolic syndrome (17 males, 30 females, mean 0.13 ± 0.34). Finally, in the group of severe OSAS patients, 244 were hypertensive (165 males, 79 females, mean 0.51 ± 0.50), and there were 138 diabetic patients (102 males, 36 females, mean 0.29 ± 0.45), 47 with dyslipidaemia (4 males, 43 females, mean 0.99 ± 0.30) and 90 with metabolic syndrome (67 males and 23 females, mean 0.19 ± 0.39). The distribution of the sample of subjects with OSAS stratified by gender is shown in Table I.
The average values and the relative standard deviation of AHI , BMI, ESS, average O2 saturation, diabetes mellitus, dyslipidaemia and metabolic syndrome are shown in Table II.
The results show different Spearman correlation patterns, Sidak adjusted (α = 0.05), in terms of statistical significance between men and women with respectively: BMI (ρs = 0.26), SO2MED (ρs = -0.51), hypertension (ρs = -0.05), dyslipidaemia (ρs = 0.22) for women, and BMI (ρs = 0.53), ESS (ρs = 0.28), SO2 MED (ρs = -0.50), hypertension (ρs = 0.17), DM (ρs = 0.28), metabolic syndrome (ρs = 0.26) for men (Fig. 1). The linear regression model showed strong statistical dependence between AHI and all the independent variables considered, as shown in Table III.
Fig. 1.
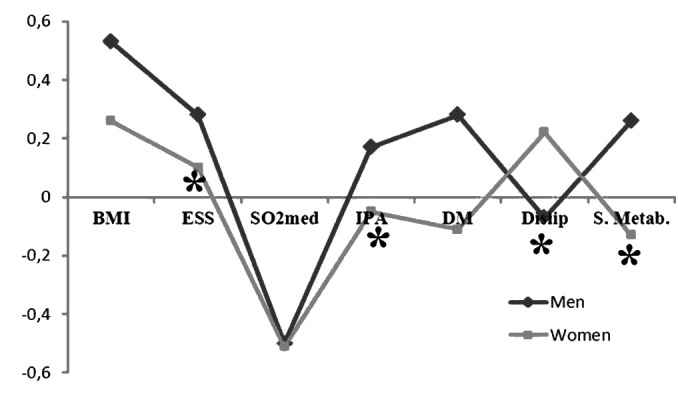
Spearman correlation patterns.
Spearman correlation patterns OSAS vs BMI, ESS, SO2 MED, IPA, DM, Dyslip, S. Metab.
BMI: body mass index, ESS: Epworth sleepiness scale, SO2MED: average O2 saturation, DM: diabetes mellitus, Dyslip: dyslipidemia, S. Metab: metabolic syndrome.
Discussion
Our study shows evidence for a correlation between OSAS and BMI, diabetes mellitus, hypertension, dyslipidaemia, metabolic syndrome, and consequently between OSAS and cardiovascular disease. Furthermore, the results demonstrated a stronger association between the predictors considered and the higher degrees of OSAS (moderate and severe), a plausible hypothesis that still needed to be statistically confirmed. A more careful interpretation of these results suggested that hypertension and dyslipidaemia are more associated with OSAS in females than in males. Conversely, ESS, diabetes mellitus and metabolic syndrome seemed to be more associated with OSAS in males than in females.
Our study supports recent evidence for a major public health impact of OSAS. However, the pathophysiologic mechanism by which OSAS contributes independently to cardiovascular and metabolic pathology remains unclear.
Patients with OSAS are always exposed to intermittent hypoxia and reoxygenation deriving from the cycles of apnoea/arousals. Recent studies suggest that sustained hypoxia would lead to oxidative stress and activation of a systemic inflammatory response 17 18, with increases in general blood antioxidant activity and in production of proinflammatory cytokines, including tumour necrosis factor α and interleukin 6 19-21. Such alterations would profoundly affect endothelial function 22, and may contribute to the development of increased platelet activity, elevated plasma fibrinogen levels and reduced fibrinolytic capacity, leading to blood hypercoagulability, atherosclerosis and cardiovascular imbalances associated with OSAS 23-25.
