Summary
Patients with cancer have an impaired T-cell response that can decrease the potential therapeutic benefit of cancer vaccines and other forms of immunotherapy. l-arginine (l-Arg) is a conditionally essential amino acid that is fundamental for the function of T lymphocytes. Recent findings in tumor-bearing mice and cancer patients indicate that increased metabolism of l-Arg by myeloid derived suppressor cells (MDSCs) producing arginase I inhibits T-lymphocyte responses. Here we discuss some of the most recent concepts how MDSC expressing arginase I may regulate T-cell function in cancer and other chronic inflammatory diseases and suggest possible therapeutic interventions to overcome this inhibitory effect.
Keywords: arginase, myeloid derived suppressor cells, T-cell function
Introduction
The clinical experiments of William Coley in the 1890s and the work of Prehn and Main in the 1950s firmly demonstrated the presence of an immune response against tumor antigens that could potentially be used in the treatment of cancer. New concepts in carcinogenesis, including the viral etiology of some malignancies, the presence of mutated onco-proteins, and the over-expression of certain normal antigens, further supports the concept that an antigen-specific immune response can be generated to control tumor growth. However, two decades of clinical trials in cancer immunotherapy have also made evident that tumor cells have sophisticated mechanisms to evade the immune response. Recent findings have characterized several molecular mechanisms triggered by the tumor microenvironment resulting in the impairment of T-cell anti-tumor function. The molecular and cellular bases underlying these mechanisms are a matter of extensive research and debate, in part because of their complexities and possible overlapping pathways. However, they represent a possible key to being able to modulate the immune response for the therapeutic benefit of patients with cancer and other chronic diseases. Even though most researchers agree that the malignant cells trigger the events that ultimately lead to T-cell tolerance, the intermediaries in this process vary and include regulatory T cells, suppressor macrophages, and more recently myeloid derived suppressor cells (MDSCs). The latter subpopulation of immune cells has become a focus of much research recently because of the novel observations demonstrating their ability to regulate T-cell responses by controlling the availability of the amino acid l-arginine (l-Arg). The demonstration of this mechanism in tumor models, patients with cancer, and various other chronic inflammatory diseases, and the potential for therapeutic intervention has created a major interest in MDSC. Here, we discuss some of the most recent concepts of how myeloid cells metabolizing l-Arg regulate T-cell function in disease and suggest possible therapeutic applications to inhibit MDSC activity.
Alterations of the immune response in cancer
A dysfunctional immune response in cancer patients manifested by the loss of delayed type hypersensitivity to bacterial and chemical antigens was demonstrated in cancer patients several decades ago (1–4). Initial explanations included the development of ‘blocking antibodies’ (5), the production of suppressor factors by tumor cells (6, 7), and the generation of suppressor macrophages and dendritic cells (DCs) (8). However, the significance of these findings on the progression of the disease and its outcome were unknown. Although cancer patients generally do not develop the characteristic opportunistic infections that affect patients immunosuppressed by the use of high dose chemotherapy or corticosteroids (which ablates granulocytes and mononuclear immune cells equally), they have indeed been shown to have an impaired delayed type hypersensitivity response to bacterial and/or chemical antigens and a poor in vitro response to mitogens (9–12). These results suggested that cancer primarily affects T-cell responses but not the myeloid response (granulocytes). Not until the advent of immunotherapy trials in cancer in the 1980s and 1990s was the real impact of the T-cell dysfunction made apparent. Several animal tumor models and many clinical trials demonstrated that immunotherapy in mice or patients with advanced tumors failed to achieve a therapeutic response as a result of the loss of T-cell responses (reviewed in 13). In addition, several vaccine trials demonstrated the progression of tumors in spite of a robust T-cell response (14). The development of cellular and molecular models leading to T-cell anergy provided important insights to understand how cancer (and other chronic inflammatory diseases) could selectively cause T-cell dysfunction (15). This development provided the basis for the discovery of new mechanisms including the role of immunoregulatory molecules in antigen-presenting cells (APCs) such as B7.1, B7.2, B7-H1, and B7-H4 (16–20), the development of regulatory T cells (21, 22) and the generation of MDSC (23–26). Although most models are in agreement that tumor cells are the initiators of the suppressor phenomenon, they also coincide that APCs, in the form of macrophages or DCs, play a central role in directly inducing T-cell anergy or generating regulatory T cells (27, 28).
Young et al. (29) demonstrated that suppressor macrophages blocked T-cell responses by producing interleukin-10 (IL-10), transforming growth factor-β (TGFβ), and prostaglandin E2 (PGE2). Gabrilovich et al. (30) demonstrated that vascular endothelial growth factor (VEGF) produced by the tumor arrested the differentiation of DCs, resulting in immature myeloid cells that induce T-cell dysfunction. These immature myeloid cells were increased in patients with breast, head and neck, and lung cancer (31, 32). More recently, Mellor and Munn (33, 34) demonstrated that an impaired T-cell response can also occur as a result of the depletion of the amino acid tryptophan by plasmacytoid DCs producing indoleamine-2, 3-dioxygenase (IDO). Tryptophan starvation induced cell cycle arrest in normal T lymphocytes and sensitized activated T cells to apoptosis before cell division (35). The mature and immature APCs can play a central role in the induction of tolerance.
Signaling alterations in anergic T cells in cancer
In the 1990s, we and others showed that T cells from cancer patients and tumor-bearing mice had multiple changes in the expression of signal transduction molecules, including a decreased expression of the T-cell receptor (TCR) ζ chain (CD3ζ), a diminished tyrosine kinases p56lck, p59fyn, and an inability to upregulate Janus kinase-3 (Jak-3), and to translocate NFκBp65, all of which resulted in a diminished in vitro T-cell response (36–38). These T-cell signal transduction alterations were accompanied by a diminished ability to mobilize Ca++ and a decreased tyrosine phosphorylation (39), and provided a possible molecular explanation for the T-cell dysfunction reported in tumor-bearing mice and cancer patients. The initial findings in tumor-bearing mice were confirmed in patients with renal cell carcinoma, melanoma, Hodgkin’s disease, ovarian cancer, colon carcinoma, and cervical cancer among others (40–42). Patients with renal cell carcinoma showed changes in CD3ζ expression in the tumor-infiltrating T cells (43), while patients with colon carcinoma showed the most significant loss of CD3ζ in the draining lymph nodes closest to the tumor (44), suggesting that the tumor microenvironment played an important role in inducing these changes. Preliminary studies also suggested an association between alterations in signal transduction and a decreased survival in cancer patients. Patients with melanoma and patients with head and neck tumors who had a decreased expression of CD3ζ had significantly shorter survival compared with patients expressing normal levels (42, 45). The absence of a mechanism to explain these changes and the apparent lack of specificity of these alterations created some initial controversy around these observations.
Mechanisms leading to a decreased CD3ζ chain in disease
Otsuji et al. (46) and Kono et al. (47, 48) were the first to demonstrate that the co-incubation of activated murine peritoneal macrophages with naive T cells induced the loss of CD3ζ chain in the latter population. This phenomenon could be blocked by the addition of oxygen radical scavengers and was therefore thought to be mediated by the release of H2O2 (49). A similar effect was suggested in a report by Schmielau et al. (36) in patients with pancreatic and breast cancer where an increase in the number of activated neutrophils and the production of H2O2 in peripheral blood was closely associated with a diminished expression of CD3ζ chain (50). Another mechanism suggested that the loss of CD3ζ chain was a consequence of Fas–Fas ligand (FasL)-induced T-cell apoptosis (51, 52). Still an additional mechanism was proposed by the work of Baniyash and colleagues (53), where they demonstrated that chronic stimulation of T cells by specific antigens led to the decreased expression of CD3ζ chain of the TCR and induction of anergy. However, none of these models reproduced the multiple alterations found in T cells of cancer patients.
