Abstract
Polymerase chain reaction (PCR) is often used to detect microorganisms, pathogens, or both, including the reproductive parasite Wolbachia pipientis (Rickettsiales: Anaplasmataceae), in mosquitoes. Natural populations of Culex pipiens L. (Diptera: Culicidae) mosquitoes are infected with one or more strains of W. pipientis, and crosses between mosquitoes harboring different Wolbachia strains provide one of the best-known examples of cytoplasmic incompatibililty (CI). When we used PCR to monitor Wolbachia in the Buckeye strain of Culex pipiens, and in a Wolbachia-cured sister colony obtained by tetracycline treatment, we noted false negative PCR reactions with DNA samples from infected mosquitoes; these results were inconsistent with direct microscopic observation of Wolbachia-like particles in gonads dissected from mosquitoes in the same population. Assays with diluted template often improved detection of positive samples, suggesting that DNA prepared from whole mosquitoes contained an inhibitor of the PCR reaction. We reconciled discrepancies between PCR and microscopy by systematic measurement of the PCR reaction in the presence of an internal standard. Mosquito decapitation before DNA extraction restored the reliability of the PCR reaction, allowing accurate determination of Wolbachia infection status in infected and tetracycline-cured mosquito populations, consistent with microscopic examination. Using PCR primers based on the Tr1 gene, we confirmed that the Wolbachia infection in the Buckeye strain of Culex pipiens belongs to the genotype designated wPip1. Finally, to explore more widely the distribution of PCR inhibitors, we demonstrated that DNA isolated from the cricket, Acheta domesticus (L.); the beetle, Tenebrio molitor L.; the honey bee, Apis mellifera L.; and the mosquito, Anopheles punctipennis Say also contained PCR inhibitors. These results underscore the importance of measuring the presence of inhibitors in PCR templates by using a known positive standard, and provide an approach that will facilitate use of PCR to monitor environmental samples of mosquitoes that harbor endosymbionts or pathogenic organisms.
Keywords: Wolbachia, wPip1, mosquito, Culex pipiens, PCR inhibitor
Wolbachia are obligate intracellular bacteria that cause reproductive distortions such as cytoplasmic incompatibility (CI), parthenogenesis, feminization, and male-killing in the various arthropods they infect (Serbus et al. 2008). Wolbachia were first described as pleomorphic, rickettsia-like organisms in Giemsa-stained smears from Culex pipiens L. gonads (Hertig 1936). The association of Wolbachia with cytoplasmic incompatibility (Yen and Barr 1971) and its potential utility as a genetic drive mechanism to control mosquito populations (Sinkins 2004) have stimulated renewed interest in these bacteria for transgenic mosquito replacement, alteration of population size or age structure, and disruption of pathogen transmission by mosquito vectors. Wolbachia infections can be detected by crosses between mosquito strains, fluorescent and electron microscopy (O’Neill et al. 1997), and western blotting (Dobson et al. 1999). The polymerase chain reaction (PCR) (O’Neill et al. 1992, Zhou et al. 1998) has been used for both qualitative and quantitative detection of Wolbachia, both in insects and in cell lines (O’Neill et al. 1997).
Although PCR provides a fast and simple method to detect Wolbachia, many considerations need to be addressed in the experimental design. For example, false negative reactions with arthropod materials are well-documented (Jeyaprakash and Hoy 2000). With mosquitoes, DNA template has been prepared from different life stages of the insect, and samples range from pools of whole insects to dissected material from individual mosquitoes. Most studies are based on the assumption that recovery of template DNA from biological samples is quantitative, that PCR reactions are uniformly efficient with each DNA template, and that all reactions remain within the “linear” range of the PCR assay, wherein band intensity is directly correlated with template copy number. These considerations are particularly important in measuring results based on quantitative PCR. For example, in their description of a 20,000-fold range in Wolbachia density in a natural population of Drosophila innubila Spencer, Unckless et al. (2009) effectively controlled for variability of PCR amplification efficiency by using serial dilutions.
