Summary
Tumor cell survival critically depends on heterotypic communication with benign cells in the microenvironment. Here, we describe a survival signaling pathway activated in stromal cells by contact to B cells from patients with chronic lymphocytic leukemia (CLL). The expression of protein kinase C (PKC)-βII and the subsequent activation of NF-κB in bone marrow stromal cells are prerequisites to support the survival of malignant B cells. PKC-β knockout mice are insusceptible to CLL transplantations, underscoring the in vivo significance of the PKC-βII-NF-κB signaling pathway in the tumor microenvironment. Upregulated stromal PKC-βII in biopsies from patients with CLL, acute lymphoblastic leukemia, and mantle cell lymphoma suggests that this pathway may commonly be activated in a variety of hematological malignancies.
Highlights
► Malignant B cells induce the expression of PKC-βII in bone marrow stromal cells ► The activation of NF-κB in tumor stromal cells strictly depends on PKC-βII ► The PKC-βII-NF-κB pathway is indispensable for survival of malignant B cells in vivo ► The PKC-βII-NF-κB pathway is activated by ALL and mantle cell lymphoma cells
Significance
Tumor-host interactions are crucial for the survival and progression of cancer cells. Specific targeting of the tumor microenvironment may therefore constitute an alternative to cytotoxic therapies. Here, we show that the expression of PKC-βII in the tumor microenvironment is induced by malignant cells from patients with CLL, ALL, and mantle cell lymphoma and required for the activation of NF-κB in bone marrow stromal cells. Interference with PKC-βII induction critically impairs the survival of CLL cells in vitro and in vivo, demonstrating that therapeutic targeting of the PKC-βII-NF-κB signaling pathway activated in the tumor microenvironment may be a meaningful treatment option.
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common B cell malignancies in adults, characterized by an accumulation of monoclonal CD5+ mature B cells in lymphoid tissues and the peripheral blood. The deletion of chromosome 13q14.3 represents the most common genetic alteration in CLL, causing autonomous B cell proliferation by affecting the expression of the microRNA cluster 15a/16-1 (Döhner et al., 2000; Klein et al., 2010). Whole-genome sequencing recently identified recurrent mutations in NOTCH1, MYD88, and SF3B1 in CLL, opening up insights in the mechanisms of clonal evolution (Fabbri et al., 2011; Puente et al., 2011; Quesada et al., 2012; Wang et al., 2011). Increased expression levels of antiapoptotic proteins have reinforced the hypothesis that a cell intrinsic defect of apoptosis is causative for B cell longevity and a steady increase in the number of malignant B cells over time (Cimmino et al., 2005; Kitada et al., 1998). However, primary CLL cells rapidly die ex vivo despite high levels of Bcl2 but can be cultured for weeks in the presence of different types of stromal cells (Burger et al., 2000; Ding et al., 2009; Pedersen et al., 2002). This indicates that the apoptosis defect in CLL is not cell autonomous but highly dependent on extrinsic signals derived from their microenvironment. Notably, this is not a static interaction in which stromal cells constitutively provide prosurvival signals to malignant cells but a dynamic process driven by bidirectional communications between the two. In the present study, we sought to investigate how CLL cells activate bone marrow stromal cells (BMSCs) and to characterize the signaling pathways and their functional consequences underlying this cell-cell communication.
Results
Stromal Cells Reminiscent of Cancer-Associated Fibroblasts Support the Survival of Malignant B Cells Derived from Patients with CLL
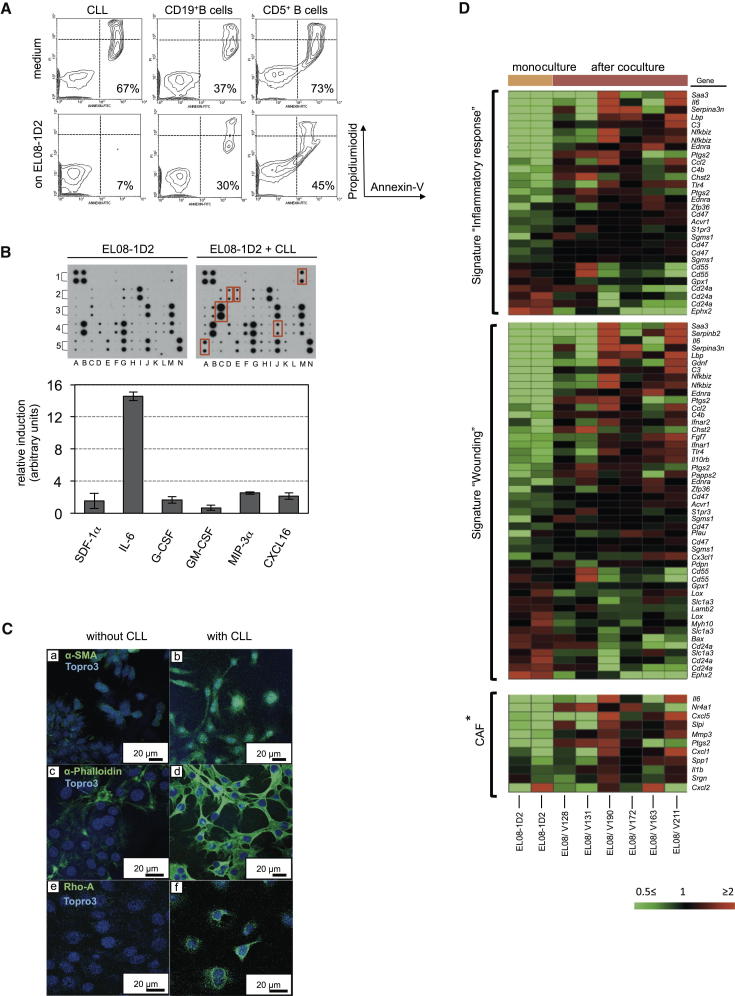
To study heterotypic cell-cell communications between stromal and CLL cells, we established a coculture system using primary leukemic B cells derived from patients’ blood and the murine cell line EL08-1D2 (Figure S1A available online), which has been carefully characterized as a stromal cell line able to maintain hematopoietic progenitor and stem cells ex vivo (Oostendorp et al., 2002). Analysis of apoptotic CLL cells after 5 days of coculture demonstrated that they were protected from spontaneous apoptosis. This antiapoptotic effect of stromal cells could not be recapitulated with CD19+ peripheral blood B cells. Notably, stromal cells provided little protection from spontaneous apoptosis of CD5+ B1 cells derived from blood of healthy donors (Figures 1A and S1B).
Figure 1.
Stromal Cells Reminiscent of CAFs Support the Survival of CLL Cells
(A) Malignant B cells from patients with CLL, or CD19+ or CD5+ B cells from healthy individuals, were cultured on the stromal cell line EL08-1D2 for 5 days before analyzing apoptotic cells by Annexin-V/PI staining.
(B) Cell-free supernatants from EL08-1D2 cells or from cocultures with CLL after 5 days were analyzed using a mouse cytokine antibody array. Results from three individual experiments with different primary CLL cells were quantified using ImageJ software. Fold induction is defined as compared to cytokine levels detected in an EL08-1D2 monoculture. Error bars show mean ± SEM. Significantly upregulated cytokines upon CLL contact are marked by red boxes.
(C) Immunofluorescent staining for α-SMA (a and b), stress fibers (c and d, indicated by the organization of actin bundles, stained by using α-phalloidin), and RhoA (e and f) in EL08-1D2 cells. CLL cells were removed from cocultures before fixation and staining the sections with antibodies.
(D) Gene expression profiles of EL08-1D2 cells without and after 5 days’ contact with CLL cells were compared. Heatmaps show the expression of gene ontologies exhibiting significant overrepresentation in the target list derived from the microarray experiment. All probe sets interrogating the respective target genes are given. The ∗CAF panel shows significantly upregulated genes in CAFs from dysplastic skin tissue (Erez et al., 2010). “V” numbers indicate encrypted patient IDs.
To define cytokines induced in CLL-stroma cocultures, supernatants from these cocultures were analyzed using a mouse-cytokine antibody array. Of the 62 cytokines measured in this assay, 6 were significantly upregulated in CLL-stroma cocultures: SDF-1α, IL-6, G-CSF, GM-CSF, MIP-3α, and CXCL16 (Figure 1B). Because all the antibodies used in this analysis, with the exception of anti-SDF-1α, were specific to mouse cytokines, the detected cytokines must have been produced by the murine stroma and not the human CLL cells. The consistent upregulation of proinflammatory cytokines by stromal cells in response to contact with leukemic B cells suggested that EL08-1D2 cells share properties akin to so-called cancer-associated fibroblasts (CAFs).
CAFs are characterized by promoting growth and invasion of epithelial tumors (Kalluri and Zeisberg, 2006), but their role in the pathogenesis of CLL is less clear. Immunofluorescence of EL08-1D2 cells demonstrated that α-SMA and stress fibers, both of which have been used to identify CAFs (Kalluri and Zeisberg, 2006; Tlsty and Coussens, 2006), were induced by contact with CLL cells (Figure 1C, a–d). This remodeling of the actin skeleton depends on the GTP-binding protein RhoA (Ridley and Hall, 1992). Correlating with the formation of stress fibers, RhoA was expressed in EL08-1D2 cells upon CLL contact (Figure 1C, e and f).
For further characterization of EL08-1D2 cells, we compared transcriptomes of EL08-1D2 cells before and after 5 days of contact with CLL cells, derived from multiple donors. We found 474 genes consistently increased (Table S1) and 347 decreased (Table S2) in stromal cells, of which 129 and 40 genes showed greater than 2-fold change, respectively. Subjecting these 821 consistently altered target probe sets to overrepresentation analysis using GeneTrail (http://genetrail.bioinf.uni-sb.de/index.php) revealed significant enrichments for the gene ontology terms “inflammatory response” (p = 6.0 × 10−5) and “response to wounding” (p = 7.2 × 10−9) (Figure 1D). It was recently reported that CAFs from skin, mammary, and pancreatic tumors in mice are characterized by a proinflammatory gene signature (Erez et al., 2010). Comparison of those genes to our results revealed significant overlap of target genes (Figure 1D, lower panel). Therefore, in coculture, EL08-1D2 cells support the survival of malignant B cells and undergo genetic and morphologic alterations reminiscent of CAFs.
