Abstract
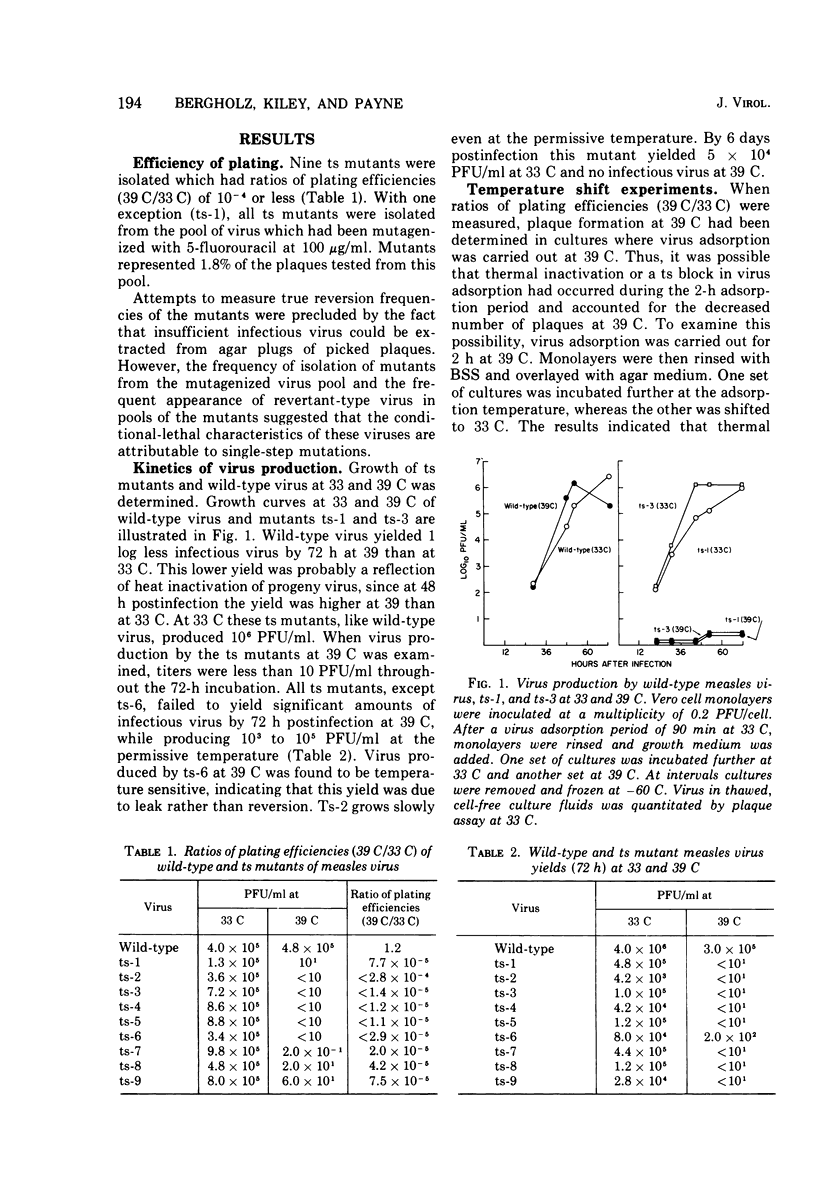
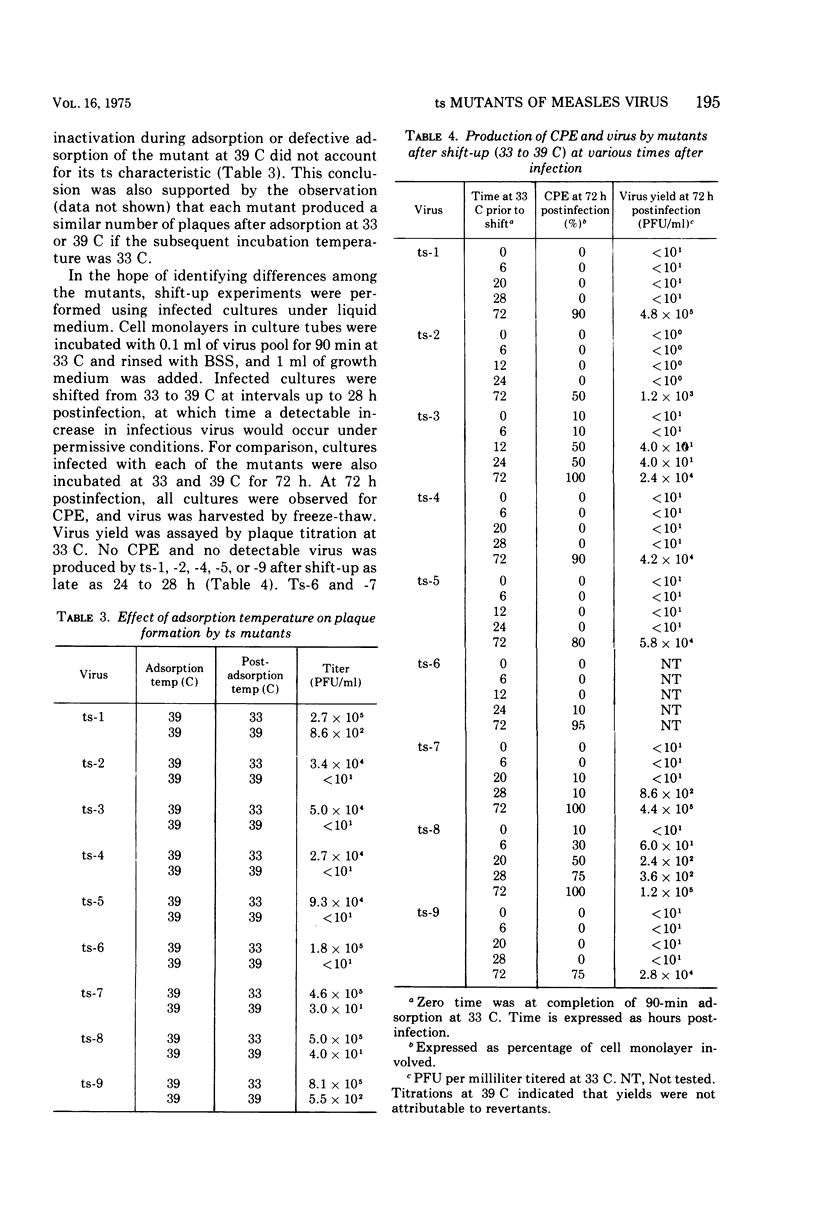
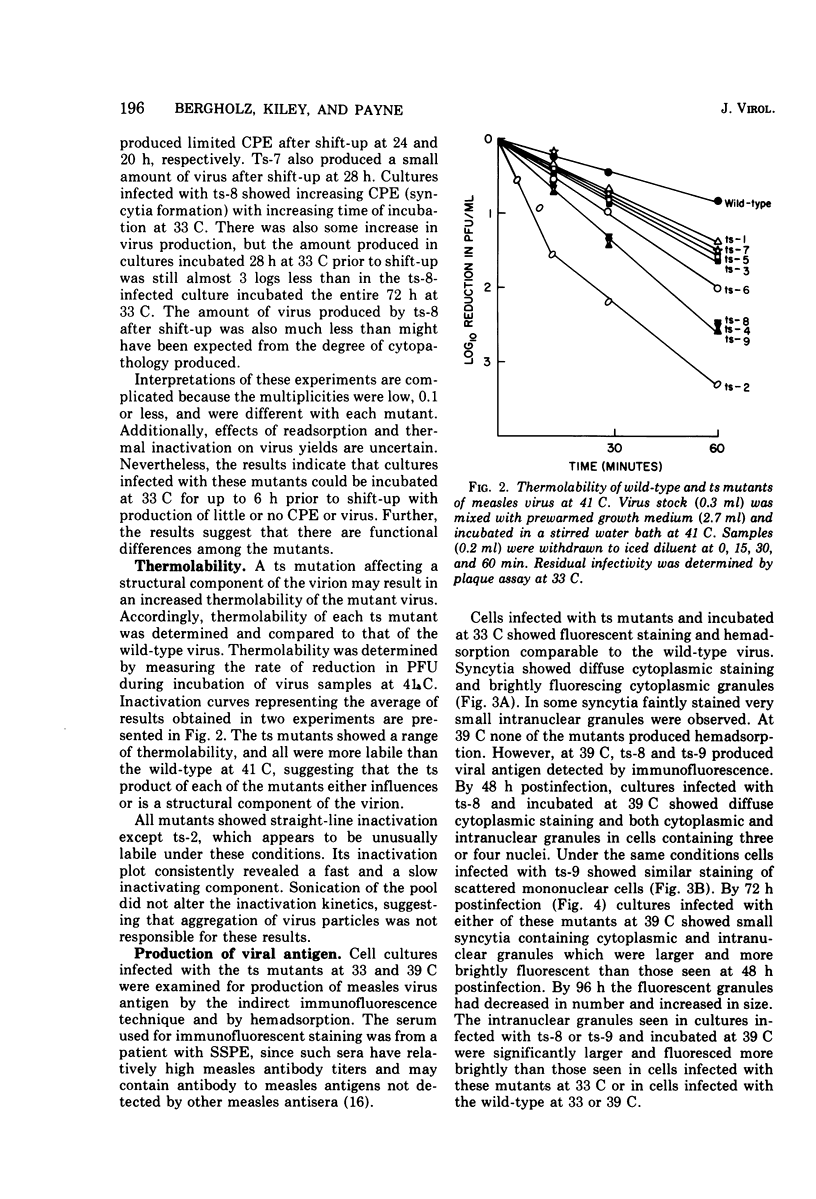
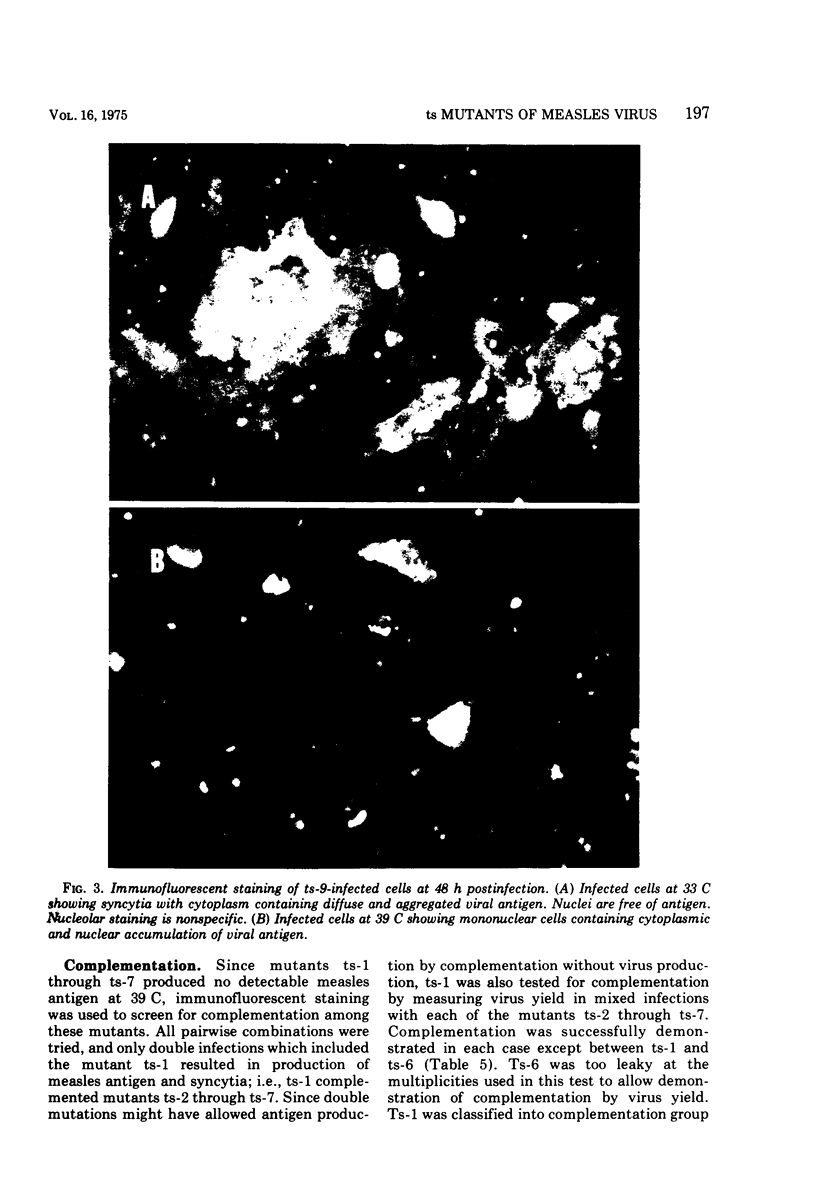
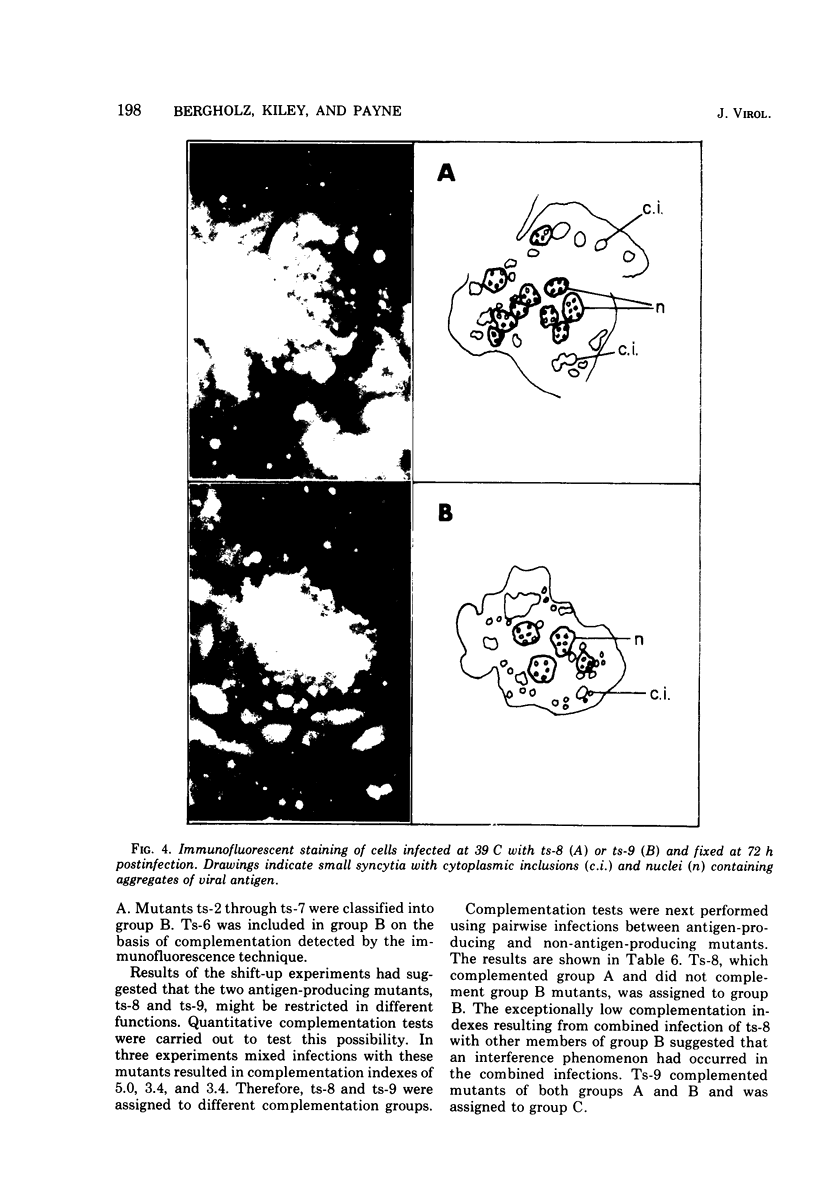
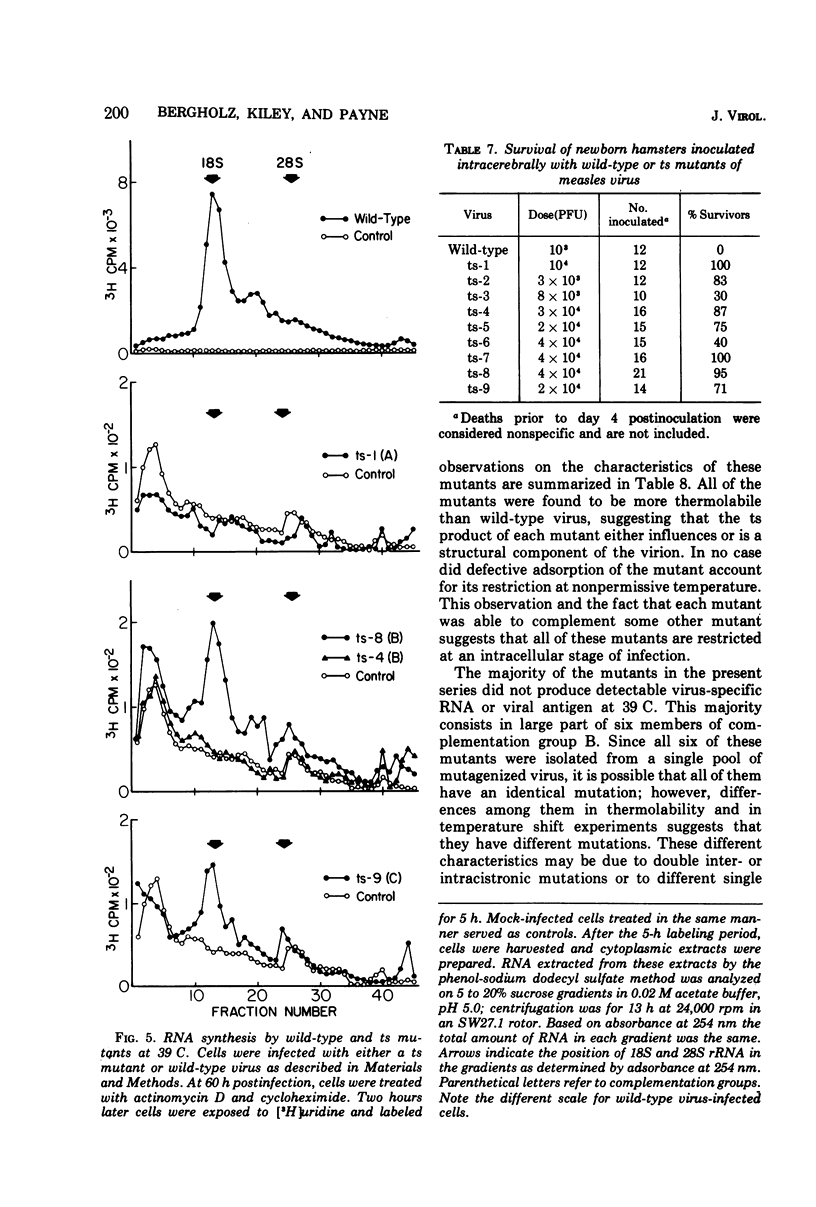
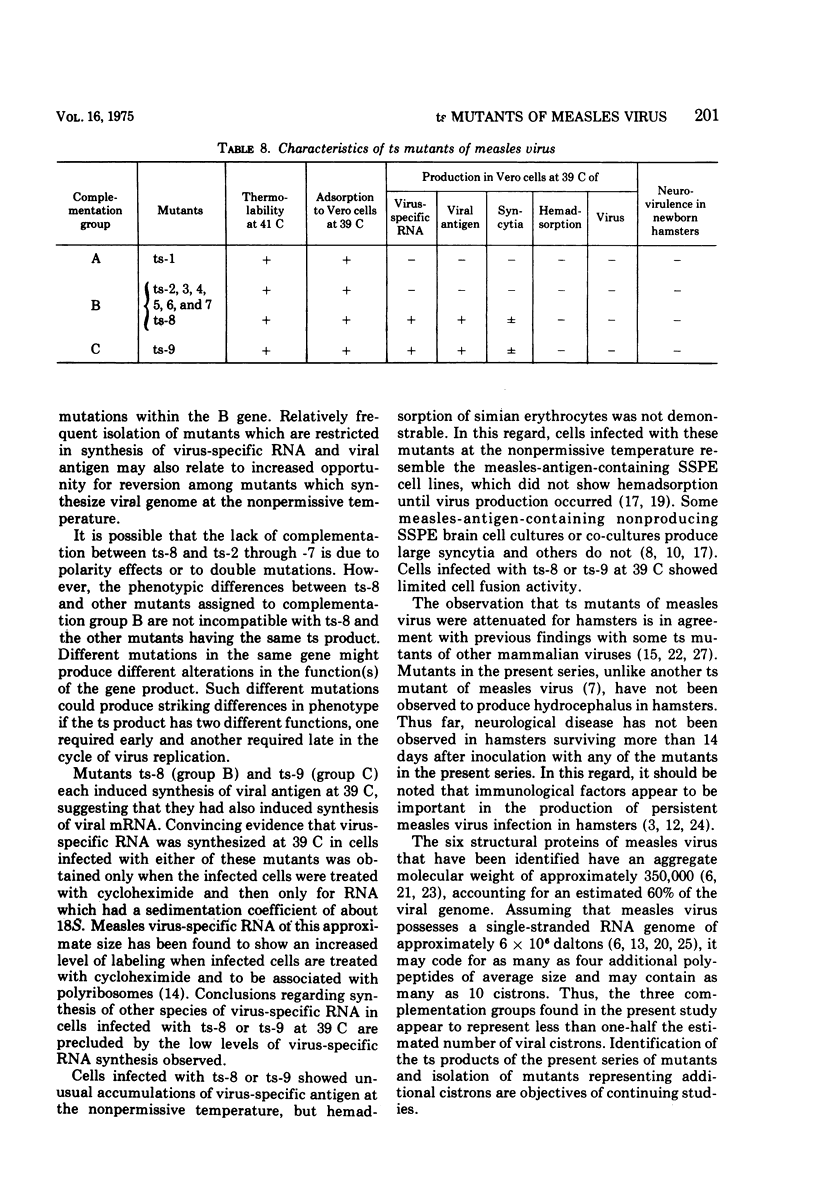
Nine temperature-sensitive (ts) mutants of nonattenuated Edmonston strain measles virus were isolated from wild-type virus which was grown in the presence of 5-fluorouracil. Adsorption, temperature shift, and complementation experiments indicated that all these mutants were restricted at an intracellular stage of infection. However, all the mutants were more rapidly inactivated at 41 C than was wild-type virus, suggesting that the ts product of each mutant either influences or is a structural component of the virus. Three complementation groups were found to be represented among the mutants. Group A contained one mutant and it did not induce synthesis of detectable amounts of viral antigen at the nonpermissive temperature (39 C). Group B consisted of six mutants which did