Abstract
The physiological functions of a tissue in the body are carried out by its complement of expressed genes. Genes that execute a particular function should be more specifically expressed in tissues that perform the function. Given this premise, we mined public microarray expression data to build a database of genes ranked by their specificity of expression in multiple organs. The database permitted the accurate identification of genes and functions known to be specific to individual organs. Next, we used the database to predict transcriptional regulators of brown adipose tissue (BAT) and validated two candidate genes. Based upon hypotheses regarding pathways shared between combinations of BAT or white adipose tissue (WAT) and other organs, we identified genes that met threshold criteria for specific or counterspecific expression in each tissue. By contrasting WAT to the heart and BAT, the two most mitochondria-rich tissues in the body, we discovered a novel function for the transcription factor ESRRG in the induction of BAT genes in white adipocytes. Because the heart and other estrogen-related receptor gamma (ESRRG)-rich tissues do not express BAT markers, we hypothesized that an adipocyte co-regulator acts with ESRRG. By comparing WAT and BAT to the heart, brain, kidney and skeletal muscle, we discovered that an isoform of the transcription factor sterol regulatory element binding transcription factor 1 (SREBF1) induces BAT markers in C2C12 myocytes in the presence of ESRRG. The results demonstrate a straightforward bioinformatic strategy to associate genes with functions. The database upon which the strategy is based is provided so that investigators can perform their own screens.
Keywords: data mining, expression microarray, tissue-specific gene expression, gene function
sophisticated bioinformatics expertise often seems necessary to extract meaningful insights from expression microarray data that has been deposited in the public domain. For example, Mootha and colleagues (5, 31) curated 1,426 microarray datasets and then performed a statistical screen for genes that show correlated expression with ones relevant to heme biosynthesis or oxidative phosphorylation. They discovered five essential and two regulatory genes for each process, respectively (5, 31). More recently, Butte and colleagues (14, 43) identified drugs currently prescribed for other conditions that may be useful for lung cancer and inflammatory bowel disease. They cross-referenced 3,113 microarrays from 176 datasets that represent 100 diseases. Then they looked for drugs whose effect on genome-wide expression patterns in cultured cell lines is anticorrelated with characteristic gene expression patterns in the profiled diseases. Computational skills are not necessary to imagine interesting gene expression patterns, but they do seem a prerequisite to mine big data sets. For investigators who lack such skills, a simple data-mining solution could help provide leads that would be otherwise inaccessible. Therefore, we describe an approach and offer a database that allows an investigator, using Microsoft Excel or a similar spreadsheet program, to associate genes and physiological functions via comparisons of the relative tissue specificity of expression of a gene in multiple organs.
The relative specificity of expression of genes in a tissue was determined from datasets pooled from the Gene Expression Omnibus (GEO) (4, 15). A rank-sum test was used to calculate a tissue-specificity rank statistic for each probe set, which represents a gene transcript, for each organ. A nonparametric method was chosen because the distribution of expression values, which is not necessarily normal and can be confounded by noise or technical differences between pooled datasets, need not be assumed (35, 49). Up to hundreds of microarrays per organ and thousands for comparison between tissues in the body were utilized to calculate the rank statistics. The results were tabulated on a spreadsheet that could be searched for interesting patterns. Tissue-specific gene expression patterns in mouse, human, and rat have been described before using microarrays, but the surveys have typically included only a few replicates per organ, which limits the power to detect relative differences between tissues (16, 18, 46, 47, 54, 59). Web-based portals permit simple queries of individual genes but not searches of genes that fit complex expression patterns (56, 59).
Ranking genes genome-wide by their tissue specificity of expression enables more flexible, customized searches. Genes act in concert to execute a molecular function. Tissues that perform the function should more specifically express the relevant genes than ones that do not. This premise logically suggests bioinformatic screens and analyses that associate genes with functions. For example, candidate genes for a function may be defined by having rank statistic scores above or below threshold levels in tissues that respectively perform the function or not. Alternatively, one may want to determine which gene among a number of candidate genes, as may be found in a chromosomal interval mapped for a disease, is involved in the function of interest. The relative specificity of expression in the affected organ could help to prioritize genes for subsequent validation experiments.
To test the utility of the database prospectively, we developed bioinformatic screens for genes that regulate brown adipocyte gene expression. Brown adipose tissue (BAT), long known to generate heat in hibernating animals, newborns and small mammals, is now recognized for its presence and function in adult humans (12, 33, 34, 50, 53). Under normal conditions, uncoupling protein 1 (Ucp1) is expressed predominantly in BAT but can be induced in the white adipose tissue (WAT) of animals exposed to the cold (3, 6, 10). UCP1 uncouples mitochondrial oxidative phosphorylation to generate heat instead of ATP. BAT shares the abilities with WAT for fat uptake and with cardiac and skeletal muscle for energy production via abundant mitochondria. The transcriptional coactivator, PRDM16, is essential for the specification of BAT from a premyogenic, Myf5+ lineage in the embryo and the induction of so-called brite or beige adipocytes from a Myf5− lineage in adult WAT (19, 21, 32, 39–41). We hypothesized that additional genes involved in the regulation of BAT could be found by comparing tissue-specific gene expression patterns in WAT, BAT, and mitochondrial-rich organs like the heart. Through a series of bioinformatic screens and biologic experiments we identified estrogen-related receptor gamma (ESRRG) and sterol regulatory element binding transcription factor 1 (SREBF1) as transcriptional regulators of Ucp1 and other BAT markers.
METHODS
Data acquisition and processing.