CLL Cells Induce the Expression of Stromal PKC-βII
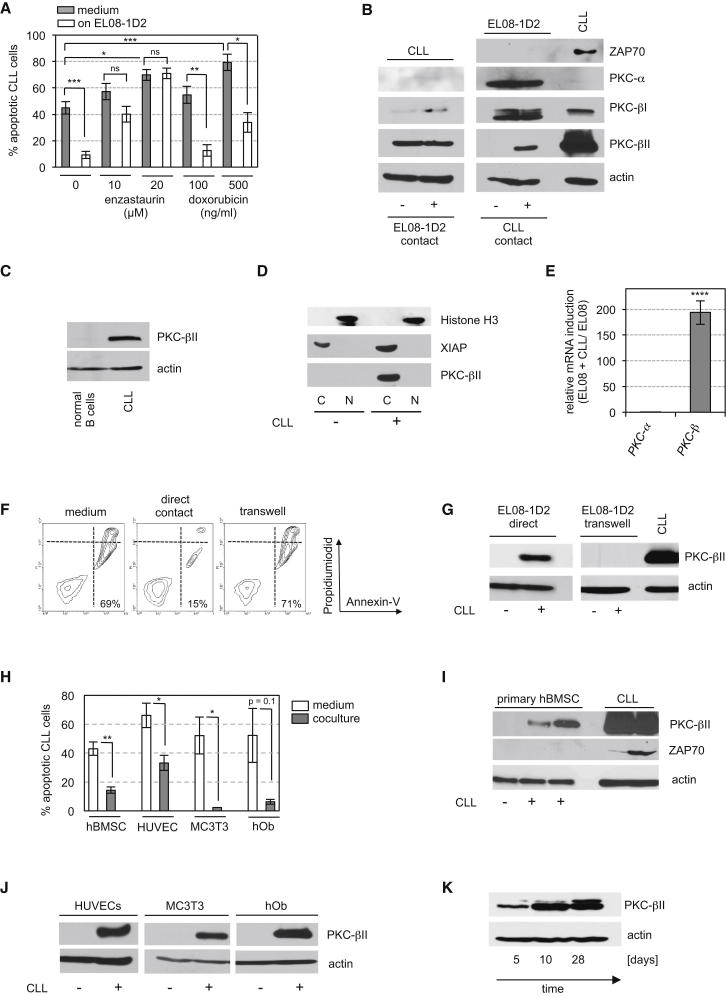
Interactions between fibroblasts and tumor cells can contribute to drug resistance by increasing the expression of antiapoptotic proteins in tumor cells (Meads et al., 2009). We previously reported that the intrinsic activation of protein kinase C (PKC)-βII in CLL cells mediates apoptosis resistance due to posttranslational modifications of Bcl2 and BimEL (zum Büschenfelde et al., 2010). Therefore, we tested whether the PKC-β inhibitor enzastaurin could overcome the protective effect of EL08-1D2 cells on CLL cells. CLL cells cultured on EL08-1D2 cells were not protected from the cytotoxic effect of enzastaurin, whereas the cytotoxicity of doxorubicin was mitigated by contact with stromal cells (Figure 2A). Importantly, neither enzastaurin nor doxorubicin significantly influenced the viability of EL08-1D2 cells (Figure S2A). These data imply that PKC-β plays an important role in stroma-CLL interactions. To further analyze this effect, we assessed the expression of classical PKC isoforms in either cell compartment before and after coculture. CLL cells predominantly express PKC-βII (Abrams et al., 2007), but its level of expression was unmodified after contact with EL08-1D2 cells (Figure 2B). In contrast, PKC-βII was not constitutively expressed in EL08-1D2 cells. However, contact with several different primary leukemic B cells induced the expression of PKC-βII in EL08-1D2 cells, but not PKC-α or PKC-βI, that were constitutively expressed in EL08-1D2 cells. The absence of ZAP70 in stromal cell lysates of coculture experiments with a ZAP70-positive CLL demonstrated that CLL contamination of stromal cell lysates is negligible in these experiments (Figures 2B and S2B). Of note, normal peripheral blood B cells from healthy donors did not induce PKC-βII in stromal cells (Figure 2C).
Figure 2.
Enhanced Survival of CLL Cells Is Associated with Induction of PKC-β in Stromal Cells
(A) CLL cells in media or after 5 days of coculture with EL08-1D2 cells were exposed to enzastaurin or doxorubicin for 48 hr. Apoptotic leukemic cells were detected by Annexin-V/PI staining. Error bars show mean ± SEM from eight (medium) and five (cocultures) individual experiments. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05; ns, statistically nonsignificant.
(B) CLL and EL08-1D2 cells were separated after 5 days’ coculture. Expression of classical PKC isoforms was analyzed in each cell compartment. One representative western blot out of three individual experiments with different CLL samples is shown.
(C) Expression of PKC-βII in EL08-1D2 cells after coculture with normal CD19+ B cells or CLL.
(D) PKC-βII in the cytoplasmic (C) or nuclear (N) fraction of EL08-1D2 cells after contact with CLL cells is detected using immunoblotting. One representative experiment out of four is shown.
(E) Quantitative RT-PCR using mouse-specific primers for PKC-β and PKC-α in EL08-1D2 cells. The relative induction of the expression of each gene by CLL contact was normalized to the expression of actin and compared to the expression in EL08-1D2 monocultures (depicted as fold induction). Results are calculated by the Pfaffl formula based on mean Ct values ±SEM from five experiments. ∗∗∗∗p < 0.0001.
(F and G) CLL cells and EL08-1D2 were directly cocultured or separated by a transwell. After 5 days, apoptotic CLL cells were detected by Annexin-V/PI staining (F), and PKC-βII expression was assessed by western blotting (G). One representative experiment out of three is shown.
(H) CLL cells were cocultured with primary hBMSCs (n = 5), HUVECs (n = 4), hObs (n = 3), and MC3T3 (n = 3) for 5 days before analyzing apoptotic CLL cells. Error bars show mean ± SEM.
(I) Induction of PKC-βII in primary hBMSCs following 5 days of coculturing with two primary CLLs. ZAP70-positive CLL cells were used as positive controls.
(J) PKC-βII expression in HUVECs and osteoblasts detected by immunoblotting at the end of the coculture experiment.
(K) PKC-βII expression in primary hBMSCs after coculturing with CLL cells at indicated time points.
See also Figure S2.
Subcellular fractioning of stromal cell proteins before and after CLL contact indicated that induced PKC-βII was located in the cytoplasm, but not the nucleus (Figure 2D). Quantitative RT-PCR using mouse-specific primers showed that upregulation of PKC-β in EL08-1D2 cells was mostly due to transcriptional regulation (Figure 2E). To further exclude the possibility of cross-contamination with PKC-βII-positive CLL cells, we assessed PKC-βII expression in stromal cells by immunofluorescence. After 5 days of coculturing CLL cells on EL08-1D2 cells, CLL cells were removed and stromal cells analyzed for the expression of PKC-βII. Stromal cells could be identified by a positive staining for Sca-1 (Oostendorp et al., 2002), which is not expressed on CLL cells. Stromal PKC-βII was detected with a perinuclear expression pattern, characteristic of activated PKC-βII (Becker and Hannun, 2003) (Figure S2C, a–d and i). PKC-βII could not be detected in EL08-1D2 monocultures (Figure S2C, e–h). We next examined whether a direct contact between CLL cells and stromal cells was required to protect CLL cells from apoptosis and to induce the expression of PKC-βII in EL08-1D2 cells, and we found that both were lost if these cell compartments were separated in a transwell experiment (Figures 2F and 2G). Furthermore, conditioned medium from EL08-1D2/CLL cocultures failed to support survival of leukemic cells (Figure S2D).
To eliminate the concern that the results seen so far were limited to coculturing human leukemic B cells on a murine cell line, we cocultured primary human CLL cells on primary human BMSCs (hBMSCs). Similar to EL08-1D2 cells, hBMSCs supported ex vivo survival of CLL cells, and accordingly, PKC-βII was induced (Figures 2H and 2I). Irradiation of EL08-1D2 cells or hBMSCs before coculturing abolished their antiapoptotic effect on CLL cells, indicating that only signaling competent stromal cells can protect CLL cells from cell death (Figure S2E).
hBMSCs are a heterogeneous mixture of different cell types. To further define the subsets of cells that were responsible for these effects, we cocultured CLL cells on human umbilical vascular endothelial cells (HUVECs), primary human osteoblasts (hObs), and a murine osteoblast-cell line (MC3T3) under comparable conditions as used for EL08-1D2/CLL cocultures. Remarkably, all cell types supported CLL survival (Figure 2H), accompanied by PKC-βII induction (Figure 2J). Finally, extended cocultures of CLL cells and hBMSCs for 4 weeks demonstrated that stromal PKC-βII induction was not transient but persistent (Figure 2K).