Reports from other diseases suggested that the loss of CD3ζ was not unique to cancer. Zea et al. (54, 55) described that patients with lepromatous leprosy or active pulmonary tuberculosis presented similar alterations in peripheral blood T cells. More recently mice with severe trauma were found to have a loss of CD3ζ chain and T-cell function (56). Furthermore, trauma patients had a rapid depletion of l-Arg levels in serum, which was paralleled by the loss of T-cell function. Animal models confirmed this observation (57) and further demonstrated that the replenishment of l-Arg by the infusion of high doses of l-Arg in mice or in trauma patients resulted in the recovery of T-cell function and an increase in the number of CD4+ cells (58–60). This finding led us to study whether regulation of the levels of l-Arg might be involved in the induction of T-cell dysfunction in patients with cancer.
l-Arg and immune response
The association of l-Arg and the immune system was initially suggested in the 1970s by reports demonstrating that the injection of l-Arg in mice undergoing extensive surgery prevented a well-described phenomenon of post-surgical thymus involution and appeared to increase the number of T cells (61). In the late 1980s, Albina and Mills (26, 62) demonstrated that l-Arg was fundamental for wound healing processes possibly by increasing the production of proline and collagen. A different but equally important association was suggested by reports showing that the rapid depletion of plasma levels of l-Arg was accompanied by a markedly decreased T-cell function in patients undergoing liver transplantation, in trauma patients, or in murine models of trauma (58–60). Furthermore, the state of anergy caused by trauma could be rapidly reversed by the enteral or parenteral supplementation of l-Arg (63).
Our initial experiments demonstrated that culturing Jurkat T cells in tissue culture medium with l-Arg levels < 50 µM resulted in the gradual loss of CD3ζ and caused a significant decrease in proliferation (64). Experiments using primary T cells (murine or human) did not show any effects of l-Arg deprivation on resting T cells. However, T cells activated in an l-Arg-free environment developed all the alterations previously described in tumor-bearing mice and cancer patients, i.e. the decreased expression of CD3ζ, an inability to upregulate Jak-3, and a decreased translocation of NFκB-p65. T cells cultured without l-Arg also failed to proliferate and did not produce interferon-γ (IFN-γ). However, these changes were selective because other functions, including the production of IL-2 and the upregulation and expression of the IL-2 receptor chains (CD25, CD122, CD132), were similar to cells cultured in medium with l-Arg (l-Arg concentration in RPMI is 1100 µM). These results suggested a potential role for l-Arg depletion as a mechanism for the induction of T-cell dysfunction.
In healthy adults, l-Arg is considered to be a non-essential amino acid, because it is synthesized endogenously from citrulline by the collaboration between the epithelial cells of the small intestine and the proximal tubules of the kidney (reviewed in 65). Normal levels of l-Arg in serum range between 50 and 150 µM. However, l-Arg is also classified as a conditional essential amino acid in certain physiological conditions involving changes in the l-Arg metabolic status, including trauma and cancer (66). l-Arg is the substrate for four enzymes, several of which exist as multiple isoforms: nitric oxide synthases (NOS1, NOS2, and NOS3), arginases (arginase I and II), arginine:glycine amidinotransferase (AGAT), and l-Arg decarboxylase (ADC) (Fig. 1). To encounter all enzymes involved in its metabolism, dietary l-Arg must be taken up by intestinal epithelial cells and must traverse the plasma membrane of all cells via the y+system of cationic amino acid transporters (CAT) (67). Once inside the cells, lArg is metabolized by NOS enzymes to produce citrulline and nitric oxide, which plays an important role in cytotoxic mechanisms and vasodilatation (68, 69). Alternatively, arginase I and arginase II metabolize l-Arg to l-ornithine and urea, the first being the precursor for the production of polyamines that are essential for cell proliferation and the second an important mechanism for detoxification of protein degradation (70). Arginase and NOS enzymes have been widely studied in vitro and in vivo; however, there is limited information about the regulation and the immunological role of ADC and AGAT. ADC converts l-Arg to agmatine, which in turn is converted to putrescine and urea by agmatinase. Mammalian ADC is highly expressed in the brain (71, 72), while AGAT is expressed in the brain and the heart (73, 74).
Fig. 1. l-Arg metabolism.
l-Arginine can be metabolized by NOS into NO and citrulline. Alternatively, arginase (I and II) can convert l-Arg into urea and ornithine, with the latter being the substrate for the synthesis of polyamines need to sustain cell proliferation. For the sake of simplicity, emphasis is on enzymes that directly metabolize l-Arg. This diagram should not be interpreted to indicate that all of these enzymes are expressed simultaneously in any given cell type.
Arginase I and NOS2 play important roles in the immune response. The expression of arginase I and NOS2 in murine macrophages is differentially regulated by T-helper 1 (Th1) and Th2 cytokines (75, 76). Stimulation of murine macrophages with IFN-γ upregulates NOS2 exclusively, while IL-4, IL-10, and IL-13 (77, 78) or TGFβ (79) induce arginase I. The mitochondrial isoform arginase II is not significantly modulated by Th1 or Th2 cytokines (80). Furthermore, inhibition of arginase I leads to an increased NOS2 expression and consequently promotes NO production (81). Conversely, upregulation of arginase I functionally inhibits NOS activity and contributes to the pathophysiology of several disease processes, including vascular dysfunction and asthma (82). The mechanisms of inhibition NOS2 expression by arginase I are partially understood. l-Arg depletion by arginase I blocks the induction of NOS2 expression and the subsequent NO production in macrophages through an arrest in the synthesis of NOS2 protein (83). In addition, very low levels of NOS can induce nitrosylation of cysteine residues of human arginase I, which increases the biological activity of arginase I and reduces l-Arg (84). This process in turn blocks the expression of NOS2 and the production of NO.
Activation of peritoneal macrophages with Th1 or Th2 cytokines also had different effects on the extracellular levels of l-Arg. Peritoneal macrophages stimulated with IL-4 + IL-13 increased the expression of arginase I and CAT-2B. This resulted in a dramatic increase in the uptake of extracellular l-Arg with the consequent reduction of l-Arg in the tissue culture medium. In contrast, macrophages stimulated with IFN-γ preferentially expressing NOS2 did not increase CAT-2B and did not deplete l-Arg levels from the tissue culture medium (80). In both culture conditions, macrophages constitutively expressed arginase II, suggesting that this enzyme does not deplete extracellular l-Arg. l-Arg depletion was not caused by the release of arginase I into the tissue culture medium by murine macrophages. In addition, data from arginase I and arginase II knockout animals suggested that the only l-Arg metabolizing enzyme able to modify serum levels of l-Arg is arginase I (85, 86). Macrophages producing arginase I or NOS2 were then co-cultured with activated T cells using transwells. Only macrophages producing arginase I, and not macrophages expressing NOS2, caused the loss of CD3ζ and inhibited T-cell proliferation. Furthermore, the addition of arginase inhibitors N-Hydroxy-nor-l-Arg (Nor-NOHA) and N-Hydroxy-l-Arg (NOHA) or exogenous l-Arg reversed the CD3ζ loss (80). Thus, arginase I expression in macrophages inhibited T-cell function by impairing the expression of CD3ζ chain of the TCR.