Sustained hypoxia, high sympathetic output, dysregulation of the hypothalamo-pituitary axis and alterations in inflammatory pathways seem to be involved in the pathogenesis of disorders of glucose metabolism in patients suffering from OSAS 26. Keckeis et al. demonstrated that a strong link exists between OSAS and insulin resistance, impaired glucose tolerance and type 2 diabetes mellitus. Nevertheless, it is difficult to separate the role of obesity and the role of OSAS in the development of glucose metabolism disorders 27.
OSAS and obesity seem to be part of a vicious circle supported by adipocytokines. Leptin has received the most attention 28 29 of all adipokines, and is a tissue-derived cytokine involved in control of body weight and a variety of biological functions. Serum levels of leptin serum are correlated with BMI and AHI 30, probably due to the intermittent low cellular oxygen tension typical of OSAS, and seem to improve after treatment with CPAP 31. Moreover, some authors found a significant correlation between a polymorphism in the leptin receptor (LEPR) and OSAS, supporting the hypothesis of a strong genetic basis in OSAS. Other studies have demonstrated that serum levels of ghrelin, a 28-amino acid polypeptide hormone with an appetite-stimulating effect, tend to be higher in patients with OSAS and are correlated with both AHI and ESS 32.
The strength of our study was examination of patients with OSAS and association with comorbidities. In many cases, patients with OSAS who did not report the investigated comorbidities might have other systemic disease (not yet diagnosed), and this misclassification could underestimate the severity of the disease.
The present study had some limitations, however. The major limitation was the absence of a control group without OSAS to evaluate the presence and prevalence of comorbidities in a normal population. We did not have data about other confounders such as smoking status, alcohol intake and exercise frequency, which is another limitation.
Conclusions
The results of the present study corroborate our initial hypothesis as a statistically significant correlation between OSAS and hypertension, diabetes, dyslipidaemia and metabolic syndrome was found. The controversial results in the literature and the lack of knowledge underlying the pathogenesis of OSAS and its comorbidities suggest the need for further studies. It will be useful to further evaluate whether and how the treatment and resolution of OSAS may influence the course of these diseases related to cardiovascular and metabolic risk factors.
References
- 1.Bixler EO, Vgontzas AN, Lin H-M, et al. Prevalence of sleep-disordered breathing in women. Am J Respir Crit Care Med. 2001;163(3Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 2.Bonsignore MR, Zito A. Metabolic effects of the obstructive sleep apnea syndrome and cardiovascular risk. Arch Physiol Biochem. 2008;114:255–260. doi: 10.1080/13813450802307451. [DOI] [PubMed] [Google Scholar]
- 3.Patil SP, Schneider H, Schwartz AR, et al. Adult obstructive sleep apnea. Pathophysiology and diagnosis. Chest. 2007;132:325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruber A, Horwood F, Sithole J, et al. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. 2006;5:22–22. doi: 10.1186/1475-2840-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: lung cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.He J, Kryger MH, Zorick FJ, et al. Mortality and apnea index in obstructive sleep apnea. Experience in 385 male patients. Chest. 1988;94:9–14. [PubMed] [Google Scholar]
- 7.Fletcher EC. Obstuctive sleep apnea and cardiovascular morbidity. Monaldi Arch Chest Dis. 1996;51:77–80. [PubMed] [Google Scholar]
- 8.Golbin JM, Somers VK, Caples SM. Obstructive sleep apnea, cardiovascular disease, and pulmonary hypertension. Proc Am Thorac Soc. 2008 Feb;:200–206. doi: 10.1513/pats.200708-143MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome, alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5:207–217. doi: 10.1513/pats.200708-139MG. [DOI] [PubMed] [Google Scholar]
- 10.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 11.Tassone F, Lanfranco F, Gianotti L, et al. Obstructive sleep apnoea syndrome impairs insulin sensitivity independently of anthropometric variables. Clin Endocrinol (Oxf) 2003;59:374–379. doi: 10.1046/j.1365-2265.2003.01859.x. [DOI] [PubMed] [Google Scholar]