Induction of Stromal PKC-β Is Required for Stromal Cell-Mediated Protection of CLL Cells
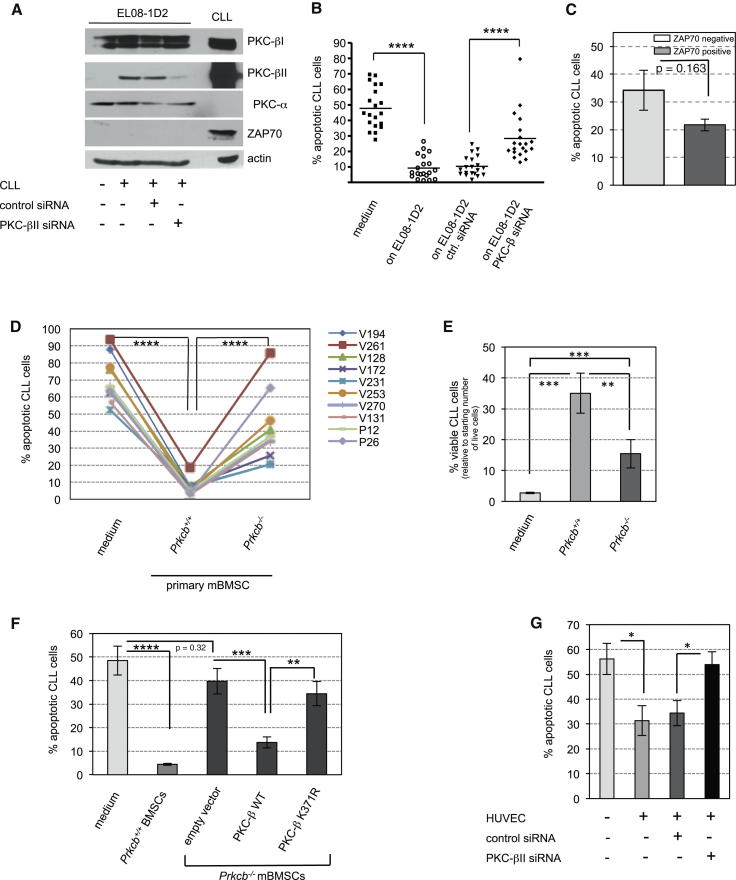
The strong correlation between PKC-βII upregulation and survival of CLL cells suggested that stromal PKC-βII was important for the antiapoptotic signals provided by EL08-1D2 cells. To test this hypothesis, PKC-βII induction in EL08-1D2 cells was suppressed using a siRNA directed against stromal PKC-βII. siRNA transfection of EL08-1D2 was performed 24 hr before coculturing with CLL cells to ensure that the PKC-βII of CLL cells was not targeted. PKC-α and PKC-β expression in EL08-1D2 cells was assessed by immunoblotting after 5 days of coculturing with CLL cells and showed that only PKC-βII was effectively targeted (Figure 3A). Analyzing the viability of CLL cells cultured on EL08-1D2 cells proficient or depleted of PKC-βII indicated that stromal PKC-βII was required for the survival of CLL cells (Figure 3B). Knockdown of stromal PKC-βII significantly impaired the survival of all samples tested from individual patients with CLL. This was particularly relevant because CLL is a heterogeneous disease with variations in the clinical course. The aberrant expression of ZAP70 in monoclonal B cells can be used as a prognostic marker of a more aggressive variant of CLL (Crespo et al., 2003). However, PKC-βII knockdown in stromal cells affected the survival of ZAP70-positive and -negative CLL, similarly (Figure 3C).
Figure 3.
Induction of PKC-βII in Stromal Cells Is Crucial for Apoptosis Protection of CLL Cells
(A) EL08-1D2 cells were transfected with siRNA directed against PKC-βII or a control siRNA. Twenty-four hours after transfection, CLL cells were cocultured on EL08-1D2 cells for 5 days. PKC-βII expression was assessed by immunoblotting performed from cell lysates of EL08-1D2 cells at the end of the coculture (n = 4 individual experiments, using different CLL samples).
(B) Survival of CLL on EL08-1D2 cells, transfected with siRNAs as indicated, was assessed by Annexin-V/PI staining of leukemic B cells after 5 days (n = 20). ∗∗∗∗p < 0.0001.
(C) Data from (B) were analyzed based on ZAP70 expression of CLL cells (n = 7 for ZAP70 positive and n = 8 for negative samples).
(D and E) Survival of CLL cells on primary BMSCs from four individual Prkcb−/− or wild-type mice. Viability was assessed by a negative Annexin-V/PI staining (D) or by trypan blue exclusion (E). Patient IDs were encrypted using V and P numbers. The following patient samples were cocultures on BMSCs from Prkcb−/−: animal 1, V261, V128, and P12; animal 2, V172 and P26; animal 3, V231, V253, and V194; and animal 4, V270 and V131. ∗∗∗p < 0.001, ∗∗p < 0.01.
(F) Ten individual CLL samples were cultured in medium or cocultured on BMSCs from wild-type or Prkcb−/− animals for 5 days. Wild-type PKC-β or a kinase-dead mutant of PKC-β (K371R) was ectopically expressed in Prkcb−/− BMSCs as indicated. Apoptotic CLL cells were analyzed by Annexin-V/PI staining after 5 days’ coculture.
(G) Survival of CLL cells was assessed by Annexin-V/PI staining after 5 days of coculture with HUVECs that were transfected with siRNAs as indicated (n = 10). The experimental conditions were similar to the experiment depicted in (A). ∗p < 0.05.
Error bars in the entire figure show mean ± SEM.
To eliminate the possibility that off-target effects of the PKC-βII siRNA accounted for the impaired survival of CLL cells, we recapitulated our experiments with primary BMSCs from wild-type and PKC-β knockout (Prkcb−/−) mice. Prkcb−/− mice, which lack both βI and βII isoforms, are characterized by an impaired immune response but have no overt phenotype in the bone marrow stromal compartment (Leitges et al., 1996). CLL cells were effectively protected from spontaneous apoptosis when cocultured on wild-type BMSCs, accompanied by induction of PKC-β in stromal cells (Figures 3D and S3A). This protection effect was significantly impaired on Prkcb−/− stromal cells (Figures 3D and 3E). Characterization of the primary CLL cells used in these experiments, based on standard cytogenetic analyses and sequencing of TP53 (exons 4–11) and NOTCH (exons 25–34), did not identify any subset of patients being more dependent on PKC-β-mediated survival than others (Table S3).
Ectopic expression of the wild-type PKC-β in Prkcb−/− BMSCs rescued their ability to provide survival cues to CLL cells (Figure 3F), confirming that PKC-β expressed in stromal cells is required for the survival of CLL cells. Moreover, expression of a kinase-dead mutant (K371R) of PKC-β (Feng and Hannun, 1998) failed to restore the survival functions of Prkcb−/− BMSCs, indicating that the kinase activity of PKC-β is essential for stroma-mediated protection of CLL cells from apoptosis (Figures 3F and S3B). The uniform upregulation of PKC-βII (Figures 2I and 2J) suggested that the antiapoptotic effect of different types of stromal cells on CLL cells was attributed to its expression. Consistent with this hypothesis, knockdown of PKC-βII in HUVECs abolished their prosurvival effects on CLL cells (Figure 3G).
PKC-βII Activates Stromal NF-κB through the Canonical Pathway
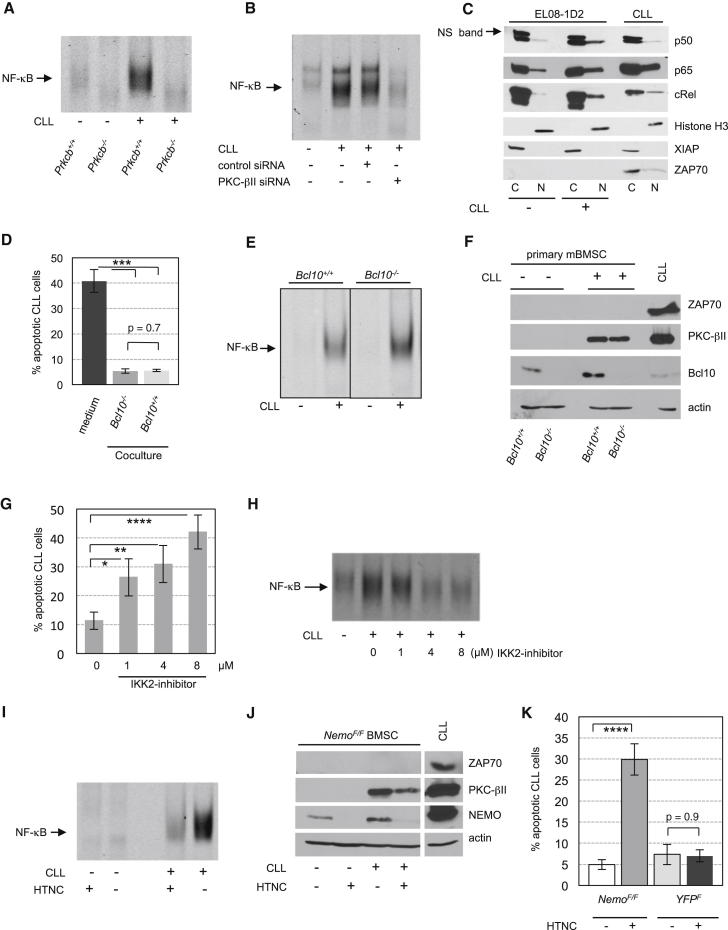
PKC-β connects the B cell receptor to canonical activation of NF-κB through a signaling complex, including Bcl10/MALT1 and NEMO/IKKγ (Zhou et al., 2004). Because stromal cells activated by CLL cells showed a proinflammatory phenotype (Figure 1), we investigated whether NF-κB activation could also occur following induction of PKC-βII in stromal cells and found that activation of NF-κB occurred in wild-type, but not in Prkcb−/− BMSCs in response to CLL contact (Figure 4A). These results were recapitulated in EL08-1D2 cells and in hBMSCs (Figures 4B and S4A).
Figure 4.
Stromal PKC-βII Is Essential for NF-κB Activation in BMSCs in Response to Contact with CLL Cells
(A) NF-κB activation was assessed by EMSA in primary mBMSCs from wild-type or Prkcb−/− mice after 5 days of coculturing with CLL cells. One out of three experiments is shown.
(B) Activation of NF-κB in EL08-1D2 cells, transfected with the indicated siRNAs, was analyzed by EMSA after 5 days of coculture with CLL.