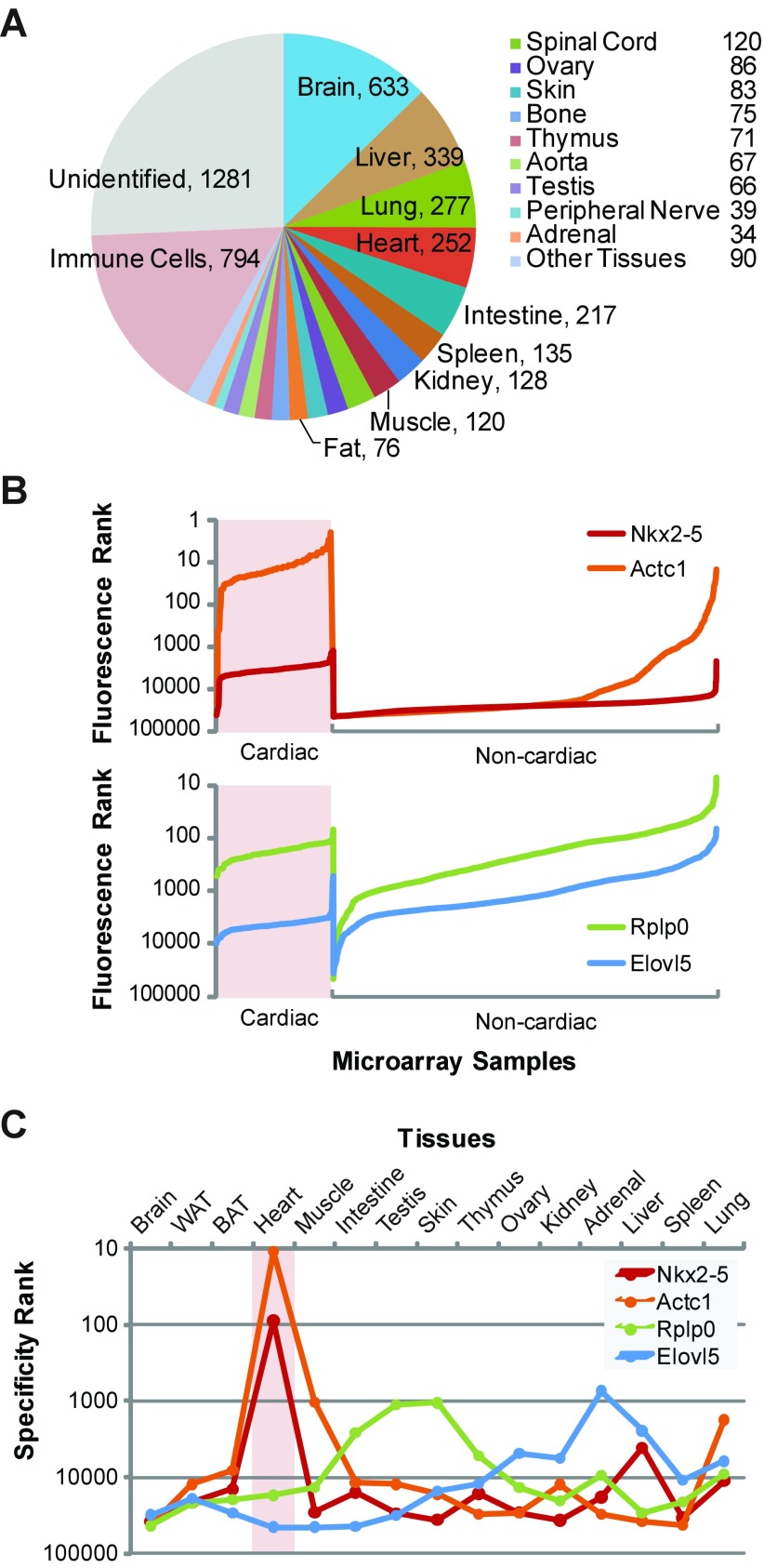
Via the GEO interface (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL1261), we downloaded 4,983 Affymetrix Mouse Genome 430A 2.0 microarray datasets (MG430Av2; Affymetrix, Santa Clara, CA; GEO platform accession GPL1261) in SOFT format. A custom Perl script parsed the SOFT file and assigned a source organ to each sample by matching a set of keywords for each organ to the sample source and the submitter's description fields. Computer-generated organ designations were manually confirmed. An overview of the microarray collection is shown (Fig. 1A). Of the 4,983 microarrays in the collection, 2,538 were excluded because the biological samples were of unknown origin, fetal in origin, cultured cell lines, or derivatized, e.g., a subpopulation of cells purified from an organ, resulting in a set of 2,445 microarray datasets used in the tissue-specific gene expression analysis. Probe set annotations (release 30) in .csv format were downloaded from the Affymetrix NetAffx portal (http://www.affymetrix.com).
Fig. 1.
A nonparametric statistical approach defines tissue-specific gene expression patterns from massively pooled microarray datasets. A: the tissue of origin for the 4,983 Affymetrix MG430Av2 microarray samples downloaded from GEO and the number of samples for each tissue used in subsequent analyses are shown. B: a gene, represented by a probe set, is ranked by its fluorescence intensity on a microarray for every sample in the collection. Top, fluorescence ranks for the cardiac-specific transcription factor Nkx2–5 (red tracing) and cardiac actin isoform Actc1 (orange) are shown. Their fluorescence ranks are generally higher in cardiac microarrays, consistent with their cardiac specificity. Bottom, fluorescence ranks for the ribosomal subunit Rplp0 (green) and fatty acid elongation protein Elovl5 (blue) are shown. The fluorescence ranks of Rplp0 are equivalent in cardiac and noncardiac samples, which is consistent with its known uniform expression in diverse tissues and its use as a reference or housekeeping gene for mRNA quantification. Elovl5 is expressed at low levels in the heart and likewise has generally lower fluorescence ranks in cardiac samples. The fluorescence ranks are plotted in reverse logarithmic scale. The noncardiac portion of the tracings is compressed horizontally to facilitate the interpretation of the plots. C: the tissue-specificity ranks of Nkx2–5, Actc1, Rplp0, and Elovl5 are shown for various organs in reverse logarithmic scale; higher points denote greater specificity.
Statistical algorithm.
To detect differential gene expression between two groups consisting of a total of m samples run on the same microarray platform, the meta-analysis algorithm first converted absolute fluorescence values (i.e., VALUE column from the SOFT formatted file) within each microarray sample into fluorescence ranks from 1 to n in order of decreasing brightness, where n is the number of probe sets represented on the microarray (Fig. 1B). For samples with fewer than n fluorescence values reported, the fluorescence ranks were scaled to range from 1 to n. For each of the n probe sets, the algorithm ranked the fluorescence rank values in descending order across the m samples to determine expression ranks from 1 to m. A Wilcoxon rank-sum test was then applied to the expression rank values for the two groups being compared with generate a Z-score, where a greater absolute Z-score represents more significant differential expression between the two groups being compared. The sign of the Z-score indicates which of the two groups has higher relative expression of the gene as measured by the probe set. To assess tissue-specific expression, the samples from the tissue of interest are compared with all others that are not. The tissue-specificity rank of a gene was determined by ranking Z-scores generated by the rank-sum tests from the most negative to the most positive; a lower Z-score reflects greater tissue specificity.