- 12.Angelico F, Ben M, Augelletti T, et al. Obstructive sleep apnoea syndrome and the metabolic syndrome in an internal medicine setting. Eur J Intern Med. 2010;21:191–195. doi: 10.1016/j.ejim.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Maggio M, Lauretani F, Ceda GP, et al. Association between hormones and metabolic syndrome in older italian men. J Am Geriatr Soc. 2006;54:1832–1838. doi: 10.1111/j.1532-5415.2006.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endeshaw Y. Clinical characteristics of obstructive sleep apnea in community-dwelling older adults. J Am Geriatr Soc. 2006;54:1740–1744. doi: 10.1111/j.1532-5415.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 15.Teramoto S, Yamamoto H, Yamaguchi Y. Obstructive sleep apnea causes systemic inflammation and metabolic syndrome. Chest. 2005;127:1074–1075. doi: 10.1378/chest.127.3.1074. [DOI] [PubMed] [Google Scholar]
- 16.Rabe-Hesketh S, Everitt BS. A handbook of statistical analyses using Stata. 3rd ed. Boca Raton, FL: Chapman & Hall/ CRC; 2003. pp. 72–72. [Google Scholar]
- 17.Lurie A. Inflammation, oxidative stress, and procoagulant and thrombotic activity in adults with obstructive sleep apnea. Adv Cardiol. 2011;46:43–66. doi: 10.1159/000325105. [DOI] [PubMed] [Google Scholar]
- 18.Büchner NJ, Quack I, Woznowski M, et al. Microvascular endothelial dysfunction in obstructive sleep apnea is caused by oxidative stress and improved by continuous positive airway pressure therapy. Respiration. 2011;82:409–417. doi: 10.1159/000323266. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg H, Ye X, Wilson D, et al. Chronic intermittent hypoxia activates nuclear factor-kappaB in cardiovascular tissues in vivo. Biochem Biophys Res Commun. 2006;343:591–596. doi: 10.1016/j.bbrc.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Basoglu OK, Sarac F, Sarac S, et al. Metabolic syndrome, insulin resistance, fibrinogen, homocysteine, leptin, and Creactive protein in obese patients with obstructive sleep apnea syndrome. Ann Thorac Med. 2011;6:120–125. doi: 10.4103/1817-1737.82440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amra B, Karbasi E, Hashemi M, et al. Endothelial dysfunction in patients with obstructive sleep apnoea independent of metabolic syndrome. Ann Acad Med Singapore. 2009;38:461–464. [PubMed] [Google Scholar]
- 22.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007;3:409–415. [PMC free article] [PubMed] [Google Scholar]
- 23.Quercioli A, Mach F, Montecucco F. Inflammation accelerates atherosclerotic processes in obstructive sleep apnea syndrome (OSAS) Sleep Breath. 2010;14:261–269. doi: 10.1007/s11325-010-0338-3. [DOI] [PubMed] [Google Scholar]
- 24.Kosseifi S, Bailey B, Price R, et al. The association between obstructive sleep apnea syndrome and microvascular complications in well-controlled diabetic patients. Mil Med. 2010;175:913–916. doi: 10.7205/milmed-d-10-00131. [DOI] [PubMed] [Google Scholar]
- 25.McNicholas WT, Bonsignore MR, Management Committee of the COST Action B26 Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29:156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 26.Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis. 2009;51:381–391. doi: 10.1016/j.pcad.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Keckeis M, Lattova Z, Maurovich-Horvat E. Impaired glucose tolerance in sleep disorders. PLoS One. 2010;5:e9444–e9444. doi: 10.1371/journal.pone.0009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanaoka M, Yu X, Urushihata K, et al. Leptin and leptin receptor gene polymorphisms in obstructive sleep apnea syndrome. Chest. 2008;133:79–85. doi: 10.1378/chest.07-1633. [DOI] [PubMed] [Google Scholar]
- 29.Zirlik S, Hauck T, Fuchs FS, et al. Leptin, obestatin and apelin levels in patients with obstructive sleep apnoea syndrome. Med Sci Monit. 2011;17:CR159–CR164. doi: 10.12659/MSM.881450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimizu K, Chin K, Nakamura T, et al. Plasma leptin levels and cardiac sympathetic function in patients with obstructive sleep apnoea-hypopnoea syndrome. Thorax. 2002;57:429–434. doi: 10.1136/thorax.57.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima AM, Franco CM, Castro CM, et al. Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respiration. 2010;79:370–376. doi: 10.1159/000227800. [DOI] [PubMed] [Google Scholar]
- 32.Ursavas A, Ilcol YO, Nalci N, et al. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: role of obesity. Ann Thorac Med. 2010;5:161–165. doi: 10.4103/1817-1737.65050. [DOI] [PMC free article] [PubMed] [Google Scholar]