(C) NF-κB subunits in cytosolic (C) and nuclear (N) fractions from EL08-1D2 cells after 5 days in contact with CLL cells were detected by immunoblotting. Ns. band, nonspecific band. One out of four experiments with different primary CLL cells is shown.
(D–F) CLL cells were cocultured on BMSCs from wild-type or aged-matched Bcl10−/− mice for 5 days. Apoptosis of CLL cells was determined by Annexin-V staining (D, n = 10). NF-κB activity was measured by EMSA (E), and PKC-βII expression was determined by immunoblotting (F). ∗∗∗p < 0.001.
(G and H) Cocultures of CLL cells and EL08-1D2 cells were exposed to an IKK2 inhibitor for 5 days at the indicated concentrations. Apoptotic CLL cells were analyzed by Annexin-V staining (G, n = 18), and NF-κB activation in EL08-1D2 cells was determined by EMSA (H). ∗∗∗∗p < 0.0001, ∗∗p < 0.01, ∗p < 0.05.
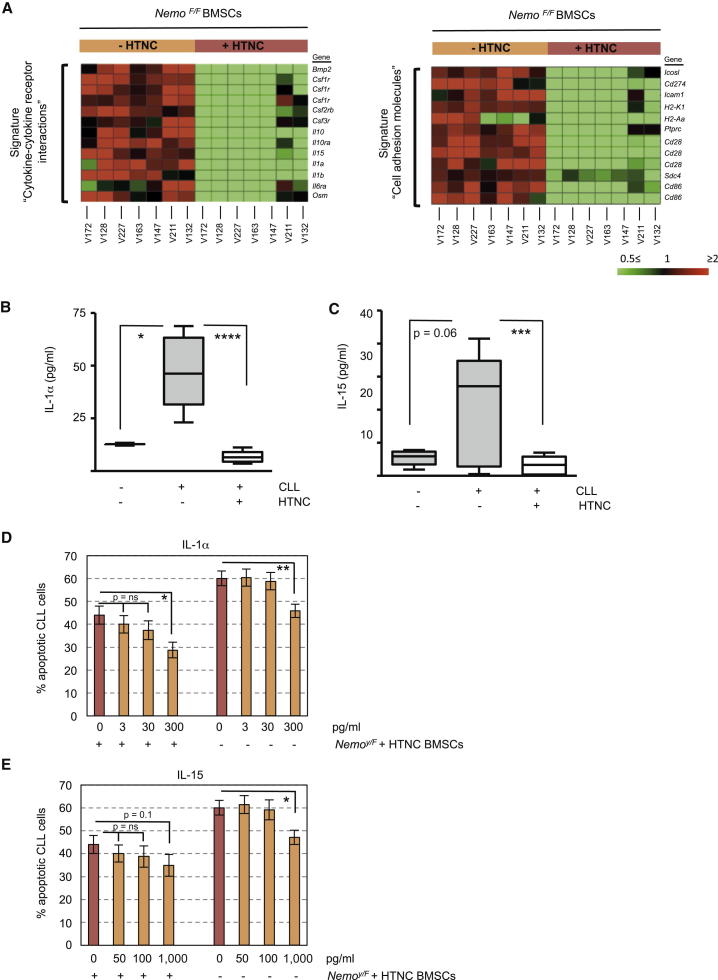
(I and J) BMSCs from NemoF/F mice were harvested, and Nemo was deleted by treating stromal cells overnight with HTNC. After propagation of stromal cells for additional two passages, cells were analyzed for their ability to activate NF-κB after contact with CLL cells for 5 days using EMSA (I). Accordingly, NEMO and PKC-βII expression was assessed in mBMSCs at the end of the experiment (J).
(K) Survival of CLL cells on HTNC-treated and untreated NemoF/F BMSCs was determined by Annexin-V staining after 5 days (n = 10). Identically treated mBMSCs from YFPF mice were used as controls.
Error bars in the entire figure show mean ± SEM.
See also Figure S4.
To test whether the loss of NF-κB activation was primarily dependent on PKC-β expression or a secondary event based on the impaired survival of CLL cells on PKC-β-deficient stromal cells, apoptosis of CLL cells was inhibited using the caspase inhibitor z.vad.fmk. Caspase inhibition rescued CLL cells cultured on PKC-β-deficient stromal cells from spontaneous apoptosis but did not result in activation of NF-κB in PKC-β-deficient stromal cells (Figures S4B and S4C), suggesting that PKC-β is directly involved in the activation of NF-κB. This activation is regulated by nuclear translocation and DNA binding of NF-κB subunits (Hayden and Ghosh, 2004). Fractionation of EL08-1D2 cell lysates showed that the NF-κB subunits p50, p65, and c-REL accumulated in the nucleus upon contact with monoclonal B cells (Figures 4C and S4D).
To further elaborate whether Bcl10 was linking PKC-βII to NF-κB activation in stromal cells, we used BMSCs from Bcl10−/− and wild-type mice for coculture experiments. Primary CLL cells survived equally well on Bcl10−/− and wild-type stromal cells (Figure 4D). Accordingly, CLL-dependent activation of NF-κB was not inhibited in Bcl10−/− stroma (Figure 4E). Notably, stromal PKC-βII was induced upon CLL contact regardless of Bcl10 (Figure 4F), excluding Bcl10 as a critical protein for PKC-βII-mediated activation of NF-κB in stroma cells.
To assess whether stromal NF-κB was important for the prosurvival effects on malignant B cells, we analyzed the viability of CLL cells in cocultures with EL08-1D2 cells in the presence of an IKK2 inhibitor (Ziegelbauer et al., 2005). IKK2 (IKKβ) is a member of the multiprotein IκB complex that also contains IKK1 (IKKα) and the essential regulatory subunit NEMO. Blockage of IKK2 significantly affected survival of CLL cells on stromal cells without affecting the viability of EL08-1D2 cells (Figures 4G and S2A). This impaired survival of CLL cells was associated with a decreased activation of NF-κB in stromal cells (Figure 4H). However, it was possible that the proapoptotic effect of the IKK2 inhibitor was exclusively related to NF-κB inhibition in CLL cells. To disentangle stroma from CLL-specific effects, we used BMSCs derived from conditional Nemo-knockout mice (NemoF) (Schmidt-Supprian et al., 2000) and as controls BMSCs from YFP reporter mice (Srinivas et al., 2001). Nemo was ablated from isolated monocultures of NemoF/F stromal cells by treatment with Cre protein (HTNC) (Figure 4J) (Peitz et al., 2002). NEMO-proficient and -deficient BMSCs were then propagated in the absence of HTNC for additional two passages before CLL cells were added. Activation of NF-κB in NEMO-deficient BMSCs was inhibited after CLL contact (Figure 4I), although PKC-βII was still induced (Figure 4J). These results indicated that PKC-βII was operating upstream of NEMO/NF-κB in BMSCs. Importantly, NEMO-deficient BMSCs failed to support the survival of primary CLL cells (Figure 4K), similar to loss of stromal PKC-βII (Figure 3). The slightly reduced induction of PKC-βII in NEMO-deficient BMSCs compared to untreated NemoF/F cells is most likely related to an impaired survival of and therefore reduced “induction signal” by CLL cells on these BMSCs (Figure 4J). These findings provide evidence that induction of PKC-βII in stromal cells precedes the activation of NF-κB and that the PKC-βII/NF-κB pathway is required for stromal cell-mediated survival of malignant B cells.
Stromal NF-κB Regulates the Expression of Proinflammatory Cytokines Required for CLL Survival
Comparing the transcriptome of HTNC-treated and -untreated NemoF/F murine bone marrow stroma cells (mBMSCs) after 5 days of coculturing with CLL revealed that 372 genes were significantly increased (≥2-fold) in NEMO-proficient BMSCs (Table S4). Accordingly, 388 genes were downregulated (≤0.5-fold) in BMSCs expressing NEMO (Table S5). Notably, BMSCs from NemoF/F mice were HTNC treated at least 8 days before the coculture with CLL cells, making it unlikely that changes in gene expression were merely related to the addition of HTNC. Using the GeneTrail software and the KEGG database to further analyze these genes, we identified a gene cluster of “cytokine and cytokine-receptors” (p = 0.029) and a cluster of “cell adhesion molecules” (p = 0.004), which were decreased in NEMO-deficient mBMSCs (Figure 5A).
Figure 5.
NF-κB Upregulates the Expression of Cytokines and Adhesion Molecules, Crucial for CLL Survival on Stromal Cells
(A) Transcriptomes from NEMO-proficient (−HTNC) BMSCs were compared to NEMO-deficient BMSCs (+HTNC) after 5 days of coculturing with primary CLL cells (n = 7, individual patients are indicated by V numbers). Data were subjected to functional pathway analysis using the GeneTrail software package. Heatmaps show two KEGG pathways exhibiting significant overrepresentation in the target list of consistently downregulated targets as determined by microarray analysis.
(B and C) Concentration of IL-1α (n = 9) and IL-15 (n = 15) was assessed in supernatants from NemoY/F-BMSC monocultures or from cocultures with CLL cells after 5 days. Boxes depict 25th−75th percentile; error bars indicate ±SEM of mean (line in the box). The Nemo gene is located on the X chromosome; therefore, hemizygous male mice (Nemoy/F) correspond genetically to homozygous NemoF/F mice. ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001, ∗p < 0.05.
(D and E) Increasing doses of murine IL-1α (D) and IL-15 (E) were supplemented to the media of CLL monocultures or CLL/NEMO-deficient BMSC cocultures for 5 days before the rate of apoptotic CLL cells was analyzed by Annexin-V/PI staining (n = 8). ∗∗p < 0.01; ns, statistically nonsignificant.
Error bars show mean ± SEM.