Here we show that an inhibitor that produces false-negative PCR reactions is found in the head of Culex pipiens mosquitoes. False-negatives can be eliminated by decapitating the mosquitoes before DNA extraction. This precaution substantially reduced PCR variability among individuals in an infected colony, and facilitated reliable discrimination between infected and antibiotic-cured individuals. In further studies, we found evidence for a PCR inhibitor in four of six additional insect species surveyed, including the mosquito Anopheles punctipennis Say. Detection of potential PCR inhibitors by using simple PCR-based assays incorporating known standards will provide a useful tool for monitoring the efficacy of Wolbachia-based strategies for control of vector populations, as well as for monitoring pathogen transmission and ecology of endosymbionts in native and transgenic mosquito populations.
Materials and Methods
Mosquitoes
Culex pipiens larvae from the Buckeye strain, collected in Columbus, OH and established in colony in 2000 (Robich and Denlinger 2005) were obtained from D. Denlinger, Department of Entomology, Ohio State University, in June 2006. Mosquitoes were maintained at 25°C with a photoperiod of 16:8 (L:D) h. Bloodmeals were provided on hamsters (University of Minnesota IACUC Protocol No. 1002A77232), anesthetized with 20% isoflurane in 1, 2-propanediol (Itah et al. 2004). From these wild type mosquitoes, we derived a cured, Wolbachia-free “sister colony” over a period of 4 mo, essentially as described by Potaro and Barr (1975). Briefly, we transferred 2-d egg rafts to distilled water containing tetracycline (12.5 μg/ml) and larval food (Escherichia coli and Kordon [Hayward, CA] fish fry food), and reared larvae from 10 to 20 egg masses in 3 liters of distilled water in the continuous presence of antibiotic. Recovery of larvae from egg masses decreased during the course of tetracycline treatment. Adults were blood-fed, and their offspring were maintained for two generations (designated G1 and G2) in the absence of tetracycline. Larvae from the G2 adults were maintained for four successive generations in the presence of tetracycline, and subsequent generations of cured mosquitoes were reared in the absence of tetracycline. Loss of Wolbachia was monitored by PCR using DNA extracted from individual mosquitoes, and by microscopic observations. With the infected Buckeye strain, we never observed egg rafts that were negative for Wolbachia by PCR (N, ≈20 egg rafts), nor did we observe ovaries or testes that failed to contain bacteria-like particles (N, ≈100 individual dissections).
Other Insects
Crickets [Acheta domesticus (L.)], and mealworms (Tenebrio molitor L.) were from un-characterized laboratory colonies; face flies (Musca autumnalis De Geer) were obtained from R. Moon, Department of Entomology, University of Minnesota; and honey bees (Apis mellifera L.), from M. Spivak, Department of Entomology, University of Minnesota. Drosophila melanogaster Meigen were from M. O’Connor, Department of Genetics, Cell Biology and Development, University of Minnesota. Anopheles punctipennis were reared from larvae collected in Afton, MN. DNA extractions were as described for Cx. pipiens.
DNA Extraction
DNA was extracted as described by Livak (1984). Whole or decapitated mosquitoes were individually homogenized in 200 μl of 120-mM Tris-HCl, pH 9, containing 0.5% SDS, 80-mM NaCl, 160-mM sucrose, and 60-mM EDTA. After 30 min at 65°C, potassium acetate (28 μl of 8 M) was added, mixed by vortexing, and the sample was incubated on ice for 30 min. Samples were centrifuged for 10 min in a microcentrifuge at 13,000 rpm, and the resulting supernatant (180 μl) was placed into a new 1.5-ml microcentrifuge tube and 360 μl of 100% ethanol was added. The samples were then briefly vortexed and held overnight at −20°C. Nucleic acids were pelleted by centrifugation at 13,000 rpm for 10 min and the pellets were dried under vacuum, before resuspension in 10-mM Tris-HCl, pH 7.5, containing 0.4-M NaCl and 10-μg boiled RNAseA (400 μl) at 37°C for 1 h. Samples were extracted with an equal volume of phenol, and the aqueous phase (380 μl) was transferred to a new microcentrifuge tube. The phenol phase was re-extracted with 400 μl of 10-mM Tris-HCl, pH 7.5, containing 0.4-M NaCl, and the combined aqueous phases were precipitated with ethanol overnight at −20°C. DNA was recovered by centrifugation, washed in 70% ethanol, dried, and dissolved in 100-μl double-distilled water by sonication in a cup-horn Misonix ultrasonic liquid processor (Qsonica LLC., Newton, CT) at 90 mA for 30-s intervals, over a total time of 7 min.