Molecular effects of l-Arg starvation on T cells
The initial data in Jurkat cells and in primary T cells cultured in medium lacking l-Arg showed a decrease of CD3ζ and a decrease in proliferation, which were not associated with an increase in apoptosis (64, 80, 87, 88). Both CD3ζ chain expression and cell proliferation rapidly recovered after replenishment of l-Arg and citrulline (88). We initially hypothesized that the main mechanism of T-cell dysfunction induced by l-Arg starvation was the loss of CD3ζ chain expression. However, the low expression of CD3ζ chain alone could not fully explain the almost complete inhibition of T-cell proliferation, even in T cells stimulated with phorbol myristate acetate (in the absence of l-Arg), which bypasses the TCR (unpublished data). In addition, T cells cultured in the absence of l-Arg had similar patterns of calcium flux and tyrosine phosphorylation (during the first 12 h of culture) as T cells cultured with l-Arg, and these cells were able to upregulate the expression of activation markers CD25 and CD69, suggesting that signaling through the TCR was intact during the early stages of culture without l-Arg (88). We therefore studied whether l-Arg deprivation specifically inhibited the mechanisms regulating T-cell proliferation.
Propidium iodide labeling of T cells’ nuclei showed that cells cultured in the absence of l-Arg were arrested G0–G1 phase of the cell cycle (without the induction of apoptosis), while cells cultured with l-Arg progressed into S and G2–M phases after 72 h of culture (89). The simple replenishment of l-Arg to physiological levels (50–150 µM) reestablished cell cycle progression even after 96 h of culture. In mammalian cells, cyclin-dependent kinase 4 (cdk4) and cdk6 are closely associated with the D-type cyclins (cyclin D1, D2, and D3) and regulate the progression through early G1 and later into the S phase of the cell cycle (90). This regulation requires inactivation of cyclin-D/cdk complex inhibitors (INK4, KIP) and the phosphorylation of the Rb protein family (91). Activated T cells cultured in the absence of l-Arg were unable to upregulate cyclin D3 and cdk4 mRNA and protein but not cdk6 (89). In fact, silencing of cyclin D3 in the human Jurkat T-cell line induced a similar inhibition of proliferation as that induced by l-Arg starvation. Results from knockout mice had demonstrated that cyclin D3 is essential for the maturation of T cells in the thymus (92) and suggested a potential and selective role in T-cell proliferation. In addition and as a consequence of cyclin D3 inhibition, T cells activated and cultured in the absence of l-Arg had a significant decrease in Rb phosphorylation and a markedly decreased nuclear translocation of E2F-1.
We further explored why l-Arg starvation negatively regulated the expression of cyclin D3 and cdk4 mRNA but not cdk6. Results demonstrated that l-Arg starvation induced a decrease in cyclin D3 mRNA transcriptional rate, as demonstrated by ‘Run-on’ experiments, as well as a decreased cyclin D3 mRNA stability. Furthermore, the l-Arg starvation impaired the translation of cyclin D3. Therefore, the expression of cyclin D3 and cdk4 in the absence of l-Arg are blocked through transcriptional, post-transcriptional, and translational mechanisms (89). What mechanism(s) may be triggered by the depletion of a non-essential amino acid to explain this apparently complex process is still a matter of ongoing research.
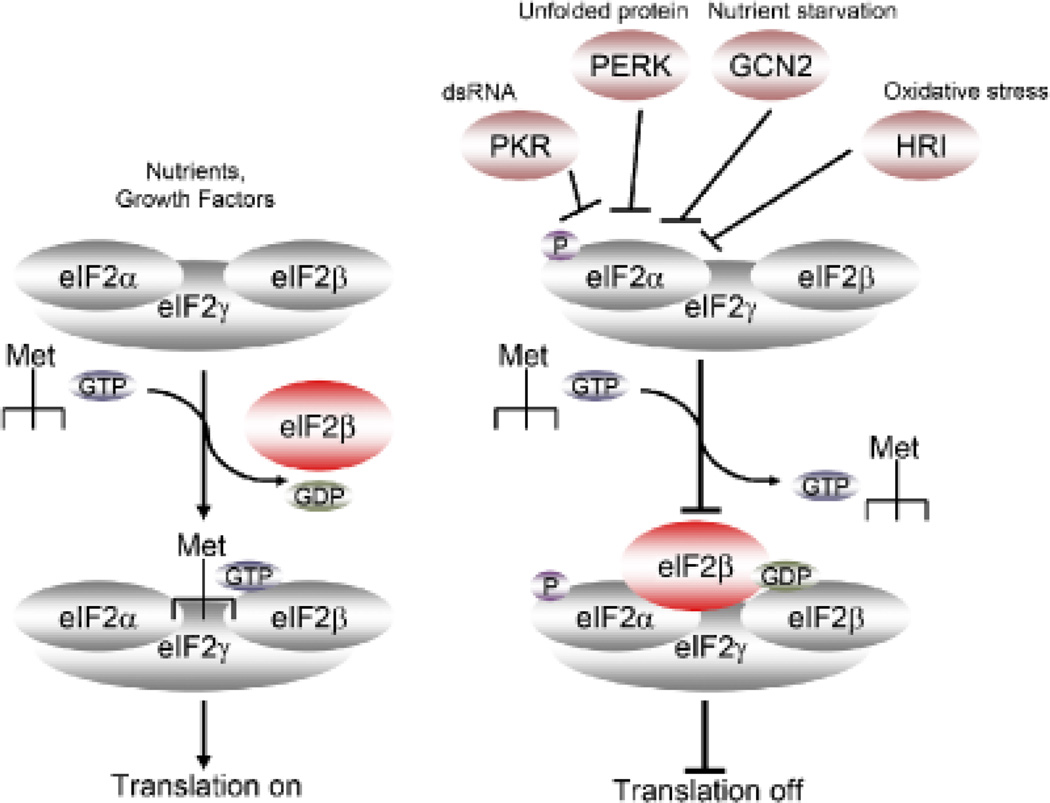
Amino acid deprivation in eukaryotes has been shown to activate mechanisms that inhibit translation. The accumulation of empty aminoacyl tRNAs caused by amino-acid starvation activates GCN2 kinase, which phosphorylates the translation initiation factor eIF2α (34). The phosphorylated form of eIF2α binds more tightly than usual to eIF2B, whose job is to exchange guanosine triphosphate (GTP) for guanosine diphosphate (GDP) in the eIF2 complex. When eIF2B is bound to the phosphorylated eIF2α, it is unable to exchange GDP for GTP, which inhibits the binding of eIF2 complex to methionine aminoacyl tRNA and finally leads to inhibition in translation initiation (Fig. 2-Translation off).
Fig. 2. Control of translation by eIF2α.
Multiple signals can trigger the phosphorylation of eIF2α, including nutrient starvation (GCN2), dsRNA (PKN), unfolded proteins (PERK), and oxidative stress (HRI). Phospho-eIF2α binds to the IF2β complex, preventing its translocation and inhibiting translation.