To validate the differential regulation of these genes, we analyzed the levels of IL-1α and IL-15, both of which have been described to support CLL survival in the absence of stromal cells (de Totero et al., 2008; Jewell et al., 1995), in supernatants from CLL and BMSC cocultures after 5 days. CLL contact induced the secretion of IL-1α by mBMSCs, which was completely abolished in NEMO-deficient BMSCs (Figure 5B). Similar to IL-1α, IL-15 was induced in a NEMO-dependent manner in stromal cells by CLL contact, although to greater variations (Figure 5C). Importantly, antibodies used in these ELISAs were specific for murine IL-1α and IL-15 and not cross-reactive to human cytokines, excluding the possibility that the detected factors were produced by human CLL cells. Upregulation of IL-1α and IL-15 mRNA in hBMSCs upon CLL contact (Figure S5A) indicated that these results were not limited to xenogeneic culture conditions.
Finally, to test that murine IL-1α, IL-1β, and IL-15 could rescue primary CLL cells from apoptosis, increasing doses of these cytokines were supplemented to CLL monocultures or CLL/NEMO-deficient BMSC cocultures. The analysis of apoptotic CLL cells after 5 days indicated that IL-1 and IL-15 partially rescued NEMO deficiency in BMSCs (Figures 5D, 5E, and S5B). Combining IL-1 and IL-15 demonstrated a stronger prosurvival effect on CLL cells (Figure S5C). Notably, cytokine doses required to partly compensate for NEMO deficiency in stromal cells exceeded the concentrations of IL-1 and IL-15 produced by stromal cells after CLL contact. This antiapoptotic effect was reproducible in CLL monocultures, indicating that these cytokines directly supported the survival of CLL cells through a paracrine mechanism (Figures 5D, 5E, and S5B). Importantly, mouse and human IL-1 and IL-15 are not entirely homologous (IL-1α, 61%; IL-1β, 69%; and IL-15, 71%), and therefore, their antiapoptotic effect may be underestimated in our experiment compared to an in vivo scenario.
Stromal PKC-β Is Indispensable for the Engraftment of TCL1/CLL In Vivo
Transgenic mice expressing TCL1 under the Eμ promotor (TCL1tg) show a clonal expansion of CD5+ B cells and die of a CLL-like disease (Bichi et al., 2002). About 40% of TCL1tg mice develop a solid malignant tumor unrelated to the expression of TCL1, mostly soft tissue sarcomas (Zanesi et al., 2006).
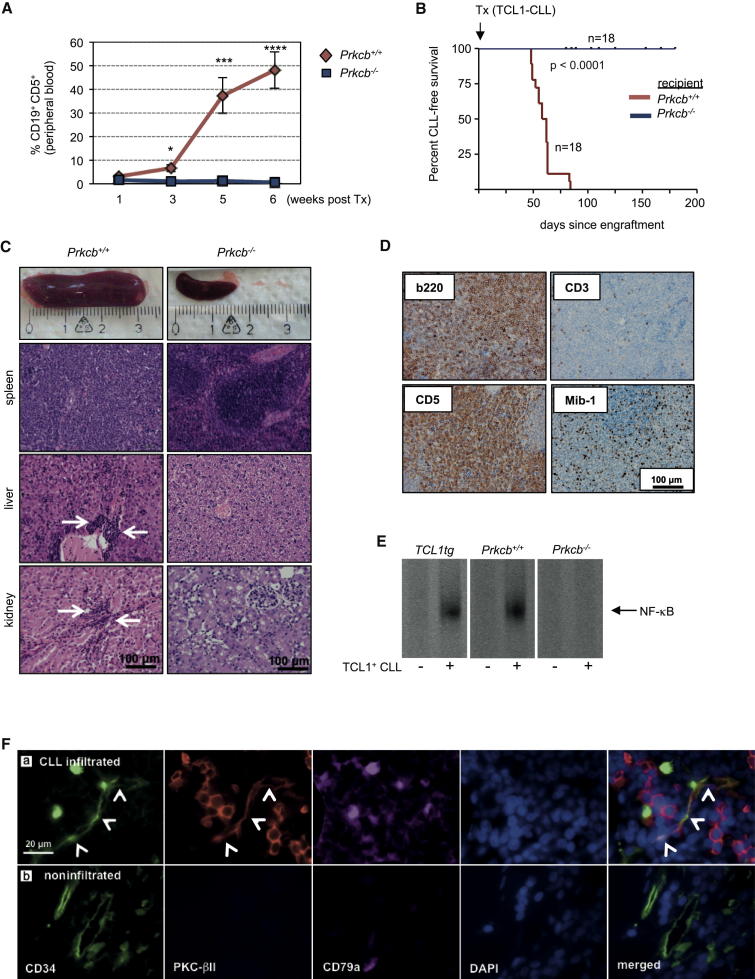
To test the relevance of PKC-β in the microenvironment for the survival of monoclonal B cells in vivo, we transplanted splenic cells from three individual lymphoma-bearing TCL1tg mice into Prkcb−/− or wild-type recipient mice. Because TCL1tg and Prkcb−/− mice were both backcrossed onto a C57BL/6 genetic background, no immunosuppression was needed before transplantation (Hofbauer et al., 2011). Tumor load was assessed weekly by analyzing the amount of CD19+CD5+ B cells in the peripheral blood of recipient mice. After 5 weeks, an increase of CD19+CD5+ cells was detected in the blood of PKC-β wild-type, but not of Prkcb−/− recipient mice (Figure 6A). All wild-type recipient mice died of a CLL-like disease after a mean of 59 days (range 48–84 days), whereas Prkcb−/− recipient mice stayed healthy and alive during that time (Figure 6B). We noticed that some of the Prkcb−/− recipient mice, all of which were transplanted with tumor 1, became moribund at much later time points (mean 125 days, range 80–180 days) (Figure S6A). Prkcb+/+ recipients showed a typical TCL1 phenotype, characterized by massive splenomegaly, hepatomegaly, lymphadenopathy, and clonal expansion of CD5+ B cells (Figures 6C and S6B). Histopathology showed a widespread lymphoma infiltration in lymphatic and extralymphatic tissues. Immunohistochemistry confirmed that these tumors were CD19+CD5+ B cell lymphomas with a proliferation index (Mib-1+) of 5%–10% (Figure 6D). Strikingly, necrobiopsy revealed that none of the Prkcb−/− recipient mice developed a CLL-like disease (Figure 6C), and spleen and lymph nodes appeared macroscopically normal. The few mice that were transplanted with tumor 1 and died showed large tumors in the peritoneal cavity. Single cells from these tumors lacked surface expression of lymphoid markers (data not shown). The presence of TCL1+CD19+CD5+ lymphocytes was assessed from spleens of Prkcb−/− and wild-type recipients at the time of sacrifice. Increased levels of CD19+CD5+ lymphocytes were only detectable in wild-type recipient mice, but not in Prkcb−/− animals (Figure S6C). Histopathology demonstrated that all tumors grown in Prkcb−/− mice were histiocytic soft tissue sarcomas (Figure S6D, a and b). Microscopically, sarcomas could also be detected in the spleen of some Prkcb+/+ recipient mice transplanted from the same primary tumor (Figure S6D, c and d).
Figure 6.
Stromal PKC-β Is Indispensable for Establishing and Propagating CLL In Vivo
(A and B) A total of 2 × 107 splenic PBMCs from three diseased TCL1tg mice was transplanted into syngeneic wild-type or Prkcb−/− recipient mice (n = 18 for each group: tumor 1 into eight Prkcb+/+ and eight Prkcb−/− mice; tumors 2 and 3 each in five recipient mice per genotype). The percentage of CD19+CD5+ B cells in the blood of recipient mice was assessed weekly by flow cytometry (A). Mice in both cohorts were followed for signs of illness and killed when moribund (B). Error bars show mean ± SEM. ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001.
(C) Necrobiopsy of Prkcb−/− (n = 12) and wild-type recipient mice (n = 16). White arrows indicate organ infiltration with lymphoma cells.
(D) Immunohistochemistry of tumors that developed in wild-type recipient animals.
(E) NF-κB activity was assessed by EMSA on nuclear extracts from TCL1tg, Prkcb+/+, and Prkcb−/− BMSCs, cultured in the absence or presence of CD19+CD5+ B cells harvested from a diseased TCL1tg mouse (n = 3).
(F) Representative images of human bone marrow trephine biopsies from 13 patients with CLL, analyzed for the expression of PKC-βII in endothelial cells (CD34+, white arrowheads) in CLL-infiltrated areas (a, CD79a+) and outside the CLL-infiltrated bone marrow (b).
See also Figure S6.
To test whether CD5+ lymphocytes from diseased TCL1tg mice were capable of inducing PKC-βII in BMSCs, we cocultured TCL1+CD19+CD5+ lymphocytes on primary murine BMSCs derived from wild-type, Prkcb−/−, and TCL1tg mice. Similar to primary CLL cells from patients, TCL1+CD19+CD5+ cells induced the expression of PKC-βII in wild-type and TCL1tg BMSCs (Figure S6E), associated with improved ex vivo survival (data not shown). Accordingly, NF-κB was activated in mBMSCs from wild-type mice, but not in Prkcb−/− stromal cells (Figure 6E). These data indicate unambiguously that PKC-β induced and expressed in the microenvironment is essential to establish a CLL-like disease in vivo, whereas stromal PKC-β seems to be dispensable for propagating soft tissue sarcomas.
To evaluate whether PKC-βII is upregulated in the microenvironment of patients with CLL, we assessed its expression in stromal cells in bone marrow trephine biopsies. To overcome the difficulties of a preponderance of malignant B cells that express PKC-βII and the lack of reliable markers for most stromal cells, we used CLL samples with a nodular, but not diffuse, infiltration of the bone marrow, allowing analyzing CLL-free and infiltrated sections in the same patient. In addition, endothelial cells are a subpopulation of stromal cells that can be reliably stained with an antibody against CD34 and can support survival of monoclonal B cells dependent on the expression of PKC-βII (Figures 2H, 2J, and 3G). Immunofluorescence of bone marrow trephine biopsies revealed that PKC-βII was only expressed in endothelial cells in sections containing CLL cell infiltrations (CD79a+), but not in those in CLL-free sections (Figure 6F).
