Abstract
Neuroadaptations associated with behavioral sensitization induced by repeated exposure to methamphetamine (MA) appear to be involved in compulsive drug pursuit and use. Increased histone acetylation, an epigenetic effect resulting in altered gene expression, may promote sensitized responses to psychostimulants. The role of histone acetylation in the expression and acquisition of MA-induced locomotor sensitization was examined by measuring the effect of histone deacetylase inhibition by sodium butyrate (NaB). For the effect on expression, vehicle or NaB (630 mg/kg, intraperitoneally) was administered 30 min prior to MA challenge in mice treated repeatedly with MA (10 days of 2 mg/kg MA) or saline (10 days), and then locomotor response to MA challenge was measured. NaB treatment increased the locomotor response to MA in both acutely MA treated and sensitized animals. For acquisition, NaB was administered 30 min prior to each MA exposure (10 days of 1 or 2 mg/kg), but not prior to the MA challenge test. Treatment with NaB during the sensitization acquisition period significantly increased locomotor activation by MA in sensitized mice only. NaB alone did not significantly alter locomotor activity. Acute NaB or MA, but not the combination, appeared to increase striatal acetylation at histone H4. Repeated treatment with MA, but not NaB or MA plus NaB, increased striatal acetylation at histone H3. Although increased histone acetylation may alter the expression of genes involved in acute locomotor response to MA and in the acquisition of MA-induced sensitization, results for acetylation at H3 and H4 showed little correspondence with behavior.
Keywords: sensitization, psychostimulant, histone acetylation, epigenetics, addiction
1. Introduction
Exposure to drugs of abuse has been shown to have lasting effects that increase the difficulty of remaining abstinent. A positron emission tomography study of recovering methamphetamine (MA) users has shown that cognitive deficits and a decrease in dopamine transporters are correlated with previous MA abuse and are persistent over months of abstinence [1]. One possible consequence of repeated psychostimulant exposure is sensitization of neural processes that influence drug and/or drug-paired cue sensitivity [2–4]. Existing evidence suggests that cellular adaptations involving changes in gene regulation underlie altered behavioral responses to repeated drug exposure [5, 6]. A behavioral measure used in animals to demonstrate the sensitizing effects of repeated psychostimulant exposure [7–10] is locomotor response to a drug challenge after one or more prior drug exposures [for review see: 11]. Heightened locomotor response, or sensitization, is a common outcome that reflects neuroadaptation to the drug.
Epigenetic mechanisms of gene regulation, including DNA methylation and histone modification, may play an important role in cellular responses to environmental events, including drug exposure. Drugs of abuse, including amphetamines, have been shown to decrease DNA methyltransferase activity [12]. Hyperacetylation of histone protein tails has been found after acute or chronic exposure to cocaine, amphetamines, and alcohol, in addition to other drugs [13–16]. Histone-tail modification is thought to regulate gene transcription by altering the charge of histone proteins that act to attract or repel DNA and mechanically prevent expression of associated genes [For review see: 17]. Histone acetylation has been shown to result in upregulation of associated gene expression [18, 19] in localized regions of the brain [20]. Thus, neuroadaptation to drug exposure may involve regulation of gene expression by histone-tail modification.
Administration of histone deacetylase (HDAC) inhibitors, such as sodium butyrate (NaB), has been shown to increase histone acetylation in the central nervous system [13, 15, 16]. However, their effects when given in combination with psychostimulant drugs have not been straightforward. Whereas the HDAC inhibitor, valproate, has been shown to reduce psychomotor sensitization [13, 21] and NaB extinguished cocaine-induced conditioned place preference [22], HDAC inhibitors have also been shown to increase behaviors induced by psychostimulant exposure [23, 24]. Hyperacetylation resulting from both MA exposure and HDAC inhibitor administration suggests the possibility that increased acetylation may be an important mechanism involved in behavioral changes following MA exposure. Cellular analyses have shown increased acetylation of histone H4 and phosphorylation of cAMP responsive element binding protein induced by amphetamine and NaB coadministration [16]. Acute administration of cocaine has been shown to increase histone H4 acetylation and H3 phosphoacetylation at the c-fos promoter, but not genome-wide acetylation of H3 [14, 15].
Data examining whether sensitized locomotor responses to repeated MA treatment are linked to histone acetylation are inconclusive. Further, no data have been collected that clearly separate the effects of HDAC inhibitors on the acquisition of sensitization from the expression of sensitization. We first investigated the effect of NaB on the expression of MA-induced sensitization by administering NaB prior to MA challenge and locomotor testing in mice receiving MA for the first time and in mice with a history of repeated MA exposures. We next investigated the effect of NaB on the acquisition of MA-induced locomotor sensitization by administering NaB prior to each saline or MA administration during the acquisition period, but not prior to the final MA challenge test. Lastly, we investigated whether acute or repeated MA and NaB exposure resulted in modification of acetylation of histone 3 at lysine 14 (H3K14) or histone 4 at lysine 12 (H4K12) in the striatum, a key brain region underlying drug stimulant responses [8, 24–26]. Because both MA and HDAC inhibitors have previously been shown to increase acetylation, we hypothesized that acute exposure to NaB would augment the locomotor response to MA challenge, regardless of history of MA exposure. With regard to chronic NaB effects, we hypothesized that NaB given just prior to each MA treatment would enhance the sensitized response to MA challenge, compared to a group repeatedly treated with vehicle and MA, but would not alter the locomotor response to acute MA in mice that had received NaB only with repeated saline. We based this prediction on the notion that NaB with MA would have sensitization-relevant combined acetylation effects that are greater than those after MA alone, but that the effects of the HDAC inhibitor alone would not be sufficient to induce sensitization to MA. Lastly, based on previous results [14–16], we hypothesized that in the striatum, acute NaB and MA would result in hyperacetylation of H4K12, whereas repeated NaB and MA would result in hyperacetylation of H3K14.