Polymerase Chain Reaction
Wolbachia primers were based on the genes of ribosomal proteins rpS12 (rpsL) and rpS7 (rpsG), which are encoded by adjacent genes in “str operon” as previously described (Fallon 2008). The PCR reaction (20 μl) contained 2.5-mM magnesium chloride, each of the four deoxy-ribonucleotide triphosphates at 0.20 mM; primers at 400 nM; Promega Go-Taq polymerase (2.5 U per reaction; Promega, Madison, WI); and 1–9 μl of template DNA. The forward primer was 5′-GCACTAAGGTGTATACTACAACTCC, and the reverse primer was 5′-GCCTTATTAGCTTCAGCCAT. PCR was carried out for 35 cycles with a denaturing step at 95°C for 1 min, annealing at 56°C for 1 min, followed by extension at 72°C for 1 min with a final extension at 72°C for 3 min. The strain designation based on the Tr1 gene (Duron et al. 2005) was based on sequence obtained with PCR primers F4N: 5′-GCCAAGTGCGTGTATAGTTGAC and R1N: 5′-ATGGAGCTGAAGGTATAGAGG as described above, using an annealing temperature of 59°C. PCR products were electrophoresed on 2% agarose gels and photographed with UV light illumination. Images were “inverted” electronically to show dark bands on a white background. DNA sequencing was carried out at the University of Minnesota BioMedical Genomics Center.
Results
Derivation of Wolbachia-Free Cx. pipiens
Culex pipiens (Buckeye strain) were provided 10% sucrose and blood-fed on hamsters. Egg rafts were collected for isolation of a Wolbachia-free sister colony, using tetracycline treatment at 12.5 μg/ml by using the modified technique of Potaro and Barr (1975). Egg hatch was poor during the first five generations of selection, presumably reflecting cytoplasmic incompatibility within random sib-matings because of loss of Wolbachia at varying rates among individual larvae, negative effect of tetracycline treatment on larvae or their microbial diet, or both. After six generations, cured and infected lines exhibited similar larval growth rates, egg hatch, and developmental time. Crosses between males from the infected line and females from the cured line failed to produce offspring.
Variability of Wolbachia PCR Detection
Wolbachia infection status of individual mosquitoes yielded unpredictable results when template DNA from whole mosquitoes was amplified by PCR using Wolbachia-specific primers. In particular, by PCR, our wild type (infected) colony appeared to contain a mixed population of infected and uninfected males and females (Fig. 1, lanes 3–18). To measure whether inconsistent recovery of DNA caused this variability, we labeled wAlbB-infected Aa23 cells (Fallon 2008) with 3H[thymidine], and monitored radioactivity throughout our DNA extraction. Consistent recovery of labeled DNA suggested that the variability in the PCR reactions was not caused by random loss of template. Likewise, to ensure that DNA was uniformly distributed in our samples, we sonicated each sample of purified DNA as a final step in our extraction. Occasionally, sonication would revert a false-negative to positive, suggesting that on occasion, the DNA pellet was not completely dissolved, but in most cases the sonication did not affect PCR results.
Fig. 1.