We first determined whether the absence of l-Arg led to the activation of GCN2 by testing eIF2α phosphorylation. A significant increase in the phosphorylation of eIF2α was observed in activated T cells cultured in the absence of l-Arg but not in cells cultured in the presence of l-Arg (89). We then tested the role of GCN2 as a central mediator of the effects on T cells induced by the absence of l-Arg, Indeed, T cells from GCN2 knockout mice did not show an arrest in cell cycle or a decreased proliferation, and they were able to upregulate the expression of cyclin D3 and cdk4 when cultured in medium without l-Arg (89). These results confirm the role of GCN2 as the sensing protein in the signaling induced by l-Arg starvation in T cells (Fig. 2). Interestingly, these results are similar to those induced by the depletion of tryptophan, an essential amino acid that when depleted by the enzyme IDO also results in the induction of a profound T-cell anergy (33, 34).
How amino acid availability decreases mRNA stability is still unclear. Some mRNA stability is increased by l-Arg starvation, which has been shown using the CAT-1 transporter where amino acid limitation increases the stability and translation of the mRNA in rat glioma cells, at a time when global protein synthesis decreases. The increased mRNA stability requires an 11 nucleotide AU-rich element within the distal 217 bases of the 3′-untranslated region (93). In addition, amino-acid starvation triggers an increased translocation of the DNA binding protein HuR from nucleus to cytoplasm which appears to increase the stability of CAT-1 mRNA (94). However, the mechanisms leading to a diminished stability of cyclin-D3 induced by l-Arg depletion remain unclear at the present time.
Arginase expression in tumors
Several tumor lines from non-small lung carcinoma and breast carcinoma have been shown to express arginase (95–97). This expression has been thought to be a mechanism for the production of polyamines needed to sustain the rapid proliferation of tumor cells. Results from our laboratory suggested instead that arginase I was preferentially expressed in myeloid cells infiltrating tumors, which inhibited T-cell function as a possible mechanism of tumor evasion (98). Myeloid cells infiltrating murine 3LL lung carcinoma and expressing arginase I have the morphology and express the markers of mature macrophages. However, the myeloid cells found in the spleen of tumor-bearing mice (including 3LL tumors) and expressing arginase I appear to be immature myeloid cells (98). Even though there has been some variation in cell morphology and maturation markers found between different tumor models and between murine and human tumors, these myeloid cells are able to suppress T-cell responses. Recently, a panel of leading investigators in the field agreed to use the common term ‘myeloid derived suppressor cells’ (MDSCs) to name these arginase I-producing cells (99). MDSC commonly express the markers CD11b and GR-1 and have a similar phenotype that of alternatively activated macrophages (also known as M2 macrophages) in the mouse. MDSC are present in the bone marrow of healthy mice; however, they accumulate in the spleen and tumors in tumor-bearing mice (with a higher ability to suppress T-cell function) (100–103). Depletion of MDSC using antibodies against GR-1 induced an anti-tumor effect, which was mediated by CD8+ T cells (104–106).
MDSC induce T-cell dysfunction by the depletion of extracellular l-Arg by arginase I metabolism and an increased uptake of l-Arg through the CAT-2B (98, 107, 108). Reduction of extracellular levels of l-Arg induces an arrest in the proliferation of activated T cells and blocks the re-expression of CD3ζ chain (98) (Fig. 3). The addition of arginase I inhibitors Nor-NOHA or NOHA in vitro or its injection into tumor-bearing mice prevented the loss of T-cell function and resulted in an immune-mediated anti-tumor response, respectively. Furthermore, tumor growth was significantly inhibited, in a dose-dependent manner, by the subcutaneous injection of the arginase inhibitor Nor-NOHA starting at the time of tumor implantation (day 0). The inhibition in tumor growth caused by Nor-NOHA, however, was lost in tumorbearing severe combined immunodeficient mice, strongly suggesting that the anti-tumor effect caused by the inhibition of arginase was dependent on lymphocyte function (98).
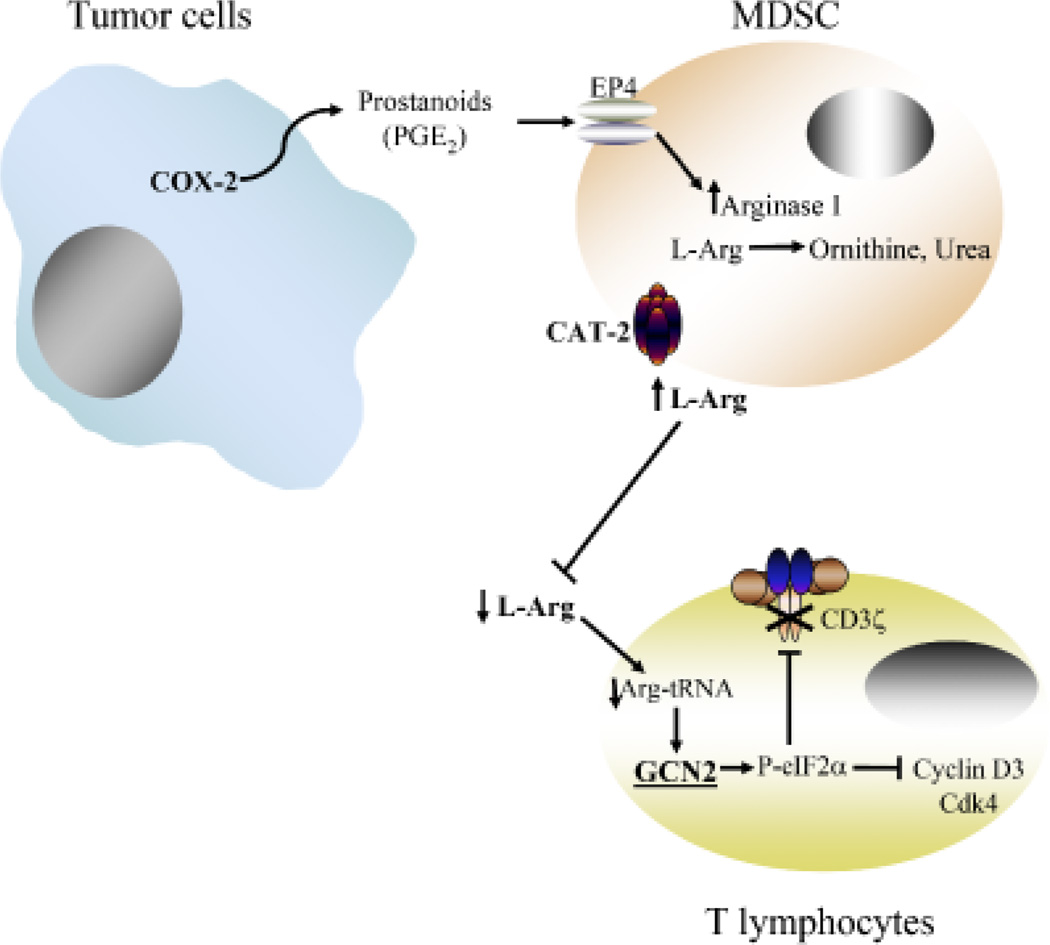
Fig. 3. T-cell dysfunction induced by arginase I.
Tumor cells expressing COX-2 and releasing PGE2 induce the expression of arginase I and CAT-2B in MDSC. This expression leads to a reduction of extracellular levels of l-Arg, which activates GCN2 and inhibits the expression of CD3ζ, cyclin D3, and cdk4 through post-transcriptional and translational mechanisms.