Gene Ontology analysis.
Gene Ontology (GO) biological process terms associated with the probe sets on the Affymetrix MG430Av2 microarray were extracted from the probe-set annotation file. The tissue specificity of a given GO term was determined by a rank-sum test. Probe sets from a tissue-specificity rank list for a particular organ were divided into two groups that were either annotated with the given GO term or not. The enrichment of the annotated group toward the top or the bottom of the rank list was described by the rank-sum test Z-score and P value. The enrichment of GO terms in a set of probe sets identified in the bioinformatic screen for potential BAT regulators was evaluated with GeneMerge (7). Significance thresholds were set at a false discovery rate of 5% for the GO analyses.
Cell culture.
The OP9 preadipocyte cell line was a generous gift from Dr. Paul Hruz (Washington University School of Medicine, St. Louis, MO). The OP9 culture was maintained and differentiated into adipocyte-like cells as previously described (55). The OP9 preadipocytes were transfected using Optifect (Invitrogen, Carlsbad, CA) as per the manufacturer's instructions. Transfected OP9 adipocytes were cultured for an additional 3 days after transfection and differentiation before harvesting. In pharmacologic experiments, differentiated OP9 cells were incubated with the PPARG agonist rosiglitazone (10 μM in DMSO; Sigma Aldrich, St. Louis, MO) and/or ESRRG antagonist 4-hydroxytamoxifen (4-OHT; 10 μM in DMSO, Sigma Aldrich) for 48 h before harvesting.
The C2C12 skeletal myoblast cell line was purchased from and maintained according to the protocol supplied by ATCC (Manassas, VA). C2C12 cells were transfected using Lipofectamine 2000 (Invitrogen) per the manufacturer's instructions. The cells were maintained for an additional 36 h after transient transfection before being harvested. In pharmacologic experiments, C2C12 cells were incubated with 4-OHT at 10 μM for 48 h before harvesting.
Expression plasmids.
A pcDNA3.1(-)-Met-Esrrg expression construct was a gift from Teresa Leone and Daniel Kelly (Burnham Institute for Medical Research, Lake Nona, FL). A pSV SPORT Srebp-1c expression construct was purchased from Addgene (Cambridge, MA). Esrrg targeting (Target gene 26381) and control shRNA constructs from the RNA interference (RNAi) Consortium library (Broad Institute, Cambridge, MA) were obtained via The Genome Institute at Washington University and the Children's Discovery Institute (St. Louis, MO).
Animal studies.
We treated 12-wk-old female FVB/N mice with intraperitoneal injection of 4-OHT (25 mg/ml in sunflower seed oil and 10% DMSO) or vehicle once daily at 0.1 mg/g/day. The mice were placed in a 4°C room 6 h after the first injection. They received food and water ad libitum. The core temperature of each mouse was measured twice daily with a K-type thermocouple probe digital thermometer. Abdominal WAT and intrascapular BAT were harvested after 72 h of cold exposure. The experiments were approved by the animal studies committee at Washington University School of Medicine.
RNA isolation and quantitative PCR.
RNA was isolated from the tissue samples using TRIzol (Invitrogen), according to the manufacturer's protocol. The precipitated RNA was resuspended in 175 μl of RNA lysis buffer from the SV Total RNA Isolation Kit (Promega, Madison, WI) and purified according to the manufacturer's protocol. Total RNA from cultured cells were isolated with SV Total RNA Isolation Kit only. cDNA was synthesized from 2.5 μg of total RNA using SuperScript VILO cDNA Synthesis Kit (Invitrogen) according to manufacturer's protocol.
Quantitative PCR of cDNA was performed in Cycler iQ system (Bio-Rad, Hercules, CA) or Mx3005P QPCR System (Stratagene, Santa Clara, CA) in a 40 μl reaction containing iQ SYBR Green Supermix (Bio-Rad) and PCR primers at a final concentration of 200 nM. Primer sequences were designed with the PerlPrimer or obtained from PrimerBank (27, 44). Primers were purchased from Sigma Aldrich or Integrated DNA Technologies (Coralville, IA). The primer sequences are available in the supplemental methods.1 Relative mRNA expression was calculated using the ΔΔ-Ct method and normalized to Hprt1.
Student's t-tests were used to compare groups in the qPCR studies. The data are reported as means ± SE.
RESULTS
Massive data mining ranks genes across the genome by their tissue specificity of expression.
Although expression microarrays are perceived to be insensitive to differences among genes expressed at low levels, a nonparametric method could rank the tissue specificity of gene expression across a wide dynamic range. First, the fluorescence rank of a gene, as represented by a probe set, was determined in order of decreasing brightness for each microarray in the pooled dataset. Second, the expression rank of a gene was determined by ranking the fluorescence ranks across all the microarrays, which in turn were classified as being from the tissue of interest or not. Third, we performed rank-sum tests of the expression ranks for every gene in the two microarray groups. The analysis generates a Z-score that lends itself to genome-wide ranking of genes (Supplementary Table S1). Negative or positive Z-scores correspond to genes specifically or counterspecifically expressed in the tissue compared with all others. The term “counterspecific” is chosen so as not to imply active repression of gene expression in a tissue. The tissue-specificity rank of each gene derives from ranking the Z-scores. The analyses were performed on datasets from a single microarray platform so that cross-platform differences in probe-set performance would not confound the fluorescence rank relationships between genes (Fig. 1). Examples of genes that are specific, counterspecific, or neither in the heart follow.
The transcription factor Nkx2–5 and cardiac actin isoform Actc1 are expressed almost exclusively in the heart (24, 29). Consistent with their respectively low and high levels of expression, their median fluorescence ranks are 3,542 and 15 among the 45,101 probe sets in 175 profiles of postnatal murine hearts and 25,449 and 25,485 in 2,270 nonheart arrays (P < 1 × 10−101 for both genes, Fig. 1B). From these data were calculated the specificity ranks. Nkx2–5 and Actc1 are ranked 89 and 11 in the heart but much lower in nonheart tissues (Fig. 1C). Their Z-scores in the heart are −21.35 and −21.79, respectively.