2. Methods and Materials
2.1 Subjects
Male, 7-week-old B6D2F1/J mice purchased from The Jackson Laboratory (Sacramento, CA) were derived from the cross of C57BL/6J (B6) females with DBA/2J (D2) males. Mice were housed in the Portland Veterans Affairs Medical Center animal facility, 4 to a cage, and testing was initiated when the mice were 50 to 60 days of age. They were housed in acrylic plastic shoe-box cages (28 × 18 × 13 cm; l × w × h) that were lined with ECOfresh bedding (Absorption Corp, Ferndale, WA) and fitted with wire tops. Colony room temperature was 20 to 22 °C, and lights were maintained on a 12:12h light:dark schedule. Food (Purina Laboratory Rodent Chow formulation 5001; Purina Mills, St. Louis, MO) and water were available ad libitum except during locomotor testing, with testing occurring no sooner than 2 h after lights on and ending no later than 2 h before lights off. All procedures were performed in accordance with the VAMC Institutional Animal Care and Use Committee, and National Institutes of Health guidelines for the care and use of laboratory animals.
2.2 Apparatus
Locomotor activity was measured using AccuScan automated activity monitors, identical to those described previously [7, 8, 25]. Interruption of photocell beams located 2 cm above the chamber floor at 8 locations along each wall were recorded and used by AccuScan software (VersaMax; V 1.80-1FFE) to calculate the horizontal distance traveled (in cm).
2.3 Drugs and reagents
All drugs for injection were prepared in 0.9% sterile saline (Baxter Healthcare, Deerfield, IL) and given intraperitoneally (i.p.) in a volume of 10 ml/kg. (+)Methamphetamine (MA) HCl (Sigma-Aldrich, St. Louis, MO) was administered at doses of 1 or 2 mg/kg, doses previously used for studies of MA-induced sensitization in B6 and D2 strains or populations derived from these strains [8, 25]. Sodium butyrate (NaB; Millipore, Billerica, MA) was given 30 min prior to saline or MA treatment at a dose of 630 mg/kg. This dose and pretreatment time were based on previous studies that examined the relationship between inhibition of histone acetylation and psychostimulant-induced locomotor behavior [13, 16]. Pharmacokinetic parameters for NaB, such as peak plasma concentration, can be difficult to predict at doses higher than this treatment dose, which may be due to saturable elimination mechanisms [27]. However, doses near or below the dose of NaB used here likely approach first-order elimination and produce more predictable maximum plasma concentrations [27]. Groups were administered vehicle (Veh) or saline (Sal) as controls for NaB and MA injection, respectively. Both Veh and Sal injections consisted of only 0.9% saline; different names were given to make clear which control injection is under discussion in subsequent sections. For anesthesia, a stock solution (Portland VAMC pharmacy) of ketamine (100 mg/ml), xylazine (20 mg/ml), and acepromazine (10 mg/ml) was diluted 1:5 with 0.9% sterile saline for an injection volume of 10 ml/kg (i.p.). All reagents used for perfusion and immunohistochemistry were obtained from Sigma-Aldrich unless otherwise indicated.
2.4 Experiment1: Effect of NaB on the expression of sensitization to 2 mg/kg MA
To test for an effect of NaB on the expression of MA-induced locomotor sensitization, a 13-day procedure adapted from one previously shown to induce locomotor sensitization to MA was used [9]. This procedure examines non-associative drug sensitization because MA treatment is not paired with the test environment. To induce sensitization, mice were weighed and injected once per day for 10 days, with either saline or 2 mg/kg MA. Repeated administration of 2 mg/kg MA has been shown to induce a sensitized response in mice [9]. Although we hypothesized an increase, based on previous reports that HDAC inhibitors have been shown to both increase [13, 16] and decrease drug-induced behavior [13, 21, 23], we anticipated the possibility that acute NaB could augment or attenuate the expression of sensitization. Thus, a moderate dose of MA was chosen that was known to induce significant sensitization, but would also allow for levels of locomotor activation to increase.
Locomotor testing began 24 h after the sensitization induction phase and was conducted over a 3-day period (days 11–13), consistent with previous work examining drug effects on locomotor behavior (e.g., [8, 28, 29]). Each day, mice were moved into the locomotor test room and allowed to acclimate for at least 45 min before being handled. Mice received two injections on each day. On days 11 and 12, mice were weighed, injected with vehicle, and then placed in individual holding cages for a 30-min period. Mice were then injected with saline and immediately placed in the center of the activity chamber, where locomotor activity was recorded for 2 h in 5-min bins. When all mice had been tested, they were returned to the colony room. Day 11 provided activity data in drug- and experiment-naïve mice, and day 12 provided baseline activity data in drug-naive mice for which the test environment and procedures were familiar. On MA challenge test day 13, procedures were identical, except that mice were administered either vehicle or 630 mg/kg NaB before being placed into the holding cages, and then received saline or 2 mg/kg MA prior to the 2-h activity test. This NaB dose and pretreatment time were based on previous studies that examined the relationship between inhibition of histone acetylation and psychostimulant-induced locomotor behavior [13, 16]. This experiment was performed in 3 cohorts of 24–64 mice (112 mice total) that were tested over a 3-month period.
2.5 Experiment 2: Effect of NaB on the acquisition of sensitization to 1 mg/kg MA
Based on the results of Experiment 1, which showed that the locomotor response to MA was augmented by NaB, a lower 1 mg/kg dose of MA was used in Experiment 2 to further reduce the possibility that a ceiling effect would limit the extent of augmentation over time. The 1 mg/kg dose of MA has been shown to result in a relatively greater magnitude of sensitization, compared with 2 mg/kg MA, likely due to the smaller initial stimulant response to the lower dose [8].