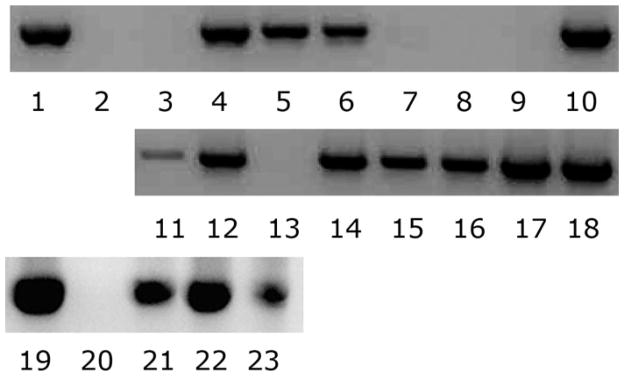
Variable PCR-based detection of Wolbachia in adult Cx. pipiens. Lanes 1 and 19: positive control; lanes 2 and 20: negative control; lanes 3–10 are females, and lanes 11–18 are males. DNA was extracted from individual mosquitoes as described in the Materials and Methods. Lanes 21–23 show positive PCR identification of Wolbachia in egg rafts: lane 21, one egg mass; lane 22, pool of five egg masses; lane 23, pool of 10 egg masses.
In some assays, PCR detection in both males and females consistently yielded 100% false-negative results and it appeared that our colony had completely lost the Wolbachia infection. In contrast, PCR results with DNA from egg rafts were always positive (Fig. 1, lanes 21–23). Microscopic examination with the cell-permeant dye, Syto-13 indicated that ovaries from our wild type colony released a halo of bacteria-like particles under hypotonic staining conditions, and contained intracellular bacteria-like particles, while ovaries from the cured strain lacked these particles. These experiments, as well as our observation of typical CI in egg rafts resulting from matings between wild type males and antibiotic cured females, showed that our wild type colony was uniformly infected by Wolbachia, which was in conflict with the PCR results from whole mosquito templates.
Evidence for a PCR Inhibitor
To measure whether purified mosquito DNA contained an inhibitor, we used a dilution series of DNA template in the PCR reaction. When the sample volume was reduced to 1 μl, we recovered a strong PCR band (Fig. 2, lane 3), and this template continued to give a positive PCR product with up to 10,000-fold further dilution (not shown). In contrast, using 2 μl of the original template substantially reduced the intensity of the positive band (Fig. 2, lane 4), and larger volumes of template DNA failed to produce a PCR product (Fig. 2, lanes 5–11). To further establish the presence of an inhibitor, we tested whether the purified whole mosquito DNA inhibited the PCR reaction of a known positive control (Fig. 2, lanes 12–18). When mixed with increasing concentrations of whole mosquito DNA extract, the PCR band from positive control DNA progressively declined (Fig. 2, compare lanes 14–18).
Fig. 2.
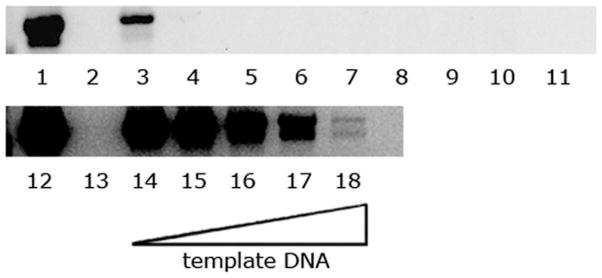
Effect of template volume on the PCR reaction. Lanes 1 and 12 show positive controls; lanes 2 and 13 are negative controls; lanes 3–11: 1 μl to 9 μl of DNA template, respectively. Lanes 14–18 all contain positive control DNA as in lane 12, with no additional mosquito DNA (lane 14) and 1 μl to 4 μl of mosquito template DNA (lanes 15–18, respectively).