At the opposite extreme, the fatty acid elongation protein Elovl5 is expressed at a much lower level in the heart than elsewhere (26). The median fluorescence ranks of the gene are 4,485 and 1,404 in the heart and nonheart arrays (P < 1 × 10−101, Fig. 1B). The tissue-specificity rank of Elovl5 in the heart is 44,970 of 45,101 probe sets. The Z-score, 17.70, is large and positive, consistent with counterspecific expression of the gene in the heart (Fig. 1C).
The ribosomal subunit Rplp0, also known as Arbp or 36B4, is expressed at comparable levels in diverse tissues (1). The median fluorescence ranks of Rplp0 were 179 and 221 in the heart and nonheart arrays (P = 1, Fig. 1B). The gene has a tissue-specificity Z-score near zero in most organs, reflecting its ubiquitous and consistent level of its expression (Fig. 1C).
Rarely is a gene expressed exclusively in a single organ. A limited pattern of expression can still be relatively tissue specific. The algorithm provides a means to judge relative specificity, as illustrated by the comparison of actin isoforms expressed in skeletal muscle. Skeletal α-actin, ACTA1, comprises ≥95% of the sarcomeric actin in skeletal muscle, whereas ACTC1 is a minor fraction (29). Acta1 and Actc1 have specificity ranks of 68 and 1,031 among the 45,101 probe sets in skeletal muscle. Hence, Actc1 is more specifically expressed in muscle than other tissues except the heart (P < 1 × 10−34, Fig. 1C).
Genome-wide rankings of tissue-specific expression offer insights into gene and organ function.
The expression pattern of a gene can offer insight into its function. Several observations suggest that our algorithm could offer similar insights. First, the tissue-specificity rank of a gene in each organ appears to correlate well with a function specific to the organ, as illustrated by the 10 transcription factors deemed most specific in 15 tissues (Fig. 2). On average, mutation or knockout of ∼6 of the 10 transcription factors is associated with a human disease or mouse phenotype related to the function of the gene in the tissue. Of course, tissue-specific genes that do not have a known mutant phenotype could still have a function. For example, myelin transcription factor 1-like (MYT1L) is the second most brain-specific transcription factor (P = 4 × 10−274, Fig. 2). Forced expression of Mytl1 and two other transcription factors, POU3F2 and ASCL1, can convert fibroblasts directly into neurons (51). Pou3f2 and Ascl1 are highly brain specific too (P < 1 × 10−107 and < 1 × 10−17, Supplementary Table S1; the least significant statistic of the two probe sets that represent each gene is presented). In combination with miR-9/9* and miR-124, MYT1L and ASCL1 potentiate the conversion of fibroblasts to neurons (58).
Fig. 2.
The 10 most specifically expressed transcription factors in 15 different tissues are listed. If several probe sets represent transcription factor, the most significant probe set is shown. Genes were identified as transcription factors if their associated Gene Ontology terms included “0006355 regulation of transcription, DNA-dependent,” or “0006350 transcription,” and “0003677 DNA binding.” The official gene symbols and P values derived from our tissue specificity rank-sum analysis are shown. Filled boxes indicate the existence of human (H) or mouse (M) mutant phenotypes related to the function of the transcription factor in the organ.
The repertoire of genes expressed by an organ determines its functions, which suggests that the tissue-specificity ranks of genes could be adapted to predict organ functions. Based on the GO annotations for each gene, we performed a rank-sum analysis of biological processes according to their distribution on the tissue-specificity rank list of an organ. Perusal of the analyses shows that the specialized functions of an organ can be predicted from pooled microarray data (Fig. 3, Supplementary Table S2). Closer inspection suggests that additional insights may be gleaned. For example, the testis and ovary produce gametes and sex hormones. Consistent with their shared functions, there is substantial overlap of the statistically significant genes and biologic processes in the two gonadal organs (Figs. 2, 3). The analyses can even suggest less obviously shared genes or functions. Genes and GO terms related to immunity are expected for the spleen and thymus, but they are also enriched in the intestine, lung, and skin, thus highlighting their common function as a barrier against pathogens (Fig. 3, Supplementary Tables S1 and S2).
Fig. 3.
The function of an organ can be predicted from the GO biological process terms assigned to genes ranked by their specificity of expression in the organ. Representative, significant functions for a number of organs are shown here with their GO accession number and P value. Significance thresholds were defined by setting a false discovery rate to 5%. The full lists of GO terms are provided in the Supplementary Table S2.
ESRRG regulates brown adipocyte gene expression in white fat.
The prior analyses of pooled microarray data revealed known tissue-specific genes and physiological processes. We next tested predictive utility based upon the premise that tissues that perform a common function utilize the same genes that are more specifically expressed in said tissues. We developed a bioinformatic screen for genes that could induce brown adipocyte gene expression in white adipose tissue in the context of two key facts. First, Prdm16 is a critical determinant of the brown fat phenotype (40). Second, the heart and BAT are the most mitochondrial-rich tissues in the body, whereas WAT is mitochondrial poor. We defined genes whose expression is more specific to BAT and heart and more counterspecific to WAT than Prdm16 in each tissue. The screen yielded 104 probe sets, representing 97 unique genes (Fig. 4A, Supplementary Table S3). The set is enriched for genes whose products localize to the mitochondria (51% vs. 9.3% of all genes on the microarray, P = 3.07 × 10−16; Fig. 4B).
Fig. 4.
ESRRG induces a BAT gene expression program in white adipocytes. A: schematic representation of the bioinformatic screen. Left: the tissue-specificity scores in BAT, WAT, and the heart are plotted for all 45,101 probe sets (gray) represented on the MG430Av2 microarray. The probe sets (blue) that are more or as specific to BAT and the heart and more or as counterspecific to WAT as Prdm16 were identified. Right: the probe sets are shown with Prdm16, Esrrg, and Esrrg-regulated genes in green, red, and yellow, respectively. B: the subset is significantly enriched for mitochondrial genes and genes regulated by ESRRG. Significance thresholds were set by a false discovery rate of 5%. C: quantitative RT-PCR analysis of Esrrg mRNA in murine BAT and WAT. *P = 0.023, n = 4. D: ESRRG induces Ucp1 mRNA expression in differentiated but not undifferentiated OP9 cells. GFP control transfections are shown for comparison. *P < 0.05, n = 3. E: ESRRG induces additional BAT markers Cidea, Pgc-1α, Pparg, and Prdm16 and suppresses the WAT marker Resistin in differentiated OP9 cells. *P < 0.05, n = 3. F: the PPARG agonist rosiglitazone induces BAT markers in differentiated OP9 cells, an effect that is suppressed by the ESRRG antagonist 4-hydroxytamoxifen (4-OHT). Data are reported relative to vehicle-treated cells. *P < 0.05 vs. vehicle-treated cells, #P < 0.05 vs. rosiglitazone-treated cells; n = 4. Error bars indicate SE. BP, biological process; CC, cellular component; MF, molecular function; ND, not detected.