Similar to Experiment 1, mice were treated with saline or MA for 10 days to induce sensitization. However, 30 min prior to each saline or MA injection, mice were treated with vehicle or 630 mg/kg NaB. Mice were then tested for locomotor behavior using the 3-day procedure described for Experiment 1, with the exception that, on day 13, all mice were treated with vehicle (none received NaB) prior to MA treatment and testing. This provided an assessment of the effect of NaB exposure during the sensitization acquisition period on the acute stimulant response to MA and on the magnitude of sensitization. This experiment was performed in 3 cohorts of 16–22 mice (56 mice total) that were tested over a 2-month period.
2.6 Experiment 3: Effect of NaB on the acquisition of sensitization to 2 mg/kg MA
Results from Experiment 2 showed that NaB given during the acquisition phase did not affect the magnitude of sensitization to 1 mg/kg MA. Based on the possibility that there is a threshold dose of MA needed to see an effect of NaB, and that a NaB effect on response to the 2 mg/kg MA dose was seen in Experiment 1, this dose was used in Experiment 3. Procedures were identical to those for Experiment 2 except for the increase in dose of MA. This experiment was performed in 3 cohorts of 14–26 mice (56 mice total) that were tested over a 2-month period.
2.7 Experiment 4: Histone H3, lysine k14 (H3K14) and Histone H4, lysine k12 (H4K12) acetylation following acute NaB and MA exposure
2.7.1 Treatment and tissue preparation
Results from Experiment 1 indicated that acute NaB accentuated locomotor behavior when administered prior to MA but not saline. To test whether changes in histone acetylation under these treatment conditions correspond with changes in behavior, mice received 2 injections that were spaced 30 min apart on 3 consecutive days, consistent with the test phase of Experiment 1. Vehicle and then saline were given on days 1 and 2, and then mice were treated with either vehicle or 630 mg/kg NaB and saline or 2 mg/kg MA on day 3. Following the second injection, brains were perfused and removed for immunohistochemistry. There were 4 mice per treatment condition.
Results from the behavioral experiments indicated that MA-induced locomotor stimulation was greatest around 30 min after MA administration. Therefore, mice were deeply anesthetized and then perfused 30 min post MA treatment, via the right atrium with 50 ml ice-cold saline followed by 50 ml ice-cold 2% paraformaldehyde in 0.01M potassium-buffered saline (PBS), pH 7.4. Brains were then removed and placed overnight at 4 °C into vials containing 2% paraformaldehyde. The next day, they were placed in 30% sucrose and 0.1% sodium azide and stored at 4 °C until sliced.
2.7.2 Immunohistochemistry
Brains were frozen on dry ice and coronally sectioned (30 microns) using a microtome (American Optical Corporation, Buffalo, NY). Sliced tissue was returned to storage at 4 °C in 30% sucrose and 0.1% sodium azide until immunohistochemistry could be performed. The immunohistochemistry procedure was consistent with a previously described protocol [30]. Tissue was washed in PBS and then exposed to 1.5% H2O2 in 0.01 M PBS solution to block endogenous peroxidase activity and remove any residual blood. After additional PBS washes, tissue was rotated for 2 h at room temperature in immunoreactive buffer comprised of normal goat serum (Vector Laboratories, Burlingame, CA) 1:33 in PBS and 0.3% Triton X-100. Rabbit monoclonal anti-acetyl-histone H3 (lys14) IgG (Cat. # 04-1044, Millipore, Billerica, MA, 1:1000 concentration in immunoreactive buffer solution) was then added, and tissue was rotated for 48 h at 4 °C. Following additional PBS washes and a 2-h rotation at room temperature in PBS, normal goat serum (1:33 concentration in PBS), 0.3% Triton X-100, and the secondary antibody, lyophilized biotinylated goat-anti rabbit IgG (Vector Laboratories, 1:200 concentration in secondary antibody solution) were added. Tissue was again washed in PBS, and then rotated at room temperature in 0.3% Triton X-100, PBS, and A+ B compound of the Vectastain ABC kit (Vector Laboratories) for 2 h. Tissue was washed in PBS and then prepared in 0.1M Tris for 5 min, before being exposed to 3,3′-diaminobenzidine with 0.1M Tris and 0.01% nickel ammonium sulfate solution for 5 min. Thirty percent H2O2 was added to react with horseradish peroxidase and expose the tissue. The reaction was stopped by washing in Tris after 90 s.
Slices were mounted on positively-charged slides and dehydrated in decreasing concentrations of ethanol and 100% CitriSolv (Fisher Scientific, Pittsburgh, PA), cover slipped with Permount (Fisher Scientific, Pittsburgh, PA), and allowed to dry for 48 h. Optical density was quantified using a Leica DFC 480 imaging system (Buffalo Grove, IL) and Image Pro Plus 7.0 software (Media Cybernetics, Bethesda, MD) for images captured by an Olympus BX60 light microscope (Center Valley, PA) at 10× and 40× objective magnification. Representative sections of the ventral and dorsal striatum [Plate 25 from Paxinos and Franklin; 31] were identified using standardized area of interest templates and averaged for each animal from three proximal slices. The ventral and dorsal striatum were chosen for their roles in the behavioral response to drug exposure [For review see: 32]and because histone H3 and H4 acetylation in the striatum has been shown to be affected by administration of psychostimulants and HDAC inhibitors [15, 16]. The experimenter was blind to treatment group at the time of quantification.
2.8 Experiment 5: Histone H3, lysine k14 (H3K14) and Histone H4, lysine k12 (H4K12) acetylation following repeated NaB and MA exposure
Results from Experiment 3 indicated that repeated NaB accentuated locomotor behavior when administered concurrently with MA, but not saline. To test whether these treatments resulted in changes in histone acetylation that corresponded with changes in behavior, mice were treated as in Experiment 3, once daily with either vehicle or 630 mg/kg NaB, and 30 min later with saline or 2 mg/kg MA for 10 days. On the 3 days that corresponded with the test phase of Experiment 3, mice were treated on 2 days with vehicle then saline spaced 30 min apart, and on the third day with vehicle followed 30 min later by 2 mg/kg MA. Mice were perfused 30 min after MA treatment, and brains were removed for immunohistochemistry. There were 4 mice per treatment condition. Immunohistochemistry was performed as described for Experiment 4.