In additional studies, we eliminated the possibility that the PCR inhibitor was an artifact of the Livak (1984) procedure, and noted that the inhibitor persisted when we prepared template with a Qiagen DNA kit (Qiagen, Valencia, CA; data not shown) developed for stool samples. We also checked whether the inhibitor was originating specifically from Wolbachia, but mosquito DNA purified from infected and uninfected individuals caused comparable levels of inhibition. Interestingly, we noted that DNA pellets commonly had a pink tinge, and reasoned that this pigment might derive from the eyes. Decapitating mosquitoes before homogenization eliminated the inhibitor (Fig. 3, lanes 4–9) whereas DNA extracted from the entire mosquito required a ninefold dilution to yield a positive PCR band (Fig. 3, compare lanes 10, 12, and 14 (1-μl template) with lanes 11, 13, and 15 (9-μl template). Before discovery of the PCR inhibitor, only 53 out of 197 Culex pipiens mosquitoes from our infected colony were shown to be infected with Wolbachia by PCR. After including decapitation in our DNA extraction protocol, 69 out of 69 Culex pipiens from the same infected colony tested positive for Wolbachia.
Fig. 3.
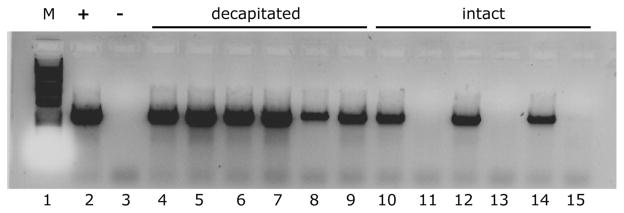
Preparation of template DNA from decapitated mosquitoes removes the inhibitor. Lanes 1, 2, and 3 show DNA ladder, positive control and negative control, respectively. Lanes 4–9 show PCR template DNA prepared from decapitated mosquitoes. For lanes 10–15, mosquitoes were homogenized intact. Even lanes used 1 μl of template DNA; odd lanes had 9 μl of template DNA.
Other Insects
To test the prevalence of PCR inhibitors in other insects, we conducted PCR assays measuring inhibition in the separated head and body of miscellaneous insects, including the mosquito, An. punctipennis(Fig. 4). We detected an inhibitor within the heads of A. mellifera (Fig. 4, lanes 21–23) and An. punctipennis (Fig. 4, lanes 29–31) but not in the body of these insects (Fig. 4, lanes 24–26; 32–34). PCR inhibitor(s) were detected in both the head and body of A. domesticus, in which levels of inhibitor were particularly variable between individuals, and seemed to be somewhat higher in the body, relative to the head (Fig. 4, lanes 9–14). With DNA from T. molitor, inhibitor was present in both heads and body (Fig. 4, lanes 15–20), but we found no evidence for inhibitors in either head or body samples from M. autumnalis (Fig. 4, lanes 3–8) and D. melanogaster (data not shown).
Fig. 4.
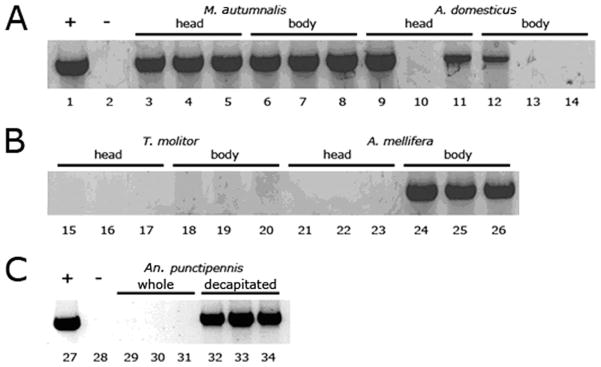
Survey for PCR inhibitors from various insects. Positive control DNA (1 μl; lanes 1 and 27) was combined with 8-μl template DNA from various extractions (lanes 3–34). Lanes with reduced PCR product, relative to the positive control show evidence for an inhibitor. Panels A and B show lanes from the same gel, whereas Panel C is from a separate gel. Lanes 2 and 28 are negative controls without DNA. Lanes 3–5 show M. autumnalis head DNA and 6–8 show M. autumnalis decapitated, whole body DNA. Lanes 9–11 show A. domesticus head DNA and 12–14 show A. domesticus decapitated, whole body DNA. Lanes 15–17 show T. molitor head DNA and 18–20 contain T. molitor decapitated, whole body DNA. Lanes 21–23 show A. mellifera head DNA and 24–26 show A. mellifera decapitated, whole body DNA. Lanes 29–30 are An. punctipennis whole mosquitoes and 32–34 are decapitated An. punctipennis mosquitoes.