MDSC not only inhibit T-cell function by the depletion of l-Arg but have also been shown to exert a strong T-cell inhibition through cell:cell contact. These mechanisms require the co-expression of arginase I and NOS2. Cultures of MDSC and activated T cells in the presence of arginase and NOS2 inhibitors completely reestablished T-cell function (109). Under limiting amounts of l-Arg (induced by arginase I), NOS2 produces peroxinitrites (ONOO2), a highly reactive oxidizing agent that nitrates proteins and induces T-cell apoptosis (110). This appears to affect the conformational flexibility of the TCR and its interaction with major histocompatibility complex (MHC) by inducing nitration of TCR proteins in CD8+ cells. Thus, MDSC directly disrupt the binding of specific peptides on MHC to CD8+ T cells (111).
The suppression effect induced by MDSC co-expressing arginase I and NOS2 has been related preferentially to the impairment of CD8+ T-cell function (98, 102, 110, 112). MDSC inhibit the function of CD8+ T cells by blocking their ability to secrete IFN-γ when stimulated with specific antigens (101, 113), which results in the induction of T-cell apoptosis (110). This suppression is dependent on cytokines IL-13 and IFN-γ (102, 114, 115) and signaling through the signal transducer and activation of transcription 1 (STAT1) (110). In addition, it has also been suggested that MDSC can induce the expansion of regulatory T cells (116), a process that appears to be dependent on the production of IFN-γ and IL-10. Blocking of stem cell factor (SCF) signaling in MDSC significantly impairs their ability to generate regulatory T cells (117). A recent report has also suggested that MDSC suppress macrophage function by switching their phenotype toward M2 macrophages (118).
MDSC in human tumors
Although MDSC have been well studied in murine models, their role in human disease has only recently started to be understood. In contrast to murine MDSC, the human MDSC phenotype varies significantly, ranging from immature DCs (119) to activated granulocytes and expressing CD11b+, CD14−, CD15+, CD34+, CD33+, and CD13+ (120). A retrospective study of patients with metastatic renal cell carcinoma (RCC) demonstrated a 6–10-fold increase in arginase activity in the peripheral blood mononuclear cells (PBMCs) as compared with normal controls (120). Separation of the different subpopulations in the PBMCs of these patients demonstrated that the cells containing all arginase activity were activated granulocytes, which separated with the PBMCs when centrifuged over ficoll-hypaque. These patients also had a significantly diminished expression of the CD3ζ chain. There also was an inverse statistical correlation between arginase activity and MDSC numbers with the expression of CD3ζ chain in T cells. Finally, T-cell proliferation and IFN-γ production were re-established (in vitro) only after the depletion of MDSC. A similar sub-population of activated granulocytes had previously been described by Schmielau and Finn (121) in patients with pancreatic cancer, where they demonstrated a correlation between the presence of activated granulocytes and alterations in T cells such as reduced CD3ζ chain expression and decreased cytokine production (121). Clinical trials with IL-2 in patients with RCC and melanoma have also shown an association of increased numbers of granulocytes in peripheral blood with a poor response and outcome in these patients (122).
Although human MDSC also express high levels of arginase I, this expression does not appear to be upregulated by cytokines or other signals once these cells are in circulation, nor is there an apparent enhanced uptake of l-Arg as was seen in murine MDSC. Instead, arginase I stored in primary (123) or gelatinase granules (124) of MDSC is released to the microenvironment, inducing a significant decrease in l-Arg levels, which impairs T-cell function and CD3ζ chain expression (120, 125, 126). In fact, the release of arginase I in placenta appears to be an important tolerance mechanisms in pregnancy (125). High release of arginase I into the sera of RCC patients induced a decrease in plasma of l-Arg levels to < 50 µM and an increase in ornithine levels (120). In addition, the low levels of l-Arg correlated with low expression of CD3ζ chain in T lymphocytes, demonstrating that arginase I not only had a metabolic effect (l-Arg depletion) but also a negative effect on the T-cell response.
Regulation and activation of MDSC in cancer
Different cytokines have been involved in the recruitment of MDSC from the bone marrow, including VEGF and granulocyte–macrophage-CSF (GM-CSF). In fact, serum levels of VEGF directly correlated with numbers of MDSC in the blood and spleen (127) and have been associated with poor prognosis in cancer patients. Tumor-derived VEGF has been previously related with arrest in DC maturation (128, 129) through inhibition in NF-κB signaling. Increased levels of GM-CSF have also been associated with MDSC-dependent suppression, which could be reversed with antibodies to GM-CSF (103). Similar effects on MDSC have been suggested with other growth factors including Fms-like tyrosine kinase 3 (Flt3) ligand (131) and FSC (117). Treatment of MDSC with all-trans retinoic acid has been shown to induce their differentiation into functional APCs (130).
We used the 3LL murine lung carcinoma model to further determine what factors might be inducing the production of arginase I in the MDSC infiltrating tumors. We initially found that enriched MDSC isolated from 3LL tumors and placed in culture in regular RPMI lose arginase I expression within 24 h. However, if freshly isolated MDSC were co-cultured in transwells with 3LL cells or with 3LL supernatants, they maintained arginase I expression, suggesting that the induction of arginase I was caused by soluble factor(s) produced by tumor cells (132). Cytokines such as IL-4, IL-13, TGFβ, and others were not detected in the supernatants of the 3LL single cell suspensions. Instead, we detected a very high expression of the inducible cyclooxygenase-2 (COX-2) and an increased production of prostaglandins including PGE2. The incubation of freshly isolated MDSC with PGE2 maintained the expression of arginase I and induced the expression in those cells that had lost it (132). Furthermore, the addition of COX-2 inhibitors into the co-cultures of 3LL tumor cells and MDSC or the silencing COX-2 in 3LL cells completely blocked their ability to induce arginase I in MDSC (132). The effect of PGE2 on MDSC was dependent on the expression of the E-prostanoid receptor (EP4) on MDSC and was associated with an increased cyclic adenosine 3′5′ monophosphate levels (132). Consequently, treatment of tumor-bearing mice with the COX-2 inhibitor sc-58125 decreased the expression of arginase I in MDSC infiltrating the tumor and induced an immune mediated antitumor effect (132). Similar results have been reported in mice bearing the 4T1 breast carcinoma. 4T1 tumor-bearing mice treated with the COX-2 inhibitor SC-58236 reduced the accumulation of MDSC in the spleen in an EP2-dependent manner (133). Similar results were found using the selective COX-2 inhibitor celecoxib in a model of induction of large intestinal tumors in Swiss mice by 1,2-dimethylhydrazine diHCl-(1,2-DMH) (134). Some other factors may also play a role in the induction of arginase in MDSC, including hypoxia-inducible factor 1 (HIF-1) and HIF-2 (reviewed in 135). In conclusion, although the mechanisms of induction of arginase I in MDSC have been partially identified in mice, the factors inducing the activation of MDSC in human have not been identified.