not induce viral antigen synthesis at 39 C and one mutant which did. Group C was represented by one mutant and it induced viral antigen synthesis at 39 C. The two mutants which induced sythesis of viral antigen also induced synthesis of relatively small amounts of virus-specific RNA at 39 C. These mutants, while producing cytoplasmic and nuclear accumulations of viral antigen at 39 C, were restricted in production of syncytia and hemadsorption. All the mutants were less neurovirulent than wild-type virus, as indicated by their inability to produce acute disease in newborn hamsters.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbanti-Brodano G., Oyanagi S., Katz M., Koprowski H. Presence of 2 different viral agents in brain cells of patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1970 May;134(1):230–236. doi: 10.3181/00379727-134-34765. [DOI] [PubMed] [Google Scholar]
- Baublis J. V., Payne F. E. Measles antigen and syncytium formtion in brain cell cultures from subacute sclerosing panencephalitis (SSPE). Proc Soc Exp Biol Med. 1968 Nov;129(2):593–597. doi: 10.3181/00379727-129-33377. [DOI] [PubMed] [Google Scholar]
- Byington D. P., Johnson K. P. Subacute sclerosing panencephalitis virus in immunosuppressed adult hamster. Lab Invest. 1975 Jan;32(1):91–97. [PubMed] [Google Scholar]
- Chen T. T., Watanabe I., Zeman W., Mealey J., Jr Subacute sclerosing panencephalitis: propagation of measles virus from brain biopsy in tissue culture. Science. 1969 Mar 14;163(3872):1193–1194. doi: 10.1126/science.163.3872.1193. [DOI] [PubMed] [Google Scholar]
- Cooper P. D. A genetic map of poliovirus temperature-sensitive mutants. Virology. 1968 Aug;35(4):584–596. doi: 10.1016/0042-6822(68)90287-0. [DOI] [PubMed] [Google Scholar]
- Hall W. W., Martin S. J. Purification and characterization of measles virus. J Gen Virol. 1973 May;19(2):175–188. doi: 10.1099/0022-1317-19-2-175. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Rapp F. Measles virus: an unwanted variant causing hydrocephalus. Science. 1975 Feb 7;187(4175):450–451. doi: 10.1126/science.1111114. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., London W. T., Jabbour J. T., Zeman W., Sever J. L. Isolation of measles virus from brain cell cultures of two patients with subacute sclerosing panencephalitis. Proc Soc Exp Biol Med. 1969 Oct;132(1):272–277. doi: 10.3181/00379727-132-34196. [DOI] [PubMed] [Google Scholar]
- Horta-Barbosa L., Fuccillo D. A., Sever J. L., Zeman W. Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature. 1969 Mar 8;221(5184):974–974. doi: 10.1038/221974a0. [DOI] [PubMed] [Google Scholar]
- Katz M., Koprowski H. The significance of failure to isolate infectious viruses in cases of subacute sclerosing panencephalitis. Brief report. Arch Gesamte Virusforsch. 1973;41(4):390–393. doi: 10.1007/BF01250213. [DOI] [PubMed] [Google Scholar]
- Katz M., Oyanagi S., Koprowski H. Subacute sclerosing panencephalitis: structures resembling myxovirus nucleocapsids in cells cultured from brain. Nature. 1969 May 31;222(5196):888–890. doi: 10.1038/222888a0. [DOI] [PubMed] [Google Scholar]
- Kibler R., Cremer N. E. Antithymocyte serum treatment of hamsters inoculated with a measles virus isolated from a patient with subacute sclerosing panencephalitis. Immunol Commun. 1973;2(3):303–321. doi: 10.3109/08820137309022802. [DOI] [PubMed] [Google Scholar]
- Kiley M. P., Gray R. H., Payne F. E. Replication of measles virus: distinct species of short nucleocapsids in cytoplasmic extracts of infected cells. J Virol. 1974 Mar;13(3):721–728. doi: 10.1128/jvi.13.3.721-728.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley M. P., Payne F. E. Replication of measles virus: continued synthesis of nucleocapsid RNA and increased synthesis of mRNA in the presence of cycloheximide. J Virol. 1974 Oct;14(4):758–764. doi: 10.1128/jvi.14.4.758-764.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J., Chanock V., Chanock R. M. Temperature-sensitive mutants of influenza virus. I. Behavior in tissue culture and in experimental animals. J Infect Dis. 1971 Feb;123(2):145–157. doi: 10.1093/infdis/123.2.145. [DOI] [PubMed] [Google Scholar]