Among the 97 genes are seven known transcription factors in addition to Prdm16, which defined the threshold Z-scores in the screen. Ppargc1a (Pgc-1α), which regulates mitochondrial biogenesis, was cloned from a brown adipocyte cDNA library (37, 57). LRPPRC forms a transcriptional regulatory complex with Pgc-1α to regulate several mitochondrial genes (9). CREB1 regulates the transition from brown preadipocytes to mature adipocytes (13). CUX1 regulates the expression of Fto, mutations of which affect adiposity (45). ESRRG regulates mitochondrial gene expression (2), but Esrrg, Whsc1, and Znf41, denoted as Riken cDNA 1700029I01, have no described function in adipocytes. Of note, 11% of the 97 genes are regulated directly or indirectly by ESRRG (2), whereas <1% of all genes on the microarray are (P = 6.38 × 10−7). The increased mRNA expression of Esrrg in BAT relative to WAT was thus verified by quantitative RT-PCR (Fig. 4C).
These observations led us to examine the ability of ESRRG to induce mRNA markers of brown fat in OP9 cells, a white adipocyte cell culture model (55). Transiently transfected Esrrg could upregulate the expression of the BAT markers Ucp1, Cidea, and Pgc-1α in differentiated OP9 adipocytes, while downregulating the WAT marker resistin (Fig. 4, D and E). ESRRG had no effect in undifferentiated OP9 preadipocytes, suggesting the requirement for an adipocyte-specific co-regulator (Fig. 4D).
BAT gene expression can be induced in white adipocytes by the PPARG agonist rosiglitazone.(36) Rosiglitazone similarly induced BAT markers as well as Esrrg in differentiated OP9 adipocytes (Fig. 4F). To determine whether induction depends upon Esrrg, we applied 4-hydroxytamoxifen (4-OHT), an ESRRG antagonist (11). The drug suppressed the rosiglitazone-mediated induction of Ucp1 and Cidea (Fig. 4F). Esrrg was likewise suppressed, which suggests either that ESRRG regulates its own expression in a positive feedback loop or that 4-OHT acts on another target that regulates Esrrg expression. Unfortunately, efforts to transfect OP9 cells with shRNA constructs, as an alternative means to knockdown ESRRG activity, were unsuccessful.
Mice subjected to the cold induce BAT genes in WAT. After housing for 3 days at 4°C, Ucp1 and other BAT markers were upregulated up to 10-fold in the WAT. The upregulation was abrogated by 4-OHT, which is consistent with a role for Esrrg in the induction of BAT in WAT (Fig. 5A). Visual inspection during gross dissection suggested that there was more intrascapular BAT after cold exposure, but the expression of Ucp1 and other markers was only slightly upregulated or unchanged (Fig. 5B). 4-OHT may not inhibit Ucp1 expression in BAT because Esrrg is expressed an order of magnitude higher than in WAT (Fig. 4C). Alternatively, BAT may have redundant mechanisms for Esrrg, just as it has for Pgc-1α, which is necessary for the expression of Ucp1 and other brown markers in WAT but not BAT (22). Treated mice maintained normal body temperature while housed in the cold, consistent with the maintenance of Ucp1 expression in BAT.
Fig. 5.
A: exposure of mice to the cold induces brown adipocyte gene expression in WAT. The ESRRG antagonist 4-OHT suppressed the effect of cold exposure. B: 4-OHT did not suppress the cold-induced induction of Ucp1 in BAT possibly because Esrrg is expressed at higher levels in BAT than WAT. Fold changes are stated relative to the WAT or BAT of vehicle-treated mice housed at room temperature. *P < 0.05 with respect to vehicle treated mice at room temperature, #P < 0.05 with respect to vehicle-treated mice exposed to the cold; n = 6 animals per group. Error bars indicate SE.
Srebf1 induces brown adipocyte gene expression in cultured myoblasts.
ESRRG induces BAT gene expression in adipocytes, but Esrrg is also highly expressed in mitochondria-rich tissues that have no UCP1 (Fig. 6A). White adipocytes may possess a co-regulator that renders them competent to express BAT markers in the presence of ESRRG. A hypothetical co-regulator should be expressed more specifically in white and brown adipose tissue than mitochondrial-rich organs. Prdm16 is probably not the co-regulator, as the brain, heart, and kidney express the gene, albeit at lower levels than in BAT (Fig. 6A). A bioinformatic screen was designed for genes that have negative tissue-specific Z-scores in WAT and BAT and positive Z-scores in brain, heart, muscle, and kidney. The screen yielded 614 annotated probe sets representing 564 unique genes, including 53 transcriptional regulators (Supplementary Table S4). Srebf1 was selected for further analysis in a muscle cell culture model because its expression pattern closely resembled the criteria for the hypothesized Esrrg co-regulator (Fig. 6B). In addition, Srebf1 regulates lipogenesis in adipocytes (38), which suggested that it could regulate additional pathways in BAT. Of two alternatively spliced forms, Srebp-1a and -1c, Srebp-1c was chosen for transfection because it is the predominant isoform in white and brown adipocytes (42).
Fig. 6.
A: Ucp1 is specifically expressed in BAT, whereas Prdm16 and Esrrg are expressed in various other tissues, suggesting that an adipocyte co-regulator is necessary for ESRRG to induce the brown adipocyte gene expression program (n = 4; ND, not detected). B: Srebf1 is more highly expressed in the liver, WAT, and BAT than other mitochondria-rich tissues (n = 4). C: SREBP-1c induces the brown adipocyte gene expression program in C2C12 myoblasts. Induction depends upon endogenous ESRRG, as shown by inhibition or knockdown by 4-OHT or shRNA, respectively. A lacZ control shRNA construct had no effect on Srebp-1c-mediated induction of BAT markers. The measurements are normalized to GFP-transfected cells. Transfections were performed in triplicate. Error bars indicate SE. *P < 0.05 compared with GFP-transfected controls, #P < 0.05 compared with Srebp-1c-transfected cells; n = 3.