MDSC: lessons from other diseases and future applications
The advent of immunotherapy of cancer made apparent that in spite of powerful biological agents that could prime tumor-specific T cells, tumors had sophisticated mechanisms to escape the immune response. Among these is the induction of MDSC, which, through the depletion of a simple non-essential amino-acid l-Arg by the enzyme arginase I, inhibit specific signaling molecules and arrest the T-cell cycle, resulting in a state of tolerance. This phenomenon however is not unique to cancer. Trauma patients and patients with chronic infections including active pulmonary tuberculosis also have increased MDSC expressing arginase I and have an inhibition of T-cell function. This finding suggests that instead of a unique mechanism triggered by tumor cells and aimed specifically at escaping the immune response, MDSC may represent a normal process triggered by tissue damage (danger signal) with the aim of protecting the integrity of the tissues and ‘healing’ the initial injury. A demonstration of this mechanism was described in the late 1980s by Albina et al. (62) studying the healing of surgical wounds. They described that the tissue surrounding a surgical wound was initially infiltrated by cells expressing iNOS, which would most likely eliminate offending agents (bacteria and dead cells) contaminating the wound. This infiltration would be followed by cells expressing arginase I, which would metabolize l-Arg to ornithine and to proline, which in turn would trigger the synthesis of collagen by fibroblasts, ultimately leading to the healing of the surgical wound. In cancer or chronic infections, tissue damage would trigger a similar response with the proliferation of fibroblasts producing collagen aimed at isolating and healing the damaged tissue (i.e. malignant growth). As a matter of fact, many tumors are surrounded by dense fibrous tissue that makes difficult its surgical excision. The major difference between the disease processes (surgical wound versus malignant tumor) would be that the surgical wound would heal, ending the role for arginase-producing MDSC. In contrast, the malignant tumor would not stop growing and destroying tissue (would not ‘heal’), which would trigger instead a chronic inflammatory process mediated by MDSC that would ultimately lead to the depletion of l-Arg from the microenvironment and the development of T-cell anergy. Therefore, it is our hypothesis that tumors ‘hijack’ a normal healing process, making it instead a vicious cycle that results in the inhibition of a potentially protective T-cell anti-tumor response (Fig. 3). Although we are sure that this is an oversimplified version of the complex mechanisms triggered in vivo, it provides a model with which to understand a complex event in the development of cancer and probably design new therapeutic approaches that may interrupt this dysfunctional response.
Much has been learned about the role of MDSC in the progression of tumors in the last 5 years. Multiple approaches have been taken to block MDSC suppression using alltransretinoic acid (130), inhibiting nitric oxide function with nitro-aspirin (136), inhibiting phosphodiesterase-5 (137), and blocking arginase activity with specific arginase inhibitors such as Nor-NOHA (98). It is likely that the appropriate combination of inhibitors blocking MDSC function and stimuli protecting T cells may overcome this powerful tumor-derived mechanism that impairs the promise of cancer immunotherapy.
Acknowledgements
This work was supported by NIH/NCI Grants 5R01CA082689, 5R01CA107974, and 5P20RR021970.
References
- 1.Miescher S, Whiteside TL, Carrel S, von FV. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986;136:1899–1907. [PubMed] [Google Scholar]
- 2.Miescher S, Stoeck M, Qiao L, Barras C, Barrelet L, von Fliedner V. Preferential clonogenic deficit of CD8-positive T-lymphocytes infiltrating human solid tumors. Cancer Res. 1988;48:6992–6998. [PubMed] [Google Scholar]
- 3.Whiteside TL, Miescher S, Moretta L, von Fliedner V. Cloning and proliferating precursor frequencies of tumor-infiltrating lymphocytes from human solid tumors. Transplant Proc. 1988;20:342–343. [PubMed] [Google Scholar]
- 4.Whiteside TL, Rabinowich H. The role of Fas/FasL in immunosuppression induced by human tumors. Cancer Immunol Immunother. 1998;46:175–184. doi: 10.1007/s002620050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellstrom I, Sjogren HO, Warner G, Hellstrom KE. Blocking of cell-mediated tumor immunity by sera from patients with growing neoplasms. Int J Cancer. 1971;7:226–237. doi: 10.1002/ijc.2910070206. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom I, Sjogren HO, Warner G, Hellstrom KE. Blocking of cell-mediated tumor immunity by sera from patients with growing neoplasms. Int J Cancer. 1971;7:226–237. doi: 10.1002/ijc.2910070206. [DOI] [PubMed] [Google Scholar]
- 7.Hellstrom KE, Hellstrom I, Nelson K. Antigen-specific suppressor (“blocking“) factors in tumor immunity. Biomembranes. 1983;11:365–388. [PubMed] [Google Scholar]
- 8.Varesio L, Giovarelli M, Landolfo S, Forni G. Suppression of proliferative response and lymphokine production during the progression of a spontaneous tumor. Cancer Res. 1979;39:4983–4988. [PubMed] [Google Scholar]
- 9.Miescher S, Whiteside TL, Carrel S, von Fliedner V. Functional properties of tumor-infiltrating and blood lymphocytes in patients with solid tumors: effects of tumor cells and their supernatants on proliferative responses of lymphocytes. J Immunol. 1986;136:1899–1907. [PubMed] [Google Scholar]
- 10.Miescher S, Stoeck M, Qiao L, Barras C, Barrelet L, von Fliedner V. Preferential clonogenic deficit of CD8-positive T-lymphocytes infiltrating human solid tumors. Cancer Res. 1988;48:6992–6998. [PubMed] [Google Scholar]
- 11.Whiteside TL, Miescher S, Moretta L, von Fliedner V. Cloning and proliferating precursor frequencies of tumor-infiltrating lymphocytes from human solid tumors. Transplant Proc. 1988;20:342–343. [PubMed] [Google Scholar]
- 12.Whiteside TL, Rabinowich H. The role of Fas/FasL in immunosuppression induced by human tumors. Cancer Immunol Immunother. 1998;46:175–184. doi: 10.1007/s002620050476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevenson FK. Update on cancer vaccines. Curr Opin Oncol. 2005;17:573–577. doi: 10.1097/01.cco.0000181406.60213.c7. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klausner RD, Lippincott-Schwartz J, Bonifacino JS. The T cell antigen receptor: insights into organelle biology. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- 17.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, costimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 18.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20:327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 19.Kryczek I, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 20.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHugh RS, Shevach EM, Margulies DH, Natarajan K. A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur J Immunol. 2001;31:2094–2103. doi: 10.1002/1521-4141(200107)31:7<2094::aid-immu2094>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 22.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 23.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA. 1995;92:6254–6258. doi: 10.1073/pnas.92.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 26.Bronte V, Zanovello P. Regulation of immune responses by l-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 27.McHugh RS, Shevach EM, Margulies DH, Natarajan K. A T cell receptor transgenic model of severe, spontaneous organ-specific autoimmunity. Eur J Immunol. 2001;31:2094–2103. doi: 10.1002/1521-4141(200107)31:7<2094::aid-immu2094>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.McHugh RS, Shevach EM. Cutting edge: depletion of CD4+CD25+ regulatory T cells is necessary, but not sufficient, for induction of organ-specific autoimmune disease. J Immunol. 2002;168:5979–5983. doi: 10.4049/jimmunol.168.12.5979. [DOI] [PubMed] [Google Scholar]
- 29.Young MR, Newby M, Wepsic HT. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987;47:100–105. [PubMed] [Google Scholar]