W. pipientis Strain Characterization
Based on DNA sequence analysis of the transposable element Tr1, Duron et al. (2005) identified five Wolbachia strains, and showed that North American populations of Culex pipiens are singly infected with wPip1 (Florida) or wPip4 (California), or doubly infected with both wPip1 and wPip4 (Minnesota). We sequenced PCR products amplified with primers F4N and R1N from three individual mosquitoes in both directions, and found complete identity with the wPip1 sequence (GenBank accession no. AJ646884) reported by Duron et al. (2005). Thus, based on the Tr1 gene, the Buckeye strain that originated from Ohio has the same Wolbachia genotype as the Florida population described by Duron et al. (2005).
Discussion
Although Laven (1967) pioneered use of Wolbachia-mediated CI to reduce vector populations >40 yr ago, symbiont-based strategies for mosquito population replacement are only recently enjoying renewed attention, due in part to advances in molecular technologies that allow relatively simple detection of Wolbachia and exploration of its effects in insect hosts. Of particular interest are recent reports that Wolbachia can be successfully transferred into mosquitoes that are uninfected in nature (Xi et al. 2005), that Wolbachia can be used to suppress dengue transmission (Hoffmann et al. 2011), and that Wolbachia inhibits development of the malaria parasite Plasmodium through stimulation of the mosquito immune system (Moreira et al. 2009, Hughes et al. 2011). Despite these remarkable advances, few investigators are investigating the Wolbachia infection in natural mosquito hosts, such as Cx. pipiens.
Cx. pipiens populations worldwide are infected with Wolbachia, and at least five Wolbachia strains can be distinguished by sequence analysis of the Tr1 gene, which encodes a transposable element (Duron et al. 2005). Only two Wolbachia strains have been described in North American populations, and strain wPip1 in the recently-colonized Buckeye population of Cx. pipiens from Ohio is among these two. As we continued to monitor the Wolbachia infection in the wild type mosquitoes, relative to that in a cured sister colony derived by antibiotic treatment, we were puzzled by PCR results that suggested an unstable Wolbachia infection in the Buckeye mosquito population. Spontaneous loss of Wolbachia in Culex colonies has not to our knowledge been reported, and despite negative PCR results, our colony continued to exhibit microscopic evidence for infection. These considerations supported our suspicion that the PCR results were in error.
While surveying the presence of Wolbachia in diverse insects, Jeyaprakash et al. (2000) noted false negative PCR results, and suggested a modification called “long PCR,” in which two different polymerases were used simultaneously. Noda et al. (2001) suspected an inhibitor while comparing Wolbachia titers in two planthopper species. In one, Laodelphax striatellus Fallen, Wobachia detection seemed to be consistent and accurate, but in the other, Sogatella furcifera (Horváth) these researchers had problems detecting Wolbachia in adult males and unsuccessfully tested for an inhibitor by running a dilution series. By measuring PCR band intensity with mosquito DNA template prepared from whole and decapitated mosquitoes and amplified in the presence of an internal positive control, we showed that mosquito heads contain an inhibitor of the PCR reaction. Preparation of template DNA by using a commercially available kit failed to remove the inhibitor, whose molecular identity remains unknown. Inhibition of PCR reactions with DNA extracts from vector mosquitoes is a cause for concern because extracts from the head and thorax are often expected to be enriched for pathogens (Vezzani et al. 2011), whose presence could be masked by the inhibitor.