Transfection of C2C12 myoblasts with Srebp-1c significantly induced the expression of Ucp1, other BAT markers and Esrrg (Fig. 6C). If SREBP-1c and ESRRG act together to induce brown adipocyte gene expression, then inhibition of ESRRG should repress the induction in C2C12 cells by SREBP-1c. Consistent with this hypothesis, 4-OHT abrogated the upregulation of BAT markers by SREBP-1c, just as it did in OP9 cells and in the WAT of cold-exposed mice. RNAi knockdown of Esrrg likewise repressed the induction of BAT markers by SREBP-1c, whereas a control shRNA had no effect. SREBP-1c had no effect on the expression of the myocyte-specific marker tropomyosin 1 alpha (Tpm1) (Fig. 6C). Although the results indicate that Srebp-1c and Esrrg are needed together to induce BAT markers in C2C12 myoblasts, they cannot differentiate between direct and indirect mechanisms of regulation.
DISCUSSION
“Big data” are revolutionizing the process of genetic discovery. Whereas an investigator once had to perform an experiment to garner a genetic foothold into a phenotype, one can now mine public databases for the first, key lead. In this way, CD44, a macrophage cell-surface receptor, was discovered to be essential to the inflammatory cascade in adipose tissue that leads from obesity to insulin resistance (23). Most scientists, however, lack the computational and statistical skills required for mining complex datasets. Hence, an experiment such as a linkage analysis or molecular biologic screen remains the first step to generate a list of candidate genes. Prioritization of the genes for subsequent validation is the next step. In lieu of or in addition to preconceived criteria, an investigator may want to take advantage of unrecognized patterns in public databases that involve the genes, which several web-based tools help to do (30). For example, a deletion at chromosome 6q24-q25, which contains 105 genes, is associated with congenital heart defects. Endeavor, a bioinformatic prioritization tool, identified TAB2 as the gene most likely to underlie the phenotype. Additional experiments in zebrafish and analyses of human patients verified the prediction (48). More than a dozen algorithms, which utilize different data sources, offer a user-friendly means to gene prioritization (30). Some investigators, however, may seek even greater flexibility than available with web-based tools but not want to write their own software code. The present work offers an intermediate solution that allows one to develop customized searches or perform ones on a spreadsheet that ranks the tissue specificity of gene expression in multiple organs. Based on knowledge of organ physiology, we sorted the spreadsheet to discover roles for two transcription factors in regulating BAT gene expression.
Our results demonstrate how GEO data mining using conceptually simple, nonparametric statistics can reveal meaningful genes and patterns. The reasons why we chose a rank-sum method are pertinent to investigators who want actionable insights but may not have sophisticated statistical or bioinformatic capabilities. Microarray data are typically normalized to remove variation that is not of biological interest, but normalization can be a vexing problem even when an experiment is well defined (25, 28). Normalization of heterogeneous, pooled datasets made more challenging by the presence of outliers, missing data, and systematic errors that may exist in individual datasets. Nonparametric statistics is robust against these issues (35, 49). Despite the greater statistical power of parametric tests, rank-sum tests applied to aggregated microarray datasets that were classified as being from a particular organ or not clearly identified tissue-specific gene expression patterns. Genes were detected across a wide dynamic range of expression, as shown by the identification of low-expressed transcription factors with organ-specific functions. Hidden higher-order biological patterns were also detected, as shown by the prediction of the specialized functions of organs.
In addition to demonstrating the utility of the statistical approach, the tissue-specificity rank lists for multiple organs can be useful in their own regard. For example, exome sequencing of individuals with a particular phenotype typically yields a list of candidate genes, only one of which is presumed to be functionally significant. One may prioritize the genes based on their specificity rank in the organ pertinent to the phenotype. Many of the highly ranked genes are associated with a disease or mutant phenotype related to the tissue-specific function of a gene. Thus, highly ranked, but as yet unassigned genes could be fruitful targets for investigation.
More broadly, the methodology should be amenable to any biological question that can be addressed by comparison of microarray samples classified into two or more groups, such as a cell type or disease. Of practical value, one can easily incorporate prior biological knowledge to define statistical thresholds in comparative analyses. The resulting rank list of genes can inform the choice of experiments, as shown by the implication of Esrrg and Srebf1 in BAT gene expression.
Numerous methods of microarray data analysis are backed by a substantial literature that assesses the merits of an algorithm by statistical modeling. The validity of the general approach presented here is more difficult to quantify on theoretical grounds because an investigator's judgment is necessary to classify datasets for comparison. One may expect that the inclusion of prior knowledge would enhance statistical power, but how much clearly depends upon the immeasurable quality of the information. We therefore chose to demonstrate the predictive power empirically with a series of simple bioinformatic screens. Esrrg was implicated in the regulation of Ucp1 and other BAT markers by its specificity of expression relative to Prdm16 in the heart and BAT but not WAT. Srebf1 was implicated as an adipocyte co-regulator by its specificity of expression in BAT and WAT but not other ESRRG-rich tissues. In vitro and in vivo experiments support the predicted functions. Neither transcription factor has been directly implicated in the regulation of BAT gene expression (8, 20), although SREBP-1c has been shown to drive a Pgc-1α promoter construct in brown adipocyte cell culture models (17).