- 30.Zarour H, et al. The majority of autologous cytolytic T-lymphocyte clones derived from peripheral blood lymphocytes of a melanoma patient recognize an antigenic peptide derived from gene Pmel17/gp100. J Invest Dermatol. 1996;107:63–67. doi: 10.1111/1523-1747.ep12298177. [DOI] [PubMed] [Google Scholar]
- 31.Almand B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 32.Almand B, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 33.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 34.Munn DH, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2, 3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Lee GK, Park HJ, Macleod M, Chandler P, Munn DH, Mellor AL. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107:452–460. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh P, et al. Gradual loss of T-helper 1 populations in spleen of mice during progressive tumor growth. J Natl Cancer Inst. 1995;87:1478–1483. doi: 10.1093/jnci/87.19.1478. [DOI] [PubMed] [Google Scholar]
- 37.Li X, et al. T cells from renal cell carcinoma patients exhibit an abnormal pattern of kappa B-specific DNA-binding activity: a preliminary report. Cancer Res. 1994;54:5424–5429. [PubMed] [Google Scholar]
- 38.Mizoguchi H, O’Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 39.Mizoguchi H, O’Shea JJ, Longo DL, Loeffler CM, McVicar DW, Ochoa AC. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 40.Finke JH, et al. Loss of T-cell receptor zeta chain and p56 lck in T-cells infiltrating human renal cell carcinoma. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 41.Kono K, et al. Decreased expression of signal-transducing zeta chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin Cancer Res. 1996;2:1825–1828. [PubMed] [Google Scholar]
- 42.Zea AH, et al. Alterations in T cell receptor and signal transduction molecules in melanoma patients. Clin Cancer Res. 1995;1:1327–1335. [PubMed] [Google Scholar]
- 43.Nakagomi H, et al. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 44.Nakagomi H, et al. Decreased expression of the signal-transducing zeta chains in tumor-infiltrating T-cells and NK cells of patients with colorectal carcinoma. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 45.Kuss I, Saito T, Johnson JT, Whiteside TL. Clinical significance of decreased zeta chain expression in peripheral blood lymphocytes of patients with head and neck cancer. Clin Cancer Res. 1999;5:329–334. [PubMed] [Google Scholar]
- 46.Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T- cell responses. Proc Natl Acad Sci USA. 1996;93:13119–13124. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kono K, et al. Decreased expression of signal-transducing zeta chain in peripheral T cells and natural killer cells in patients with cervical cancer. Clin Cancer Res. 1996;2:1825–1828. [PubMed] [Google Scholar]
- 48.Kono K, et al. Hydrogen peroxide secreted by tumor-derived macrophages down-modulates signal-transducing zeta molecules and inhibits tumor-specific T cell- and natural killer cell-mediated cytotoxicity. Eur J Immunol. 1996;26:1308–1313. doi: 10.1002/eji.1830260620. [DOI] [PubMed] [Google Scholar]
- 49.Corsi MM, Maes HH, Wasserman K, Fulgenzi A, Gaja G, Ferrero ME. Protection by l-2-oxothiazolidine-4-carboxylic acid of hydrogen peroxide-induced CD3zeta and CD16zeta chain down-regulation in human peripheral blood lymphocytes and lymphokine-activated killer cells. Biochem Pharmacol. 1998;56:657–662. doi: 10.1016/s0006-2952(98)00085-9. [DOI] [PubMed] [Google Scholar]
- 50.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 51.Rabinowich H, Reichert TE, Kashii Y, Gastman BR, Bell MC, Whiteside TL. Lymphocyte apoptosis induced by Fas li. J Clin Invest. 1998;101:2579–2588. doi: 10.1172/JCI1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uzzo RG, et al. Mechanisms of apoptosis in T cells from patients with renal cell carcinoma. Clin Cancer Res. 1999;5:1219–1229. [PubMed] [Google Scholar]
- 53.Bronstein-Sitton N, et al. Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nat Immunol. 2003;4:957–964. doi: 10.1038/ni975. [DOI] [PubMed] [Google Scholar]
- 54.Zea AH, et al. Changes in expression of signal transduction proteins in T lymphocytes of patients with leprosy. Infect Immun. 1998;66:499–504. doi: 10.1128/iai.66.2.499-504.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zea AH, et al. Decreased expression of CD3zeta and nuclear transcription factor kappa B in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J Infect Dis. 2006;194:1385–1393. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- 56.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- 57.Carraway MS, Piantadosi CA, Jenkinson CP, Huang YC. Differential expression of arginase and iNOS in the lung in sepsis. Exp Lung Res. 1998;24:253–268. doi: 10.3109/01902149809041533. [DOI] [PubMed] [Google Scholar]
- 58.Kirk SJ, Regan MC, Wasserkrug HL, Sodeyama M, Barbul A. Arginine enhances T-cell responses in athymic nude mice. J Parenter Enteral Nutr. 1992;16:429–432. doi: 10.1177/0148607192016005429. [DOI] [PubMed] [Google Scholar]
- 59.Ochoa JB, et al. Trauma increases extrahepatic arginase activity. Surgery. 2000;127:419–426. doi: 10.1067/msy.2000.104745. [DOI] [PubMed] [Google Scholar]
- 60.Ochoa JB, Strange J, Kearney P, Gellin G, Endean E, Fitzpatrick E. Effects of l-arginine on the proliferation of T lymphocyte subpopulations. JPEN J Parenter Enteral Nutr. 2001;25:23–29. doi: 10.1177/014860710102500123. [DOI] [PubMed] [Google Scholar]
- 61.Barbul A, Rettura G, Levenson SM, Seifter E. Arginine: a thymotropic and wound-healing promoting agent. Surg Forum. 1977;28:101–103. [PubMed] [Google Scholar]
- 62.Albina JE, Caldwell MD, Henry WL, Jr, Mills CD. Regulation of macrophage functions by l-arginine. J Exp Med. 1989;169:1021–1029. doi: 10.1084/jem.169.3.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Barbul A. Arginine and immune function. Nutrition. 1990;6:53–58. [PubMed] [Google Scholar]
- 64.Taheri F, et al. l-Arginine regulates the expression of the T-cell receptor zeta chain (CD3zeta) in Jurkat cells. Clin Cancer Res. 2001;7:958s–965s. [PubMed] [Google Scholar]
- 65.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr. 2004;134:2791S–2795S. doi: 10.1093/jn/134.10.2791S. [DOI] [PubMed] [Google Scholar]
- 66.Nieves C, Jr, Langkamp-Henken B. Arginine and immunity: a unique perspective. Biomed Pharmacother. 2002;56:471–482. doi: 10.1016/s0753-3322(02)00291-3. [DOI] [PubMed] [Google Scholar]
- 67.Closs EI, Simon A, Vekony N, Rotmann A. Plasma membrane transporters for arginine. J Nutr. 2004;134:2752S–2759S. doi: 10.1093/jn/134.10.2752S. [DOI] [PubMed] [Google Scholar]
- 68.Amber IJ, Hibbs JB, Jr, Parker CJ, Johnson BB, Taintor RR, Vavrin Z. Activated macrophage conditioned medium: identification of the soluble factors inducing cytotoxicity and the l-arginine dependent effector mechanism. J Leukoc Biol. 1991;49:610–620. doi: 10.1002/jlb.49.6.610. [DOI] [PubMed] [Google Scholar]
- 69.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 70.Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- 71.Iyo AH, Zhu MY, Ordway GA, Regunathan S. Expression of arginine decarboxylase in brain regions and neuronal cells. J Neurochem. 2006;96:1042–1050. doi: 10.1111/j.1471-4159.2005.03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhu MY, Iyo A, Piletz JE, Regunathan S. Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim Biophys Acta. 2004;1670:156–164. doi: 10.1016/j.bbagen.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cullen ME, et al. Myocardial expression of the arginine:glycine amidinotransferase gene is elevated in heart failure and normalized after recovery: potential implications for local creatine synthesis. Circulation. 2006;114:I16–I20. doi: 10.1161/CIRCULATIONAHA.105.000448. [DOI] [PubMed] [Google Scholar]
- 74.Item CB, et al. Arginine:glycine amidinotransferase deficiency: the third inborn error of creatine metabolism in humans. Am J Hum Genet. 2001;69:1127–1133. doi: 10.1086/323765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hesse M, et al. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of l-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 76.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- 77.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- 78.Rutschman R, Lang R, Hesse M, Ihle JN, Wynn TA, Murray PJ. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J Immunol. 2001;166:2173–2177. doi: 10.4049/jimmunol.166.4.2173. [DOI] [PubMed] [Google Scholar]