Upregulated Stromal PKC-βII Is a Recurrent Finding in Hematological Malignancies
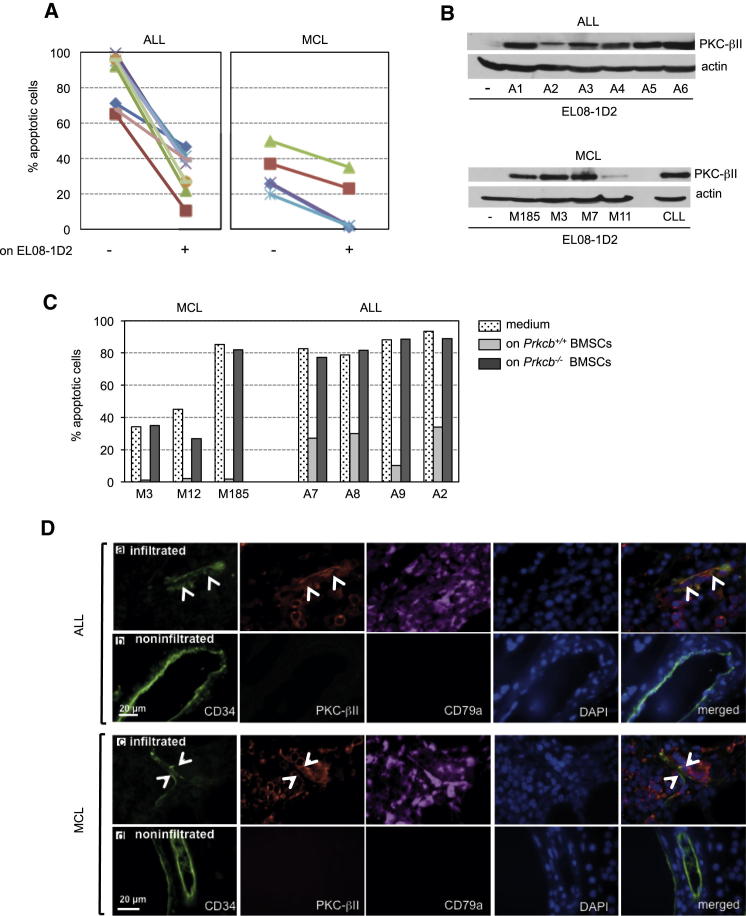
To test whether heterotypic interactions between BMSCs and the tumor are also important for the survival and propagation of other malignant cells, we cocultured primary human acute lymphoblastic leukemia (ALL) cells and mantle cell lymphoma (MCL) B cells for 5 days on EL08-1D2 cells. Although ALL cells died at a significant higher rate than MCL cells in vitro, contact with stromal cells reduced spontaneous apoptosis of both ALL and MCL cells (Figure 7A). Similar to CLL cells, primary ALL and MCL cells induced the expression of PKC-βII in EL08-1D2 cells (Figure 7B), suggesting that the PKC-βII/NF-κB pathway is commonly activated in B cell malignancies. Indeed, we found activated NF-κB in EL08-1D2 cells after coculture with ALL and MCL cells, similar to coculture with CLL cells (Figure S7). The importance of stromal PKC-βII for the survival of ALL and MCL cells was confirmed by the support of survival of ALL and MCL cells by the wild-type but not the Prkcb−/− BMSCs (Figure 7C). To evaluate whether PKC-βII was induced in stromal cells in vivo, we analyzed human bone marrow trephine biopsies from patients with a mature ALL and MCL for the expression of PKC-βII in endothelial cells. Endothelial cells surrounded by ALL and MCL cells expressed PKC-βII, whereas those outside did not (Figure 7D). These data show that stromal PKC-βII is upregulated in a variety of lymphoproliferative malignancies and suggest that activation of the PKC-βII pathway could represent a general response of the tumor microenvironment to support leukemogeneis/lymphomagenesis.
Figure 7.
Stromal PKC-βII Is Expressed in Lymphoproliferative Diseases
(A and B) Primary malignant lymphocytes from the blood of patients with ALL (n = 9) and MCL (n = 5) were cultured in medium or cocultured on EL08-1D2 cells for 5 days. Apoptotic B cells were quantified by Annexin-V/PI staining (A), and the expression of PKC-βII in EL08-1D2 cells was analyzed by immunoblotting (B). Patients’ ID were encrypted by A or M numbers.
(C) Primary B cells from three patients with MCL and four patients with ALL were cultured in medium or cocultured on confluent wild-type or Prkcb−/− BMSCs for 5 days. Apoptotic cells were detected by flow cytometry and Annexin-V/PI positivity. Patients’ ID were encrypted by A or M numbers.
(D) Representative images of endothelial cells (CD34+, white arrowheads) in bone marrow sections from ten patients with a mature form of ALL and ten with MCL, analyzed for the expression of PKC-βII. Infiltrated areas (a and c, CD79+) were compared to noninfiltrated areas of the bone marrow (b and d).
See also Figure S7.
Discussion
It has become more appreciated that tumors are not merely an accumulation of monoclonal cells but rather are heterogeneous complex structures, composed of malignant cells and various host cells. This cancer-associated microenvironment contributes significantly to tumor progression by supplying prosurvival factors crucial for the propagation of malignant cells. Therefore, targeting the tumor microenvironment constitutes a treatment option to bypass cancer cells’ intrinsic drug resistance.
Here, we report that the induction of PKC-βII in stromal cells is required for the survival of leukemic B cells. Studies on PKC-β knockout mice indicated that its function was restricted to normal B cell physiology by linking the B cell receptor to NF-κB activation (Leitges et al., 1996; Su et al., 2002). Reports of PKC-β in the context of tumorigenesis are limited to intrinsic high expression levels in B cell malignancies (Decouvelaere et al., 2007) and the promotion of colon carcinogenesis through a mechanism involving the accumulation of β-catenin (Fields et al., 2009).
The induction of PKC-βII in the microenvironment by CLL cells was neither species restricted nor limited to a subtype of stromal cells. This suggests that CLL cells can communicate with stromal cells through highly conserved and ubiquitously expressed receptors, or the upregulation of PKC-βII is related to metabolic alterations. The latter is supported by data showing that a metabolic interplay between stromal cells and CLL cells can promote survival of CLL cells (Zhang et al., 2012). Although it is very reasonable to assume that the functional consequences of PKC-βII activation differ between different subsets of stromal cells, the induced expression of PKC-βII in all cell types marks its potential as a therapeutic target. We observed that the expression of PKC-βII in endothelial cells in vivo was restricted to vessels within the infiltrated bone marrow. Previously, activation of PKC-βII in endothelial cells was observed as a response to hypoxia in a nontumor mouse model and was required for neovascularization (Suzuma et al., 2002). In this regard, the expression of PKC-βII in endothelial cells may also be accountable for the reported increase in bone marrow angiogenesis in patients with CLL (Kini et al., 2000).
Our data demonstrate that PKC-βII, but not PKC-βI, is induced in stromal cells activated by contact with monoclonal B cells. This raises the important question of what upstream events and signaling cascades are involved in the isoform-specific regulation of PKC-β. PKC-β activity is modulated by the presence of lipid second messengers, calcium, and protein modifications, but little is known about the regulation of its protein level. Quantification of PKC-β transcripts indicates that the increase in PKC-βII protein is transcriptionally regulated. Importantly, both isoforms are encoded by a single gene and arise via alternative splicing (Ono et al., 1987). This suggests that specific stabilization of PKC-βII mRNA is accountable for an increase in protein levels, similar to the regulation of PKC-β isoforms by glucose (Patel et al., 1999) and insulin (Chalfant et al., 1995). However, soluble factors were eliminated as the only determinants to induce PKC-βII. It is important to mention that PKC-βII protein was detectable by immunoblotting at the earliest 3 days after coculture with CLL cells (data not shown). This delayed upregulation almost certainly excludes that PKC-βII is a direct target gene engaged by an immediately activated signaling pathway. Long-term cultures of hBMSCs and CLL cells showed that PKC-βII levels steadily increased over time. This ruled out that PKC-βII is subjected to a negative feedback loop as reported for other PKC isoforms (Kang et al., 2000; Parker et al., 1995) and is an important observation for the development of PKC-β-directed therapies.
We show that NF-κB is linked through the canonical pathway to PKC-βII in stromal cells, but Bcl10 is dispensable, demonstrating that the mechanism by which PKC-βII activates NF-κB in stromal cells is distinct from that utilized in normal B cells. Alternative pathways linking PKC-β to NF-κB independently of Bcl10 include the PKC-associated kinase (PKK) (Muto et al., 2002) and Hsp27-mediated interaction with IKKβ (Park et al., 2003). Although, to our knowledge, the precise mechanism of NF-κB activation remains unsolved, our results indicate that PKC-βII operates upstream of canonical NF-κB activation and that its expression is a prerequisite to activate NF-κB in stromal cells.
We have identified that stromal NF-κB regulates the expression of cytokines and of adhesion molecules. CLL cells can bind to ICAM1 expressed on stromal cells via αLβ2 integrins. Therefore, the reduced expression of Icam1 in NEMO-deficient cells suggests that impaired survival of CLL cells on these stromal cells could also be related to reduced binding. Conversely NF-κB may support the survival of leukemic B cells through enforced expression of adhesion molecules. Surprisingly, we found genes generally known to play an important role for the interaction between B and T cells expressed in NEMO-proficient BMSCs after contact with CLL cells. Importantly, primary murine BMSCs are a heterogeneous cell population, constituted of nonhematopoietic cells and antigen-presenting cells (APCs). Decreased expression of CD86 in NEMO-deficient BMSCs may therefore reflect an exquisite dependence of APCs on functional NF-κB. In addition, some of these antigens, such as CD28, can also be expressed in nonlymphoid stromal cells (Gray Parkin et al., 2002). In addition to adhesion molecules, NEMO regulated the production and secretion of proinflammatory cytokines. As a proof of principle, we have demonstrated that IL-1 and IL-15 can rescue leukemic B cells from cell death. However, we assume that in vivo, numerous cytokines in collaboration with enhanced expression of adhesion molecules on the surface of stromal cells stimulate CLL cells in parallel and presumably act synergistically on malignant B cells to block apoptosis.