2.9 Statistics
Locomotor activity difference scores were used to index drug treatment effects. Difference scores were calculated by subtracting distance traveled (cm) on baseline day 12 from distance traveled (cm) on drug challenge day 13. This provides a measure that takes individual differences in baseline activity into account, even in the absence of group differences, and creates a drug response score that can be easily interpreted as increased activity for values above 0 and decreased activity for values below 0. Difference score data were analyzed across time using repeated measures ANOVA, with NaB dose (0 and 630 mg/kg) and MA dose (0 and 1 or 2 mg/kg) as between-groups factors. Sources of significant two-way interactions were examined using simple main effect analysis, and the Neuman-Keuls test was used for post-hoc mean comparisons. Optical densities from immunohistochemical stains in Experiment 4 were analyzed by ANOVA, with NaB dose and MA dose as between-groups factors; follow-up analyses were performed as described for the behavioral data. For Experiment 5, optical density data were examined for acetylation changes from control using Dunnett’s test. Alpha level was set at 0.05, and statistical analyses were performed using the Statistica 9.1 software package (StatSoft, Inc., Tulsa, OK).
3. Results
Data from 2 mice were removed from Experiment 2 prior to analysis because of procedural errors that occurred during locomotor testing. Age and body weights were not found to significantly differ among groups on any test day in any experiment. Habituation to the test procedure and environment was suggested by significantly lower levels of locomotor activity on saline test day 12 (baseline) compared with day 11 (when the environment was novel) in all experiments (data not shown). However, treatment groups did not significantly differ in locomotor activity level on baseline day 12, indicating that repeated MA or NaB treatment prior to this test did not have significant effects on basal activity levels.
3.1 Experiment1: Effect of NaB on the expression of sensitization to 2 mg/kg MA
Shown in Figure 1A are locomotor activity difference scores for all treatment groups over the course of the 2-h activity session. Repeated measures ANOVA identified two significant three-way interactions that included time (time × NaB dose on test day × MA dose on test day, F[23, 2392]=4.78, p<0.0001; time × MA dose during acquisition × MA dose on test day, F[23, 2392]=1.85, p<0.01). To examine time-dependent effects of particular treatments, further analyses were conducted that included fewer factors.
Figure 1.
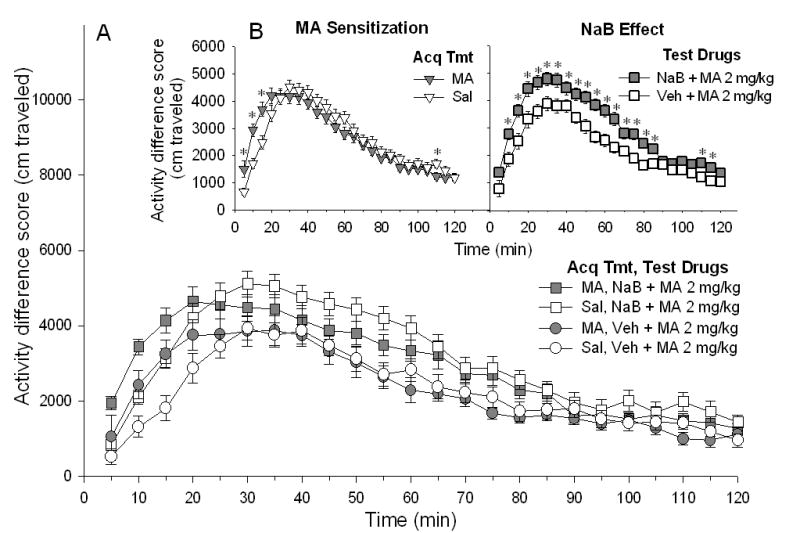
Acute NaB enhances the stimulant response to MA. Mice were treated with saline (Sal) or MA 2 mg/kg (Acq Tmt) on 10 consecutive days prior to locomotor testing. A. Shown are mean ± SEM activity difference scores created by subtracting baseline day (day 12) data (cm traveled) from challenge day (day 13) data for each individual animal that received a MA 2 mg/kg challenge. On the challenge day, vehicle (Veh) or NaB 630 mg/kg was administered, 30 min prior to Sal or MA 2 mg/kg (Test Drugs). B Left panel. Shown is sensitization to MA. Mice treated with MA during the sensitization acquisition period exhibited a significantly larger locomotor response to MA challenge largely at early time points, compared to mice treated with Sal during the acquisition period. Data are collapsed on the Veh vs. NaB treatment factor to clearly illustrate the sensitized response. * p<0.05 for the difference between Sal and MA treatment conditions. B Right panel. Shown is the effect of NaB treatment given on MA challenge day. Pretreatment with NaB increased the locomotor response to MA, at some time points. Data are collapsed on the Sal vs. MA Acq Tmt factor to clearly demonstrate the effect of NaB. * p<0.05 for the difference between Veh and NaB treatment conditions.
The effect of 10 days of saline vs. MA pretreatment on response to saline or MA challenge was examined, with data for the vehicle and NaB treatment groups combined. This analysis provided evidence for significant MA-induced sensitization (see Figure 1B, MA Sensitization). There was a significant interaction of time x MA dose during acquisition (F[23, 1242]=5.30, p<0.0001); repeatedly MA-treated mice exhibited a larger response to MA challenge than did repeatedly saline-treated mice, mostly at early time points. Locomotor data after saline challenge are not shown, because there were no significant differences among groups, indicating that prior MA treatment had no significant effect on basal activity levels.