We included the honey bee in our survey for PCR inhibitors, because most honey bee pathogens are diagnosed by PCR. For example, Chen et al. (2006) investigated transmission dynamics of deformed wing virus (DWV) by PCR assays on dissected tissues. Virus-positive samples were detected in every tissue, including feces, hemolymph, gut, ovaries, spermatheca, and eviscerated body, but not in the honey bee head. Similarly, Yue and Genersch (2005) detected DWV in the thorax and abdomen of symptomatic and asymptomatic bees, but never detected viral RNA in heads except in symptomatic bees where viral titers were extreme. Although these results suggested that DWV cannot replicate in head tissues, Zioni et al. (2011) recently showed that a recombinant form of DWV does replicate in the honey bee head, suggesting that in at least some studies, others have unknowingly encountered an inhibitor of the PCR reaction in honey bee heads. We note that in studies with Plasmodium, the presence of inhibitors from mosquitoes interfered with detection of low parasite numbers (Schriefer et al. 1991, Arez et al. 2000).
In the absence of appropriate positive controls for the PCR reaction, qualitative differences in the abundance of Wolbachia under different conditions can be difficult to measure, as PCR inhibition could, for example, mimic a low bacterial load. Echaubard et al. (2010) used quantitative PCR to investigate whether the Wolbachia load in a population of insecticide resistant Cx. pipiens mosquitoes changed, relative to measurements 36 generations earlier (Berticat et al. 2002). An apparent decrease in Wolbachia density in insecticide-resistant mosquitoes, both in the lab and in the field, was attributed to attenuation of the Wolbachia infection in the insecticide resistant strains. Given the apparent variability of Wolbachia density with diverse factors such as host and Wolbachia genotype, environment, age, larval density, and other variables that may be difficult to control (Unckless et al. 2009), results based on quantitative PCR could be strengthened by incorporating additional controls with internal standards, and showing that the quantitative results ‘add up’ as expected. Such an approach might lead to a better understanding of Wolbachia’s effects on host physiology and fitness.
Acknowledgments
This work was supported by NIH grant AI081322 and by the University of Minnesota Agricultural Experiment Station, St. Paul, MN. We thank Cassandra Kurtz for help with mosquito rearing, and G. D. Baldridge for helpful discussions.
References Cited
- Arez AP, Lopes D, Pinto J, Franco AS, Snounou G, do Rosario VE. Plasmodium sp.: optimal protocols for PCR detection of low parasite numbers from mosquito (Anopheles sp.) samples. Exp Parasitol. 2000;94:269–272. doi: 10.1006/expr.2000.4496. [DOI] [PubMed] [Google Scholar]
- Berticat C, Rousset F, Raymond M, Berthomieu A, Weill M. High Wolbachia density in insecticide-resistant mosquitoes. Proc R Soc Lond B. 2002;269:1413–1416. doi: 10.1098/rspb.2002.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YP, Pettis JS, Collins A, Feldlaufer MF. Prevalence and transmission of honeybee viruses. Appl Environ Microbiol. 2006;72:606–611. doi: 10.1128/AEM.72.1.606-611.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson SL, Bourtzis K, Braig HR, Jones BF, Zhou W, Rousset F, O’Neill SL. Wolbachia infections are distributed throughout insect somatic and germ line tissues. Insect Biochem Mol Biol. 1999;29:153–160. doi: 10.1016/s0965-1748(98)00119-2. [DOI] [PubMed] [Google Scholar]
- Duron O, Lagnel J, Raymond M, Bourtzis K, Fort P, Weill M. Transposable element polymorphism of Wolbachia in the mosquito Culex pipiens: evidence of genetic diversity, superinfection and recombination. Mol Ecol. 2005;14:1561–1573. doi: 10.1111/j.1365-294X.2005.02495.x. [DOI] [PubMed] [Google Scholar]
- Echaubard P, Duron O, Agnew P, Sidobre C, Noel V, Will M, Michalakis Y. Rapid evolution of Wolbachia density in insecticide resistant Culex pipiens. Heredity. 2010;104:15–19. doi: 10.1038/hdy.2009.100. [DOI] [PubMed] [Google Scholar]