Mechanistic questions remain regarding the role of Esrrg and Srebf1 in the regulation of the BAT phenotype. For example, we cannot conclude that Esrrg or Srebf1 directly regulates Ucp1 gene expression. Either could induce the expression of transcription factors that directly regulate BAT markers. SREBP-1c is not strongly adipogenic in cell culture models (38), however, which suggests that SREBP-1c does not control a master switch, as PRDM16 does, of BAT gene expression in C2C12 cells (39). We also note that the positive results of ESRRG and SREBP-1c in the present experiments do not exclude functions for the other transcription factors revealed in the bioinformatic screens. The experimental results do prospectively demonstrate the yield of a bioinformatic strategy based upon straightforward statistical analyses of public microarray data. Other genes that were identified in the same screens as Esrrg and Srebf1 likely have relevant functions. For example, Pgc-1α was identified in the screen for genes specific to the heart and BAT but not WAT. Originally cloned from a brown fat cDNA library, Pgc-1α is essential to mitochondrial biogenesis and thermogenesis (37, 57). In the screen for genes specific to BAT and WAT but not other mitochondrial-rich tissues, Tle3 was represented by two probe sets along with Srebf1 and 51 other transcriptional regulators. Tle3 was discovered in a screen of 18,292 cDNA vectors to co-regulate adipogenesis with Pparg. Cebpa, a positive control in the cDNA screen, was also identified by the bioinformatic screen (52). One can imagine how the bioinformatic results could have accelerated screening of the cDNA library.
Obviously, no bioinformatic or experimental screen is perfect or all-encompassing. Sensitivity is limited by the design of the screen. For example, the present analyses would not identify ubiquitously expressed genes that regulate BAT gene expression. Specificity is a concern too, but seemingly false positive results could be genes that have functions unintentionally captured by a bioinformatic screen. Hence, the screen that yielded Esrrg included genes that perform mitochondrial functions, which is consistent with the high abundance of mitochondria in the heart and BAT. Despite the limitations, we suggest that they are outweighed by the relative simplicity of the rank-sum approach to public expression microarray data and the consequent ease of extracting biological insights from the output as tabulated on a spreadsheet.
GRANTS
I. D. Chen was supported in part by the Lucille P. Markey Pathway in Human Pathobiology. This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-105857, Washington University Nutrition Obesity Research Center (P30DK-056341), the American Heart Association, Edward Mallinckrodt Jr. Foundation, and Children's Discovery Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: I.-b.D.C. and P.Y.J. conception and design of research; I.-b.D.C., V.K.R., D.S.D., and P.Y.J. performed experiments; I.-b.D.C. and P.Y.J. analyzed data; I.-b.D.C. and P.Y.J. interpreted results of experiments; I.-b.D.C. and P.Y.J. prepared figures; I.-b.D.C. and P.Y.J. drafted manuscript; I.-b.D.C., V.K.R., and P.Y.J. edited and revised manuscript; I.-b.D.C., V.K.R., D.S.D., and P.Y.J. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We thank Anatoly Tzekov for technical advice on OP9 cell culture, Dr. Paul Hruz for the OP9 cells, Teresa Leone and Dr. Daniel Kelly for expression constructs, and Maria Efimova for graphic illustrations. We appreciate helpful comments from Drs. Todd Druley, Alan Schwartz, and David Wilson.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Akamine R, Yamamoto T, Watanabe M, Yamazaki N, Kataoka M, Ishikawa M, Ooie T, Baba Y, Shinohara Y. Usefulness of the 5′ region of the cDNA encoding acidic ribosomal phosphoprotein P0 conserved among rats, mice, and humans as a standard probe for gene expression analysis in different tissues and animal species. J Biochem Biophys Methods 70: 481–486, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6: 13–24, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 298: E1244–E1253, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Barrett T, Troup DB, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Muertter RN, Holko M, Ayanbule O, Yefanov A, Soboleva A. NCBI GEO: archive for functional genomics data sets–10 years on. Nucleic Acids Res 39: D1005–D1010, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baughman JM, Nilsson R, Gohil VM, Arlow DH, Gauhar Z, Mootha VK. A computational screen for regulators of oxidative phosphorylation implicates SLIRP in mitochondrial RNA homeostasis. PLoS Genet 5: e1000590, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cannon B, Hedin A, Nedergaard J. Exclusive occurrence of thermogenin antigen in brown adipose tissue. FEBS Lett 150: 129–132, 1982. [DOI] [PubMed] [Google Scholar]
- 7. Castillo-Davis CI, Hartl DL. GeneMerge–post-genomic analysis, data mining, and hypothesis testing. Bioinformatics 19: 891–892, 2003. [DOI] [PubMed] [Google Scholar]
- 8. Collins S, Yehuda-Shnaidman E, Wang H. Positive and negative control of Ucp1 gene transcription and the role of beta-adrenergic signaling networks. Int J Obes (Lond) 34, Suppl 1: S28–S33, 2010. [DOI] [PubMed] [Google Scholar]
- 9. Cooper MP, Qu L, Rohas LM, Lin J, Yang W, Erdjument-Bromage H, Tempst P, Spiegelman BM. Defects in energy homeostasis in Leigh syndrome French Canadian variant through PGC-1alpha/LRP130 complex. Genes Dev 20: 2996–3009, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 103: 931–942, 1992. [DOI] [PubMed] [Google Scholar]
- 11. Coward P, Lee D, Hull MV, Lehmann JM. 4-Hydroxytamoxifen binds to and deactivates the estrogen-related receptor gamma. Proc Natl Acad Sci USA 98: 8880–8884, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cypess AM, Zhang H, Schulz TJ, Huang TL, Espinoza DO, Kristiansen K, Unterman TG, Tseng YH. Insulin/IGF-I regulation of necdin and brown adipocyte differentiation via CREB- and FoxO1-associated pathways. Endocrinology 152: 3680–3689, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dudley JT, Sirota M, Shenoy M, Pai RK, Roedder S, Chiang AP, Morgan AA, Sarwal MM, Pasricha PJ, Butte AJ. Computational repositioning of the anticonvulsant topiramate for inflammatory bowel disease. Sci Transl Med 3: 96ra76, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30: 207–210, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ge X, Yamamoto S, Tsutsumi S, Midorikawa Y, Ihara S, Wang SM, Aburatani H. Interpreting expression profiles of cancers by genome-wide survey of breadth of expression in normal tissues. Genomics 86: 127–141, 2005. [DOI] [PubMed] [Google Scholar]
- 17. Hao Q, Hansen JB, Petersen RK, Hallenborg P, Jorgensen C, Cinti S, Larsen PJ, Steffensen KR, Wang H, Collins S, Wang J, Gustafsson JA, Madsen L, Kristiansen K. ADD1/SREBP1c activates the PGC1-alpha promoter in brown adipocytes. Biochim Biophys Acta 1801: 421–429, 2010. [DOI] [PubMed] [Google Scholar]