To examine the effect of vehicle vs. NaB treatment given prior to saline or MA challenge on test day 13, data for the saline and MA acquisition treatment groups were combined (see Figure 1B, NaB Effect). This was justified because there was no significant three-way interaction of time x MA dose during acquisition x NaB dose on test day, indicating that the effect of NaB on response to MA challenge was not dependent upon prior experience with MA. There was a significant interaction of time x NaB challenge dose (F[23, 1242]=2.04, p<0.005); NaB pretreatment was associated with significantly increased locomotor activity at multiple time points after MA challenge. Locomotor data after saline challenge are not shown, because there were no significant effects of NaB given prior to the saline test.
3.2 Experiment 2: Effect of NaB on the acquisition of sensitization to 1 mg/kg MA
Shown in Figure 2A are difference score data for the response to MA challenge in mice that received vehicle or NaB prior to each repeated saline or 1 mg/kg MA treatment during the sensitization acquisition period. The 1 mg/kg dose of MA induced significant sensitization during earlier time periods, as indicated by a significant time x repeated MA treatment interaction (F[23, 1196]=11.20, p<0.0001). However, the magnitude of acquired sensitization was not affected by NaB exposure. Figure 2B shows data combined for the vehicle and NaB treatment groups to more clearly illustrate the MA-induced sensitization.
Figure 2.
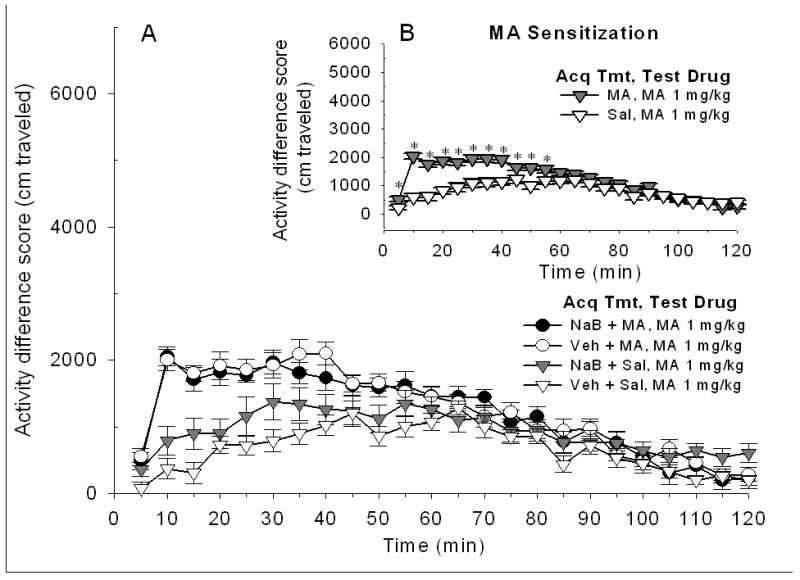
NaB given during the sensitization acquisition period does not alter the sensitized response induced by repeated MA 1 mg/kg treatment. Mice were treated with Veh or NaB 630 mg/kg, 30 min before Sal or MA 1 mg/kg (Acq Tmt) on 10 consecutive days prior to locomotor testing. A. Shown are mean ± SEM activity difference scores for all groups on challenge day, when all mice were treated with MA 1 mg/kg (Test Drug), 30 min after Veh injection, and then immediately placed into the locomotor chambers. B. Shown is sensitization to MA. Mice treated with MA during the sensitization acquisition period exhibited a significantly larger locomotor response to MA challenge at early time points, compared to mice treated with Sal during the acquisition period. Data are collapsed on the Veh vs. NaB treatment factor to clearly illustrate the sensitized response. * p<0.05 for the difference between Sal and MA Acq Tmt conditions.
3.3 Experiment 3: Effect of NaB on the acquisition of sensitization to 2 mg/kg MA
Shown in Figure 3A are difference score data for the response to MA challenge in mice that received vehicle or NaB prior to each repeated saline or 2 mg/kg MA treatment during the sensitization acquisition period. There was a significant three-way interaction (time x NaB dose during acquisition x MA dose during acquisition; F[23,1196]=2.96, p<0.0001), indicating that patterns of activity over time were dependent upon whether mice had received repeated MA or saline and NaB or vehicle. To examine these effects further, patterns across time were analyzed in separate ANOVAs for NaB acquisition dose and for MA acquisition dose.
Figure 3.
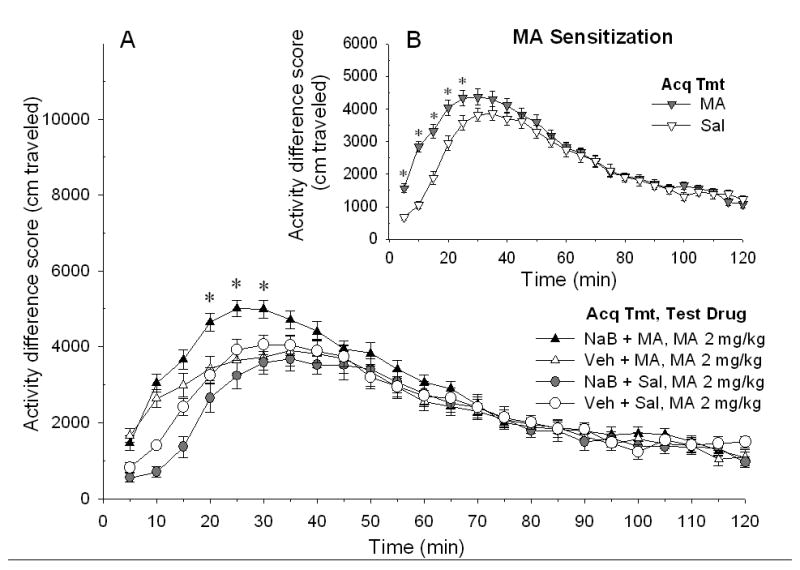
Repeated NaB enhances locomotor sensitization induced by repeated MA 2 mg/kg treatment. Mice were treated with Veh or NaB 630 mg/kg, 30 min before Sal or MA 2 mg/kg (Acq Tmt) on 10 consecutive days prior to locomotor testing. On challenge day, all mice were treated with MA 2 mg/kg (Test Drug), 30 min after Veh injection, and then immediately placed into the locomotor chambers. A. Shown are mean ± SEM activity difference scores for all groups. * p<0.05 for the difference between repeated NaB + MA and Veh + MA in response to MA 2 mg/kg challenge. There was no time x NaB dose interaction for the groups that received Sal during the acquisition period. B. Shown is sensitization to MA. Mice treated with MA during the sensitization acquisition period exhibited a significantly larger locomotor response to MA challenge at early time points, compared to mice treated with Sal during the acquisition period. Data are collapsed on the Veh vs. NaB treatment factor to clearly illustrate the sensitized response. * p<0.05 for the difference between Sal and MA treatment conditions.