The observation that expression of PKC-βII in the microenvironment is essential to promote the survival of CLL cells provides a reason to target PKC-βII therapeutically. Motivated by the observation that specific PKC isoforms are intrinsically overexpressed in numerous different tumors, several PKC inhibitors have already been developed. Enzastaurin, an orally available PKC-β inhibitor, demonstrated clinical activity in a phase II trial on patients with lymphoma with negligible toxicity (Robertson et al., 2007). Numerous clinical studies are currently exploring the usefulness of enzastaurin for the treatment of a broad variety of solid and hematopoietic malignancies (listed on http://clinicaltrials.gov).
Based on our data, we propose a dual function of PKC-βII in the pathogenesis of CLL: intrinsic expression and activation of PKC-βII in CLL cells augment PKB/AKT signaling leading to apoptosis resistance (Barragán et al., 2006), whereas its induction in stromal cells mediates microenvironment-dependent survival. The crucial role of PKC-β for the pathogenesis of CLL was recently demonstrated by crossing TCL1tg mice with Prkcb−/− mice (Holler et al., 2009). However, this experiment does not allow deciphering the relative contribution of PKC-β expressed in CD19+CD5+ monoclonal B cells and in stromal cells to the development of the disease.
The recently reported activation of NF-κB in CAFs from mammary and pancreatic adenocarcinomas (Erez et al., 2010) suggests that stromal PKC-βII could also operate upstream of NF-κB in other tumor-associated stromal cells. This hypothesis is further supported by the finding of overexpressed PKC-β in stromal cells of human basal cell carcinomas (Reynolds et al., 1997). Therefore, induced expression of PKC-β may be a common mechanism of malignant cells to harness stromal cells for tumor-promoting purposes. Therapeutic strategies aiming at tumor cell intrinsic oncogenic pathways have been limited in their success due to the genetic instability of tumor cells and the evolution of mutations conferring drug resistance. In contrast, cancer-associated stromal cells presumably lack these escape mechanisms and may therefore constitute an alternative therapeutic target. Further studies are needed to explore whether inhibition of PKC-β, expressed in tumor-associated stromal cells, displays clinical significance.
Experimental Procedures
Human and Mouse Samples
After informed patients’ consent and in accordance with the Helsinki declaration, peripheral blood was obtained from untreated patients with a diagnosis of CLL, ALL, and MCL. hBMSCs were collected from individuals who underwent bone marrow aspiration for diagnostic purposes and in whom subsequently a hematological disease was ruled out. Our studies on CLL, MCL cells, and hBMSCs were approved for ex vivo experiments by the local ethical committee of the Technical University Munich (project number 1894/07). The collection and use of primary ALL and normal blood samples were approved for ex vivo experiments by the local ethic committee of the University Hospital of Essen (project numbers 10-4380 and 04-2459). Monoclonal B cells from patients with MCL and ALL were isolated from peripheral blood at the time of first diagnosis. PBMCs were isolated from heparinized blood or bone marrow samples by centrifugation over a Ficoll-Hypaque layer (Biochrom, Berlin) of 1.077 g/ml density. T cells and monocytes were removed with anti-CD2 and anti-CD14 magnetic beads (Dynabeads M450; Dynal, Oslo). A total of 2 × 104 BM-mononuclear cells was plated in 24-well tissue plates using the media conditions described in the Supplemental Information. Adhering cells were propagated at least for 3 weeks before experiments were performed. mBMSCs were established from femora and tibiae of 4- to 8-week-old mice accordingly. Each mouse provided about 5 × 107 bone marrow cells.
Syngeneic Transplantation of TCL1-CLL
As previously described by Hofbauer et al. (2011), a massively enlarged spleen was removed from an aged and moribund Eμ-Tcl1tg mouse (provided by C. Croce, Columbus, Ohio, USA), and single cells were obtained by homogenizing the tissue and filtering cells through a 100 μm nylon cell strainer (BD Falcon, Heidelberg, Germany). After separation of PBMCs by centrifugation over a Ficoll-Hypaque layer, 20 × 106 cells were injected intraperitoneally into 6- to 8-week-old syngeneic Prkcb+/+ or Prkcb−/− mice. Experiments were performed under approval from the Austrian Animal Ethics Committee. Mice were housed in a specific pathogen-free facility and followed for signs of illness. Moribund mice were killed and samples for histology and flow cytometric analysis collected.
Statistical Analysis
Statistical analyses of the results were performed by one-way ANOVA followed by Student’s t test. Throughout the manuscript, statistical significance was defined as p < 0.0001 (∗∗∗∗), p < 0.001 (∗∗∗), p < 0.01 (∗∗), p < 0.05 (∗), or ns (statistically nonsignificant).
Acknowledgments
We would like to thank Laura Soucek and Jonathan Whitfield for fruitful scientific discussions and their help with the manuscript. The research was supported by grants from the Deutschen Forschungsgemeinschaft (DFG, SFB-TRR54, TPC3) to I.R. and (DFG, SFB-TRR54, TPA3) to M.S.-S., and grants from the Austrian Science Fund (FWF) P19481-B12 and L488-B13 to A.E. and T516-B13 to N.Z. T. Kocher is a recipient of a DOC fellowship of the Austrian Academy of Sciences (23137).
Published: January 14, 2013
Footnotes
Supplemental Information includes seven figures, five tables, and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.ccr.2012.12.003.
Accession Numbers
The microarray data discussed in this study have been deposited in the NCBI Gene Expression Omnibus (GEO) and are accessible at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi (GEO series accession GSE36416).
Supplemental Information
References
- Abrams S.T., Lakum T., Lin K., Jones G.M., Treweeke A.T., Farahani M., Hughes M., Zuzel M., Slupsky J.R. B-cell receptor signaling in chronic lymphocytic leukemia cells is regulated by overexpressed active protein kinase CbetaII. Blood. 2007;109:1193–1201. doi: 10.1182/blood-2006-03-012021. [DOI] [PubMed] [Google Scholar]
- Barragán M., de Frias M., Iglesias-Serret D., Campàs C., Castaño E., Santidrián A.F., Coll-Mulet L., Cosialls A.M., Domingo A., Pons G., Gil J. Regulation of Akt/PKB by phosphatidylinositol 3-kinase-dependent and -independent pathways in B-cell chronic lymphocytic leukemia cells: role of protein kinase Cbeta. J. Leukoc. Biol. 2006;80:1473–1479. doi: 10.1189/jlb.0106041. [DOI] [PubMed] [Google Scholar]
- Becker K.P., Hannun Y.A. cPKC-dependent sequestration of membrane-recycling components in a subset of recycling endosomes. J. Biol. Chem. 2003;278:52747–52754. doi: 10.1074/jbc.M305228200. [DOI] [PubMed] [Google Scholar]
- Bichi R., Shinton S.A., Martin E.S., Koval A., Calin G.A., Cesari R., Russo G., Hardy R.R., Croce C.M. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc. Natl. Acad. Sci. USA. 2002;99:6955–6960. doi: 10.1073/pnas.102181599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J.A., Tsukada N., Burger M., Zvaifler N.J., Dell’Aquila M., Kipps T.J. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell-derived factor-1. Blood. 2000;96:2655–2663. [PubMed] [Google Scholar]
- Chalfant C.E., Mischak H., Watson J.E., Winkler B.C., Goodnight J., Farese R.V., Cooper D.R. Regulation of alternative splicing of protein kinase C beta by insulin. J. Biol. Chem. 1995;270:13326–13332. doi: 10.1074/jbc.270.22.13326. [DOI] [PubMed] [Google Scholar]
- Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcik S.E., Aqeilan R.I., Zupo S., Dono M. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo M., Bosch F., Villamor N., Bellosillo B., Colomer D., Rozman M., Marcé S., López-Guillermo A., Campo E., Montserrat E. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N. Engl. J. Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- Decouvelaere A.V., Morschhauser F., Buob D., Copin M.C., Dumontet C. Heterogeneity of protein kinase C beta(2) expression in lymphoid malignancies. Histopathology. 2007;50:561–566. doi: 10.1111/j.1365-2559.2007.02666.x. [DOI] [PubMed] [Google Scholar]
- de Totero D., Meazza R., Capaia M., Fabbi M., Azzarone B., Balleari E., Gobbi M., Cutrona G., Ferrarini M., Ferrini S. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008;111:517–524. doi: 10.1182/blood-2007-04-087882. [DOI] [PubMed] [Google Scholar]
- Ding W., Nowakowski G.S., Knox T.R., Boysen J.C., Maas M.L., Schwager S.M., Wu W., Wellik L.E., Dietz A.B., Ghosh A.K. Bi-directional activation between mesenchymal stem cells and CLL B-cells: implication for CLL disease progression. Br. J. Haematol. 2009;147:471–483. doi: 10.1111/j.1365-2141.2009.07868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhner H., Stilgenbauer S., Benner A., Leupolt E., Kröber A., Bullinger L., Döhner K., Bentz M., Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- Erez N., Truitt M., Olson P., Arron S.T., Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Fabbri G., Rasi S., Rossi D., Trifonov V., Khiabanian H., Ma J., Grunn A., Fangazio M., Capello D., Monti S. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J. Exp. Med. 2011;208:1389–1401. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Hannun Y.A. An essential role for autophosphorylation in the dissociation of activated protein kinase C from the plasma membrane. J. Biol. Chem. 1998;273:26870–26874. doi: 10.1074/jbc.273.41.26870. [DOI] [PubMed] [Google Scholar]