- 18. Hsiao LL, Dangond F, Yoshida T, Hong R, Jensen RV, Misra J, Dillon W, Lee KF, Clark KE, Haverty P, Weng Z, Mutter GL, Frosch MP, MacDonald ME, Milford EL, Crum CP, Bueno R, Pratt RE, Mahadevappa M, Warrington JA, Stephanopoulos G, Gullans SR. A compendium of gene expression in normal human tissues. Physiol Genomics 7: 97–104, 2001. [DOI] [PubMed] [Google Scholar]
- 19. Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, Spiegelman BM. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature 460: 1154–1158, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab 11: 257–262, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL, Chin S, Tempst P, Lazar MA, Spiegelman BM. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 22: 1397–1409, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci USA 109: 9635–9640, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kodama K, Horikoshi M, Toda K, Yamada S, Hara K, Irie J, Sirota M, Morgan AA, Chen R, Ohtsu H, Maeda S, Kadowaki T, Butte AJ. Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes. Proc Natl Acad Sci USA 109: 7049–7054, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Komuro I, Izumo S. Csx: a murine homeobox-containing gene specifically expressed in the developing heart. Proc Natl Acad Sci USA 90: 8145–8149, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leek JT, Scharpf RB, Bravo HC, Simcha D, Langmead B, Johnson WE, Geman D, Baggerly K, Irizarry RA. Tackling the widespread and critical impact of batch effects in high-throughput data. Nat Rev Genet 11: 733–739, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leonard AE, Bobik EG, Dorado J, Kroeger PE, Chuang LT, Thurmond JM, Parker-Barnes JM, Das T, Huang YS, Mukerji P. Cloning of a human cDNA encoding a novel enzyme involved in the elongation of long-chain polyunsaturated fatty acids. Biochem J 350: 765–770, 2000. [PMC free article] [PubMed] [Google Scholar]
- 27. Marshall OJ. PerlPrimer: cross-platform, graphical primer design for standard, bisulphite and real-time PCR. Bioinformatics 20: 2471–2472, 2004. [DOI] [PubMed] [Google Scholar]
- 28. Mecham BH, Nelson PS, Storey JD. Supervised normalization of microarrays. Bioinformatics 26: 1308–1315, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Minty AJ, Alonso S, Caravatti M, Buckingham ME. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell 30: 185–192, 1982. [DOI] [PubMed] [Google Scholar]
- 30. Moreau Y, Tranchevent LC. Computational tools for prioritizing candidate genes: boosting disease gene discovery. Nat Rev Genet 13: 523–536, 2012. [DOI] [PubMed] [Google Scholar]
- 31. Nilsson R, Schultz IJ, Pierce EL, Soltis KA, Naranuntarat A, Ward DM, Baughman JM, Paradkar PN, Kingsley PD, Culotta VC, Kaplan J, Palis J, Paw BH, Mootha VK. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab 10: 119–130, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15: 395–404, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, Scheinin M, Taittonen M, Niemi T, Enerback S, Virtanen KA. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab 14: 272–279, 2011. [DOI] [PubMed] [Google Scholar]
- 34. Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest 122: 545–552, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park PJ, Pagano M, Bonetti M. A nonparametric scoring algorithm for identifying informative genes from microarray data. Pac Symp Biocomput 52–63, 2001. [DOI] [PubMed] [Google Scholar]
- 36. Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 285: 7153–7164, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92: 829–839, 1998. [DOI] [PubMed] [Google Scholar]
- 38. Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev 14: 1293–1307, 2000. [PubMed] [Google Scholar]
- 39. Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest 121: 96–105, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab 6: 38–54, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shimomura I, Shimano H, Horton JD, Goldstein JL, Brown MS. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J Clin Invest 99: 838–845, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sirota M, Dudley JT, Kim J, Chiang AP, Morgan AA, Sweet-Cordero A, Sage J, Butte AJ. Discovery and preclinical validation of drug indications using compendia of public gene expression data. Sci Transl Med 3: 96ra77, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 38: D792–D799, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, Zeltser LM, Chung WK, Leibel RL. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 294: R1185–R1196, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA 99: 4465–4470, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA 101: 6062–6067, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thienpont B, Zhang L, Postma AV, Breckpot J, Tranchevent LC, Van Loo P, Mollgard K, Tommerup N, Bache I, Tumer Z, van Engelen K, Menten B, Mortier G, Waggoner D, Gewillig M, Moreau Y, Devriendt K, Larsen LA. Haploinsufficiency of TAB2 causes congenital heart defects in humans. Am J Hum Genet 86: 839–849, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Troyanskaya OG, Garber ME, Brown PO, Botstein D, Altman RB. Nonparametric methods for identifying differentially expressed genes in microarray data. Bioinformatics 18: 1454–1461, 2002. [DOI] [PubMed] [Google Scholar]
- 50. van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508, 2009. [DOI] [PubMed] [Google Scholar]
- 51. Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463: 1035–1041, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Villanueva CJ, Waki H, Godio C, Nielsen R, Chou WL, Vargas L, Wroblewski K, Schmedt C, Chao LC, Boyadjian R, Mandrup S, Hevener A, Saez E, Tontonoz P. TLE3 is a dual-function transcriptional coregulator of adipogenesis. Cell Metab 13: 413–427, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med 360: 1518–1525, 2009. [DOI] [PubMed] [Google Scholar]
- 54. Walker JR, Su AI, Self DW, Hogenesch JB, Lapp H, Maier R, Hoyer D, Bilbe G. Applications of a rat multiple tissue gene expression data set. Genome Res 14: 742–749, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Park C, Choi K, Bickel PE. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. J Lipid Res 47: 450–460, 2006. [DOI] [PubMed] [Google Scholar]
- 56. Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. [DOI] [PubMed] [Google Scholar]
- 58. Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476: 228–231, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang W, Morris QD, Chang R, Shai O, Bakowski MA, Mitsakakis N, Mohammad N, Robinson MD, Zirngibl R, Somogyi E, Laurin N, Eftekharpour E, Sat E, Grigull J, Pan Q, Peng WT, Krogan N, Greenblatt J, Fehlings M, van der Kooy D, Aubin J, Bruneau BG, Rossant J, Blencowe BJ, Frey BJ, Hughes TR. The functional landscape of mouse gene expression. J Biol 3: 21, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.