Similar to the results for 1 mg/kg MA shown in Figure 2, mice repeatedly treated with 2 mg/kg MA exhibited significant sensitization during earlier time periods after MA challenge (Figure 3B, MA Sensitization). This was supported by a significant interaction of time x MA dose during acquisition (F[23,1242]=7.26, p<0.0001). Unlike the results for 1 mg/kg MA, NaB given during the acquisition period had a significant effect on magnitude of sensitization (see white and black triangles in Figure 3A). This was supported by a significant interaction of time x NaB dose within the repeated MA treatment group (F[1,23]=2.73, p<0.0001), but not within the repeated saline treatment group (see white and grey circles in Figure 3A).
3.4 Experiment 4: Immunohistochemistry after acute NaB and MA administration
Shown in Figure 4A are nuclear stain optical density data for the effects of NaB and MA on acetylation of histone H3K14. There was a significant effect of NaB treatment on optical density in both the ventral (F[1, 12]=4.77, p<0.05) and dorsal (F[1, 12]=5.48, p<0.05) striatum that was not dependent on MA treatment. The acute injection of MA alone did not have a significant effect on histone H3K14 acetylation in either region of the striatum. However, as shown in Figure 5A, there was a significant NaB dose x MA dose interaction (F[1, 12]=5.02, p<0.05) on acetylation of histone H4K12 in the ventral striatum. No significant treatment effects were found for the dorsal striatum. Lastly, there were no effects of NaB or MA treatment on immunoreactivity for histone H3 (Figure 5B), which suggests that the difference detected in acetylated histone H3 (H3K14 in Figure 4A) between NaB treated and non-treated mice was not due to variation in the level of the total H3 protein present in the cell.
Figure 4.
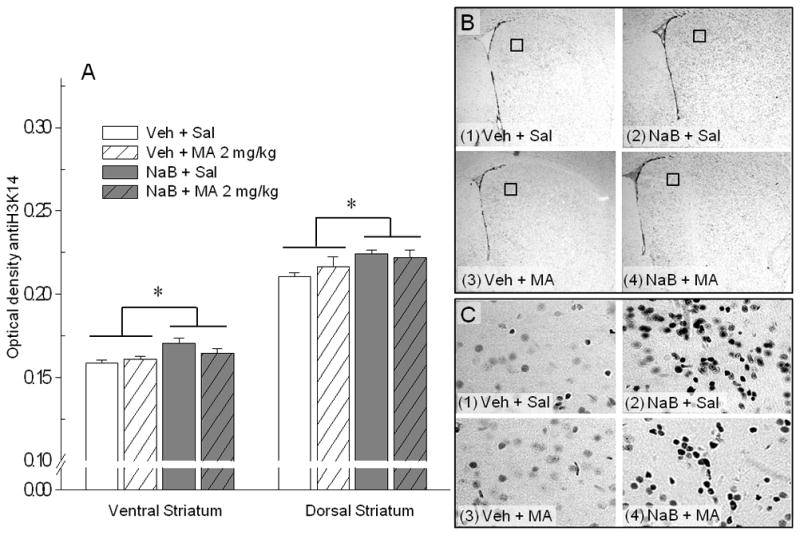
Acute NaB increases acetylation at histone H3 in the dorsal and ventral striatum. Mice were treated with Veh or NaB 630 mg/kg, 30 min prior to Sal or MA 2 mg/kg. Tissue was perfused 30 min after the final treatment and stained for acetylation at histone H3K14. A. Shown are optical density data (mean ± SEM from 3 averaged samples per individual), reflecting acetylation of H3K14 for the ventral and dorsal striatum. * p<0.05 for the main effect of NaB. B. Representative images of nuclear stains for dorsal striatal sections from (1) Veh + Sal, (2) NaB + Sal, (3) Veh + MA, and (4) NaB + MA groups. Images were captured with identical camera and software settings, converted to be monochromatic, and calibrated for standard optical density before analysis. Representative images included in the figure have been identically adjusted for maximum and minimum intensity to better illustrate treatment group differences in nuclear staining. (C) Higher magnification (40× objective) images of nuclear-stained sections from regions indicated by boxes in (B).
Figure 5.