- Fallon AM. Cytological properties of an Aedes albopictus mosquito cell line infected with Wolbachia strain wAlbB. In Vitro Cell Dev Biol Anim. 2008;44:154–161. doi: 10.1007/s11626-008-9090-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertig M. The rickettsia, Wolbachia pipientis (Gen. Et SP.N.) and associated inclusions of the mosquito Culex pipiens. Parasitology. 1936;28:453–86. [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, Greenfield M, Durkan M, Leong YS, Dong Y, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7:e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itah R, Gitelman I, Davis C. A replacement for methoxyflurane (Metofane) in open-circuit anaesthesia. Lab Anim. 2004;38:280–285. doi: 10.1258/002367704323133664. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash A, Hoy MA. Long PCR improves Wolbachia DNA amplification: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol. 2000;9:393–405. doi: 10.1046/j.1365-2583.2000.00203.x. [DOI] [PubMed] [Google Scholar]
- Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics. 1984;107:611–634. doi: 10.1093/genetics/107.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, Rocha BC, Mendelin S, Day A, Riegler M, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Noda H, Koizumi Y, Zhang Q, Deng K. Infection density of Wolbachia and incompatibility level in two planthopper species, Laodelphax striatellus and Sogatella furcifera. Insect Biochem Mol Biol. 2001;31:727–737. doi: 10.1016/s0965-1748(00)00180-6. [DOI] [PubMed] [Google Scholar]
- O’Neill S, Giordano R, Colbert AME, Karr TL, Robertson HM. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill SL, Pettigrew MM, Sinkins SP, Braig HR, Andreadis TG, Tesh RB. In vitro cultivation of Wolbachia pipientis in an Aedes albopictus cell line. Insect Mol Biol. 1997;6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- Potaro JK, Barr AR. “Curing” Wolbachia infections in Culex pipiens. J Med Entomol. 1975;12:265. doi: 10.1093/jmedent/12.2.265. [DOI] [PubMed] [Google Scholar]
- Robich RM, Denlinger DL. Diapause in the mosquito Culex pipiens evokes a metabolic switch from blood feeding to sugar gluttony. Proc Natl Acad Sci USA. 2005;102:15912–15917. doi: 10.1073/pnas.0507958102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriefer ME, Sacci JB, Wirtz RA, Azad AF. Detection of polymerase chain reaction-amplified malarial DNA in infected blood and individual mosquitoes. Environ Parasitol. 1991;73:311–316. doi: 10.1016/0014-4894(91)90102-3. [DOI] [PubMed] [Google Scholar]
- Serbus LR, Casper-Lindley C, Landmann F, Sullivan W. The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet. 2008;42:683–707. doi: 10.1146/annurev.genet.41.110306.130354. [DOI] [PubMed] [Google Scholar]
- Sinkins SP. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect Biochem Mol Biol. 2004;34:723–729. doi: 10.1016/j.ibmb.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Unckless RL, Boelio LM, Herren JK, Jaenike J. Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proc R Soc B. 2009;276:2805–2811. doi: 10.1098/rspb.2009.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani D, Mesplet M, Eiras DF. PCR detection of Dirofilaria immitis in Aedes aegypti and Culex pipiens from urban temperate Argentina. Parasitol Res. 2011;108:985–989. doi: 10.1007/s00436-010-2142-1. [DOI] [PubMed] [Google Scholar]
- Xi Z, Khoo CCH, Dobson SL. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science. 2005;310:326–328. doi: 10.1126/science.1117607. [DOI] [PubMed] [Google Scholar]
- Yen JH, Barr AR. New hypothesis of the cause of cytoplasmic incompatibility in Culex pipiens. Nature. 1971;232:657–658. doi: 10.1038/232657a0. [DOI] [PubMed] [Google Scholar]
- Yue C, Genersch E. RT-PCR analysis of deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor) J Gen Virol. 2005;86:3419–3424. doi: 10.1099/vir.0.81401-0. [DOI] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O’Neill SL. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zioni N, Soroker V, Chejanovsky N. Replication of Varroa destructor Virus 1 (VDV-1) and a Varroa destructor Virus 1– deformed wing virus recombinant (VDV-1-DWV) in the head of the honeybee. Virology. 2011;417:106–112. doi: 10.1016/j.virol.2011.05.009. [DOI] [PubMed] [Google Scholar]