- Fields A.P., Calcagno S.R., Krishna M., Rak S., Leitges M., Murray N.R. Protein kinase Cbeta is an effective target for chemoprevention of colon cancer. Cancer Res. 2009;69:1643–1650. doi: 10.1158/0008-5472.CAN-08-3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray Parkin K., Stephan R.P., Apilado R.G., Lill-Elghanian D.A., Lee K.P., Saha B., Witte P.L. Expression of CD28 by bone marrow stromal cells and its involvement in B lymphopoiesis. J. Immunol. 2002;169:2292–2302. doi: 10.4049/jimmunol.169.5.2292. [DOI] [PubMed] [Google Scholar]
- Hayden M.S., Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Hofbauer J.P., Heyder C., Denk U., Kocher T., Holler C., Trapin D., Asslaber D., Tinhofer I., Greil R., Egle A. Development of CLL in the TCL1 transgenic mouse model is associated with severe skewing of the T-cell compartment homologous to human CLL. Leukemia. 2011;25:1452–1458. doi: 10.1038/leu.2011.111. [DOI] [PubMed] [Google Scholar]
- Holler C., Piñón J.D., Denk U., Heyder C., Hofbauer S., Greil R., Egle A. PKCbeta is essential for the development of chronic lymphocytic leukemia in the TCL1 transgenic mouse model: validation of PKCbeta as a therapeutic target in chronic lymphocytic leukemia. Blood. 2009;113:2791–2794. doi: 10.1182/blood-2008-06-160713. [DOI] [PubMed] [Google Scholar]
- Jewell A.P., Lydyard P.M., Worman C.P., Giles F.J., Goldstone A.H. Growth factors can protect B-chronic lymphocytic leukaemia cells against programmed cell death without stimulating proliferation. Leuk. Lymphoma. 1995;18:159–162. doi: 10.3109/10428199509064937. [DOI] [PubMed] [Google Scholar]
- Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- Kang B.S., French O.G., Sando J.J., Hahn C.S. Activation-dependent degradation of protein kinase C eta. Oncogene. 2000;19:4263–4272. doi: 10.1038/sj.onc.1203779. [DOI] [PubMed] [Google Scholar]
- Kini A.R., Kay N.E., Peterson L.C. Increased bone marrow angiogenesis in B cell chronic lymphocytic leukemia. Leukemia. 2000;14:1414–1418. doi: 10.1038/sj.leu.2401825. [DOI] [PubMed] [Google Scholar]
- Kitada S., Andersen J., Akar S., Zapata J.M., Takayama S., Krajewski S., Wang H.G., Zhang X., Bullrich F., Croce C.M. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia: correlations with In vitro and In vivo chemoresponses. Blood. 1998;91:3379–3389. [PubMed] [Google Scholar]
- Klein U., Lia M., Crespo M., Siegel R., Shen Q., Mo T., Ambesi-Impiombato A., Califano A., Migliazza A., Bhagat G., Dalla-Favera R. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- Leitges M., Schmedt C., Guinamard R., Davoust J., Schaal S., Stabel S., Tarakhovsky A. Immunodeficiency in protein kinase cbeta-deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Meads M.B., Gatenby R.A., Dalton W.S. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat. Rev. Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- Muto A., Ruland J., McAllister-Lucas L.M., Lucas P.C., Yamaoka S., Chen F.F., Lin A., Mak T.W., Núñez G., Inohara N. Protein kinase C-associated kinase (PKK) mediates Bcl10-independent NF-kappa B activation induced by phorbol ester. J. Biol. Chem. 2002;277:31871–31876. doi: 10.1074/jbc.M202222200. [DOI] [PubMed] [Google Scholar]
- Ono Y., Kikkawa U., Ogita K., Fujii T., Kurokawa T., Asaoka Y., Sekiguchi K., Ase K., Igarashi K., Nishizuka Y. Expression and properties of two types of protein kinase C: alternative splicing from a single gene. Science. 1987;236:1116–1120. doi: 10.1126/science.3576226. [DOI] [PubMed] [Google Scholar]
- Oostendorp R.A., Harvey K.N., Kusadasi N., de Bruijn M.F., Saris C., Ploemacher R.E., Medvinsky A.L., Dzierzak E.A. Stromal cell lines from mouse aorta-gonads-mesonephros subregions are potent supporters of hematopoietic stem cell activity. Blood. 2002;99:1183–1189. doi: 10.1182/blood.v99.4.1183. [DOI] [PubMed] [Google Scholar]
- Park K.J., Gaynor R.B., Kwak Y.T. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J. Biol. Chem. 2003;278:35272–35278. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- Parker P.J., Bosca L., Dekker L., Goode N.T., Hajibagheri N., Hansra G. Protein kinase C (PKC)-induced PKC degradation: a model for down-regulation. Biochem. Soc. Trans. 1995;23:153–155. doi: 10.1042/bst0230153. [DOI] [PubMed] [Google Scholar]
- Patel N.A., Chalfant C.E., Yamamoto M., Watson J.E., Eichler D.C., Cooper D.R. Acute hyperglycemia regulates transcription and posttranscriptional stability of PKCbetaII mRNA in vascular smooth muscle cells. FASEB J. 1999;13:103–113. doi: 10.1096/fasebj.13.1.103. [DOI] [PubMed] [Google Scholar]
- Pedersen I.M., Kitada S., Leoni L.M., Zapata J.M., Karras J.G., Tsukada N., Kipps T.J., Choi Y.S., Bennett F., Reed J.C. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- Peitz M., Pfannkuche K., Rajewsky K., Edenhofer F. Ability of the hydrophobic FGF and basic TAT peptides to promote cellular uptake of recombinant Cre recombinase: a tool for efficient genetic engineering of mammalian genomes. Proc. Natl. Acad. Sci. USA. 2002;99:4489–4494. doi: 10.1073/pnas.032068699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente X.S., Pinyol M., Quesada V., Conde L., Ordóñez G.R., Villamor N., Escaramis G., Jares P., Beà S., González-Díaz M. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada V., Conde L., Villamor N., Ordóñez G.R., Jares P., Bassaganyas L., Ramsay A.J., Beà S., Pinyol M., Martínez-Trillos A. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat. Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- Reynolds N.J., Todd C., Angus B. Overexpression of protein kinase C-alpha and -beta isozymes by stromal dendritic cells in basal and squamous cell carcinoma. Br. J. Dermatol. 1997;136:666–673. [PubMed] [Google Scholar]
- Ridley A.J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Robertson M.J., Kahl B.S., Vose J.M., de Vos S., Laughlin M., Flynn P.J., Rowland K., Cruz J.C., Goldberg S.L., Musib L. Phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory diffuse large B-cell lymphoma. J. Clin. Oncol. 2007;25:1741–1746. doi: 10.1200/JCO.2006.09.3146. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M., Bloch W., Courtois G., Addicks K., Israël A., Rajewsky K., Pasparakis M. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol. Cell. 2000;5:981–992. doi: 10.1016/s1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T.T., Guo B., Kawakami Y., Sommer K., Chae K., Humphries L.A., Kato R.M., Kang S., Patrone L., Wall R. PKC-beta controls I kappa B kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 2002;3:780–786. doi: 10.1038/ni823. [DOI] [PubMed] [Google Scholar]
- Suzuma K., Takahara N., Suzuma I., Isshiki K., Ueki K., Leitges M., Aiello L.P., King G.L. Characterization of protein kinase C beta isoform’s action on retinoblastoma protein phosphorylation, vascular endothelial growth factor-induced endothelial cell proliferation, and retinal neovascularization. Proc. Natl. Acad. Sci. USA. 2002;99:721–726. doi: 10.1073/pnas.022644499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T.D., Coussens L.M. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- Wang L., Lawrence M.S., Wan Y., Stojanov P., Sougnez C., Stevenson K., Werner L., Sivachenko A., DeLuca D.S., Zhang L. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N. Engl. J. Med. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanesi N., Aqeilan R., Drusco A., Kaou M., Sevignani C., Costinean S., Bortesi L., La Rocca G., Koldovsky P., Volinia S. Effect of rapamycin on mouse chronic lymphocytic leukemia and the development of nonhematopoietic malignancies in Emu-TCL1 transgenic mice. Cancer Res. 2006;66:915–920. doi: 10.1158/0008-5472.CAN-05-3426. [DOI] [PubMed] [Google Scholar]
- Zhang W., Trachootham D., Liu J., Chen G., Pelicano H., Garcia-Prieto C., Lu W., Burger J.A., Croce C.M., Plunkett W. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 2012;14:276–286. doi: 10.1038/ncb2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Wertz I., O’Rourke K., Ultsch M., Seshagiri S., Eby M., Xiao W., Dixit V.M. Bcl10 activates the NF-kappaB pathway through ubiquitination of NEMO. Nature. 2004;427:167–171. doi: 10.1038/nature02273. [DOI] [PubMed] [Google Scholar]
- Ziegelbauer K., Gantner F., Lukacs N.W., Berlin A., Fuchikami K., Niki T., Sakai K., Inbe H., Takeshita K., Ishimori M. A selective novel low-molecular-weight inhibitor of IkappaB kinase-beta (IKK-beta) prevents pulmonary inflammation and shows broad anti-inflammatory activity. Br. J. Pharmacol. 2005;145:178–192. doi: 10.1038/sj.bjp.0706176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zum Büschenfelde C.M., Wagner M., Lutzny G., Oelsner M., Feuerstacke Y., Decker T., Bogner C., Peschel C., Ringshausen I. Recruitment of PKC-betaII to lipid rafts mediates apoptosis-resistance in chronic lymphocytic leukemia expressing ZAP-70. Leukemia. 2010;24:141–152. doi: 10.1038/leu.2009.216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.