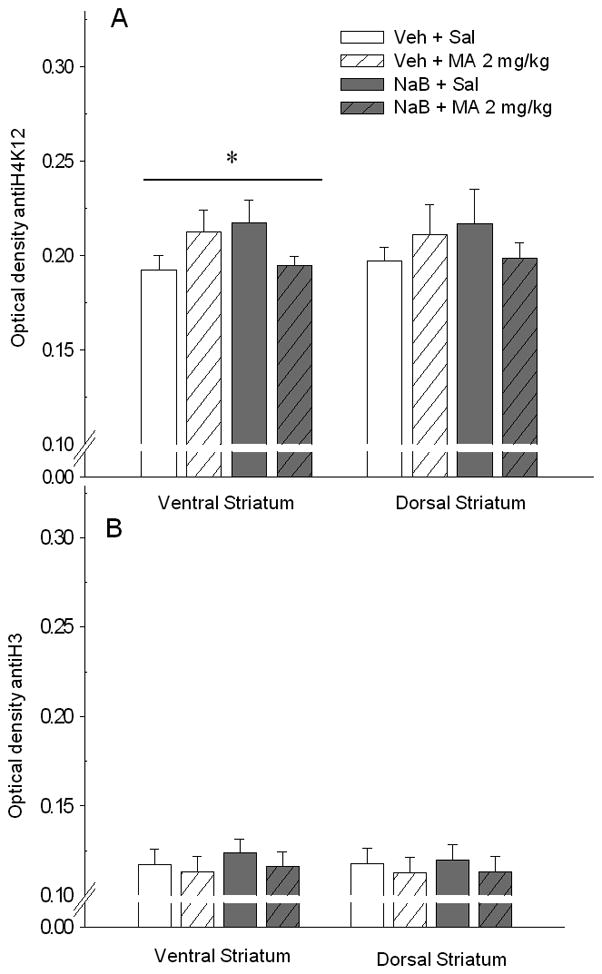
Effects of acute MA and NaB on acetylation at histone H4K12 in the ventral and dorsal striatum. Mice were treated with Veh or NaB 630 mg/kg, 30 min prior to Sal or MA 2 mg/kg. Tissue was perfused 30 min after the final treatment and stained for acetylation at histone H4K12 or non acetyl-specific H3. A. Shown are optical density data (mean ± SEM from 3 averaged samples per individual), reflecting acetylation of H4K12 for the ventral and dorsal striatum. * p<0.05 for the NaB dose x MA dose interaction. B. Shown are optical density data (mean ± SEM from 3 averaged samples per individual), reflecting concentration of total H3 protein for the ventral and dorsal striatum.
3.5 Experiment 5: Immunohistochemistry after repeated NaB and MA administration
Nuclear stain optical density data for the effects of repeated drug exposures on H3K14 acetylation are shown in Figure 6A. There was a significantly higher level of acetylation at H3K14 in the ventral and dorsal striatum of mice in the repeated MA group compared with non-drug-treated controls. There was no significant effect of NaB alone or NaB plus MA on acetylation at H3K14 compared with controls, and no significant treatment effects on acetylation at H4K12 were found (Figure 6B).
Figure 6.
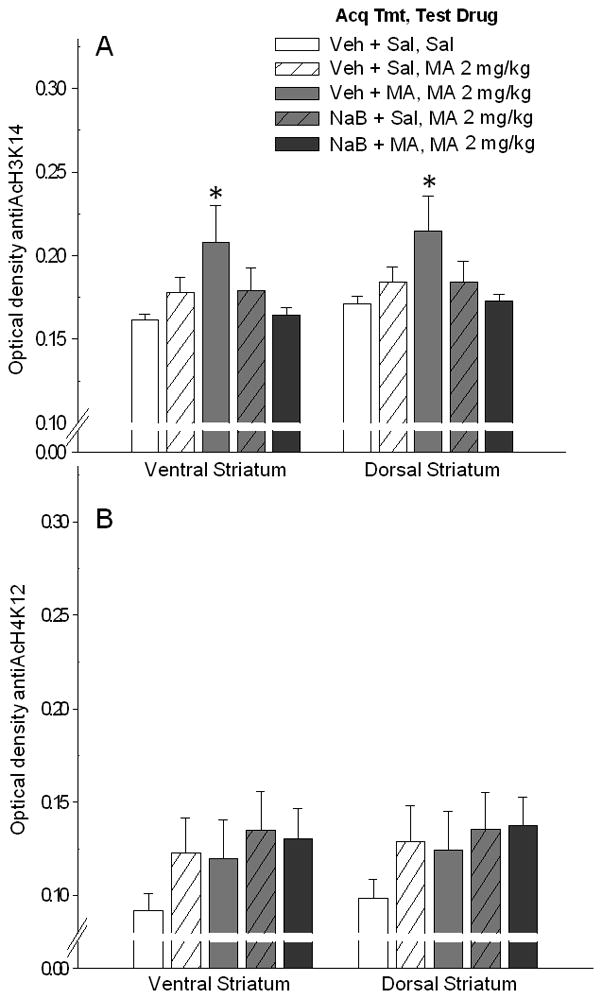
Repeated MA increases acetylation at histone H3K14 in the ventral and dorsal striatum. Mice were treated with either NaB 630 mg/kg or Veh, 30 min prior to MA 2 mg/kg or saline (Acq Tmt) for 10 days, then received two injections of saline on two consecutive days. Finally, all groups except controls (Sal) were administered MA 2 mg/kg on day 13 (Test Drug). Tissue was perfused 30 min after the final Sal or MA treatment and stained for acetylation at histone H3K14 and H4K12. A. Shown are optical density data (mean ± SEM from 3 averaged samples per individual), reflecting acetylation of H3K14 for the ventral and dorsal striatum. * p<0.05 for the difference between the indicated repeated MA treatment group (Veh + MA, MA 2 mg/kg) and control group (Veh + Sal, Sal). B. Shown are optical density data (mean ± SEM from 3 averaged samples per individual), reflecting acetylation of H4K12 for the ventral and dorsal striatum.
4. Discussion
Repeated treatment with 1 or 2 mg/kg MA resulted in significant locomotor sensitization to MA challenge. Treatment with NaB during acquisition of MA-induced sensitization augmented the sensitized response to 2 mg/kg MA, but not 1 mg/kg MA, and acute NaB administered 30 minutes prior to MA challenge increased the locomotor response to MA, regardless of history of previous MA exposure. A history of repeated NaB exposure did not alter the stimulant response to MA challenge, when MA was given in the absence of NaB pretreatment. Neither acute nor repeated administration of NaB significantly altered locomotor activity levels in saline-treated mice. Thus, the increases in locomotor activity following NaB in MA-pre-exposed and MA-challenged mice were not due to effects of NaB alone on locomotor behavior. Lastly, results for histone acetylation at H3 and H4 showed little correspondence with behavioral results, although there were significant independent effects of NaB and MA exposure.