- Palosuo T., Salmi A. A., Pettay O. Measles antibodies detected by platelet aggregation and gel precipitation in patients with subacute sclerosing panencephalitis. Arch Gesamte Virusforsch. 1971;35(1):45–53. doi: 10.1007/BF01249751. [DOI] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V. Decreased reactivity of SSPE strains of measles virus with antibody. J Infect Dis. 1973 May;127(5):505–511. doi: 10.1093/infdis/127.5.505. [DOI] [PubMed] [Google Scholar]
- Payne F. E., Baublis J. V., Itabashi H. H. Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med. 1969 Sep 11;281(11):585–589. doi: 10.1056/NEJM196909112811103. [DOI] [PubMed] [Google Scholar]
- Schluederberg A., Chavanich S., Lipman M. B., Carter C. Comparative molecular weight estimates of measles and subacute sclerosing panencephalitis virus structural polypeptides by simultaneous electrophoresis in acrylamide gel slabs. Biochem Biophys Res Commun. 1974 Jun 4;58(3):647–651. doi: 10.1016/s0006-291x(74)80467-5. [DOI] [PubMed] [Google Scholar]
- Schluederberg A. Measles virus RNA. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1012–1015. doi: 10.1016/0006-291x(71)90004-0. [DOI] [PubMed] [Google Scholar]
- Wagner R. R. Pathogenicity and immunogenicity for mice of temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1974 Aug;10(2):309–315. doi: 10.1128/iai.10.2.309-315.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters D. J., Bussell R. H. Polypeptide composition of measles and canine distemper viruses. Virology. 1973 Oct;55(2):554–557. doi: 10.1016/0042-6822(73)90202-x. [DOI] [PubMed] [Google Scholar]
- Wear D. J., Rapp F. Latent measles virus infection of the hamster central nervous system. J Immunol. 1971 Dec;107(6):1593–1598. [PubMed] [Google Scholar]
- Winston S. H., Rustigian R., Bratt M. A. Persistent infection of cells in culture by measles virus. 3. Comparison of virus-specific RNA synthesized in primary persistent infection in HeLa cells. J Virol. 1973 Jun;11(6):926–932. doi: 10.1128/jvi.11.6.926-932.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygraich N., Lobmann M., Huygelen C. Inoculation of hamsters with a temperature sensitive (ts) mutant of parainfluenza 3 virus. J Hyg (Lond) 1972 Jun;70(2):229–234. doi: 10.1017/s0022172400022282. [DOI] [PMC free article] [PubMed] [Google Scholar]