- 79.Boutard V, Havouis R, Fouqueray B, Philippe C, Moulinoux JP, Baud L. Transforming growth factor-beta stimulates arginase activity in macrophages. Implications for the regulation of macrophage cytotoxicity. J Immunol. 1995;155:2077–2084. [PubMed] [Google Scholar]
- 80.Rodriguez PC, et al. l-arginine consumption by macrophages modulates the expression of CD3zeta chain in T lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 81.Chicoine LG, Paffett ML, Young TL, Nelin LD. Arginase inhibition increases nitric oxide production in bovine pulmonary arterial endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L60–L68. doi: 10.1152/ajplung.00194.2003. [DOI] [PubMed] [Google Scholar]
- 82.Zhang C, et al. Upregulation of vascular arginase in hypertension decreases nitric oxide-mediated dilation of coronary arterioles. Hypertension. 2004;44:935–943. doi: 10.1161/01.HYP.0000146907.82869.f2. [DOI] [PubMed] [Google Scholar]
- 83.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Santhanam L, et al. Inducible NO Synthase dependent S-nitrosylation and activation of arginase1 contribute to age-related endothelial dysfunction. Circ Res. 2007;101:692–702. doi: 10.1161/CIRCRESAHA.107.157727. [DOI] [PubMed] [Google Scholar]
- 85.Deignan JL, et al. Ornithine deficiency in the arginase double knockout mouse. Mol Genet Metab. 2006;89:87–96. doi: 10.1016/j.ymgme.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 86.Iyer RK, et al. Mouse model for human arginase deficiency. Mol Cell Biol. 2002;22:4491–4498. doi: 10.1128/MCB.22.13.4491-4498.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor CD3 zeta chain expression by l-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 88.Zea AH, et al. l-Arginine modulates CD3zeta expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez PC, Quiceno DG, Ochoa AC. l-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kato JY. Control of G1 progression by D-type cyclins: key event for cell proliferation. Leukemia. 1997;11(Suppl 3):347–351. [PubMed] [Google Scholar]
- 91.Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–684. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 92.Sicinska E, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell. 2003;4:451–461. doi: 10.1016/s1535-6108(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 93.Yaman I, et al. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J Biol Chem. 2002;277:41539–41546. doi: 10.1074/jbc.M204850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yaman I, et al. Nutritional control of mRNA stability is mediated by a conserved AU-rich element that binds the cytoplasmic shuttling protein HuR. J Biol Chem. 2002;277:41539–41546. doi: 10.1074/jbc.M204850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang CI, Liao JC, Kuo L. Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity. Cancer Res. 2001;61:1100–1106. [PubMed] [Google Scholar]
- 96.Suer GS, Yoruk Y, Cakir E, Yorulmaz F, Gulen S. Arginase and ornithine, as markers in human non-small cell lung carcinoma. Cancer Biochem Biophys. 1999;17:125–131. [PubMed] [Google Scholar]
- 97.Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G. Arginase activity in human breast cancer cell lines: N(omega)-hydroxy-l- arginine selectively inhibits cell proliferation and induces apoptosis in MDA-MB-468 cells. Cancer Res. 2000;60:3305–3312. [PubMed] [Google Scholar]
- 98.Rodriguez PC, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 99.Gabrilovich DI, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bronte V, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 101.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 102.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 103.Bronte V, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 104.Seung LP, Rowley DA, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA. 1995;92:6254–6258. doi: 10.1073/pnas.92.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Holda JH, Maier T, Claman HN. Murine graft-versus-host disease across minor barriers: immunosuppressive aspects of natural suppressor cells. Immunol Rev. 1985;88:87–105. doi: 10.1111/j.1600-065x.1985.tb01154.x. [DOI] [PubMed] [Google Scholar]
- 107.Rodriguez PC, Zea AH, Ochoa AC. Mechanisms of tumor evasion from the immune response. Cancer Chemother Biol Response Modif. 2003;21:351–364. doi: 10.1016/s0921-4410(03)21018-8. [DOI] [PubMed] [Google Scholar]
- 108.Rodriguez PC, Ochoa AC. T cell dysfunction in cancer: role of myeloid cells and tumor cells regulating amino acid availability and oxidative stress. Semin Cancer Biol. 2005;105:2549–2556. doi: 10.1016/j.semcancer.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 109.Bronte V, et al. IL-4-Induced Arginase 1 Suppresses Alloreactive T Cells in Tumor-Bearing Mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 110.Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 111.Nagaraj S, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Ginderachter JA, et al. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) ligands reverse CTL suppression by alternatively activated (M2) macrophages in cancer. Blood. 2006;108:525–535. doi: 10.1182/blood-2005-09-3777. [DOI] [PubMed] [Google Scholar]
- 113.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 114.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 115.Gallina G, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang B, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 117.Pan PY, et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid derived suppressor cell development by blockade of SCF function. Blood. 2008;111:219–228. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 119.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zea AH, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 121.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–4760. [PubMed] [Google Scholar]
- 122.Rodgers S, Rees RC, Hancock BW. Changes in the phenotypic characteristics of eosinophils from patients receiving recombinant human interleukin-2 (rhIL-2) therapy. Br J Haematol. 1994;86:746–753. doi: 10.1111/j.1365-2141.1994.tb04824.x. [DOI] [PubMed] [Google Scholar]
- 123.Munder M, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 124.Jacobsen LC, Theilgaard-Monch K, Christensen EI, Borregaard N. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood. 2007;109:3084–3087. doi: 10.1182/blood-2006-06-032599. [DOI] [PubMed] [Google Scholar]
- 125.Kropf P, et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol. 2007;37:935–945. doi: 10.1002/eji.200636542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Munder M, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 127.Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–272. doi: 10.1385/IR:23:2-3:263. [DOI] [PubMed] [Google Scholar]
- 128.Gabrilovich D, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–4166. [PubMed] [Google Scholar]
- 129.Oyama T, et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factorkappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–1232. [PubMed] [Google Scholar]
- 130.Kusmartsev S, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 131.Solheim JC, et al. Spleen but not tumor infiltration by dendritic and T cells is increased by intravenous adenovirus-Flt3 ligand injection. Cancer Gene Ther. 2007;14:364–371. doi: 10.1038/sj.cgt.7701018. [DOI] [PubMed] [Google Scholar]
- 132.Rodriguez PC, et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–4513. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 134.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol. 2007;7:140–151. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 135.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 136.De SC, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci USA. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Serafini P, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]