Studies examining the effects of the HDAC inhibitor valproate on MA-induced sensitization have presented discrepant results, with both increases [13, 16], like those seen here with NaB treatment, and decreases [13, 21, 23]. There are several factors that could contribute to these discrepancies. First, different HDAC inhibitors may have different effects. Like NaB, valproate has been shown to inhibit histone deacetylase, such as HDAC 1 [33]. However, while neither valproate [34] nor NaB [35] work exclusively to prevent deacetylation of histones, a major action of valproate is to increase GABAergic activity [36, 37]. Mechanisms other than HDAC inhibition may contribute to the behavioral effects of these drugs. GABAB agonists have been shown to inhibit amphetamine-induced sensitization [38], and others have suggested that valproate may inhibit MA-induced sensitization via stimulation of GABAergic processes [27].
Additional factors that could contribute to discrepancies across studies are the study design, the particular psychostimulant under examination, and the genotype of mouse used. Two papers [13, 16] reported an increase in locomotor activation following combined administration of valproate (175 mg/kg) and amphetamine compared with amphetamine alone, whereas two other papers [21, 23] reported an effect in the opposite direction following valproate (150 mg/kg) and MA compared with MA alone. These divergent findings may indicate that behavior resulting from MA exposure responds differently to HDAC inhibitors than behavior resulting from amphetamine exposure. Alternatively, the studies that utilized amphetamine [13, 16] used inbred C57BL/6 mice, whereas the MA studies [21, 23] used outbred Kunming mice and CD-1 mice, respectively. This raises the possibility of genotype-dependent actions of HDAC inhibitors on the effects of these psychostimulants.
An important distinction between the current and previous similar studies is the separation of effects of the HDAC inhibitor on the acquisition of sensitization (effects of repeated HDAC exposure) from the expression of sensitization (effects of an acute exposure). If acute and chronic exposures to HDAC inhibitors and psychostimulant drugs affect histone modifications and cellular cascades differently, as suggested by Maze et al. [39], then these processes should be examined independently. To test whether histone acetylation affects acquisition of MA-induced behavioral sensitization, it is important to administer the inhibitor concurrently with repeated MA exposure but not prior to MA challenge on the test day. To examine effects on expression only, NaB should be administered only on the MA challenge day and not during acquisition. Previous studies administered an HDAC inhibitor plus amphetamine both during repeated administration of amphetamine and on the test day [13, 16]. Independent effects of the HDAC inhibitor on acquisition and expression of drug-induced sensitization cannot be determined using this design. In another experiment, Kalda et al. [13] administered amphetamine to animals for 8 days without an HDAC inhibitor and then administered an HDAC inhibitor without amphetamine for 6 days followed by amphetamine challenge in the absence of the HDAC inhibitor on the locomotor test day. This experiment is difficult to interpret with regard to possible effects of the HDAC inhibitor on either the acquisition or expression of amphetamine-induced sensitization. Part of the time, amphetamine was given without the HDAC inhibitor, so sensitization may have already developed before the inhibitor was ever administered. The results of the experiment suggested that repeated administration of an HDAC inhibitor, following the acquisition of sensitization, can decrease the locomotor response to amphetamine challenge.
In the current study, acute NaB increased H3K14 acetylation in both the ventral and dorsal striatum, indicating that this dose of NaB had detectable effects. However, acute MA did not affect H3K14 acetylation alone, and the effect of NaB was not influenced by acute MA administration. On the other hand, repeated exposure to MA increased H3K14 acetylation in both the ventral and dorsal striatum, but effects of NaB were not seen when it was given repeatedly during the MA exposure period, rather than shortly before tissue was taken for immunohistochemistry. Further, the effect of repeated MA was seen only when given without prior administration of NaB. For H4K12 acetylation, a statistical interaction appeared to indicate independent increasing effects of acute NaB and acute MA in the ventral striatum that were absent when both drugs were administered. Effects on H4K12 acetylation from the repeated drug administration study showed non-significant and non-specific higher acetylation levels in all drug-treated groups in both brain regions compared with the non-drug-treated control. These results are not straightforward, and do not show a clear correspondence with the behavioral results. However, modification at specific histones or lysines on histones may be temporally specific to MA exposure, and this will require further investigation.
The difference in the effect of acute vs. chronic MA on H3K14 acetylation is consistent with previous results showing that cocaine has varying effects on histones, depending on whether exposure was acute or chronic [For review see: 39, 40]. For example, previous work [15] reported increased acetylation at histone H4 at the fosB promoter after acute cocaine administration, whereas chronic cocaine administration led to increased acetylation at H3 histones proximal to the cyclin-dependent kinase 5 (cdk5), brain-derived neurotrophic factor (bdnf), and fosB gene promoters. Additionally, previous studies reported that repeated exposure to amphetamine altered acetylation at H4K12 [13], and chronic cocaine administration induced acetylation at the H3, fosB promoter [41]. However, these findings were for psychostimulants other than MA, and it remains to be seen whether MA has similar or different epigenetic effects in relation to specific genes.
Although repeated MA increased H3K14 acetylation, this effect was not seen when MA was combined with NaB pretreatment. These results do not correspond with the locomotor data; mice repeatedly treated with NaB plus MA exhibited higher levels of MA-induced stimulation compared with mice that had been repeatedly treated with MA alone (Figure 6). It is likely that NaB and MA have interactive or independent effects that mask global changes in H3 and/or H4 acetylation. Increased acetylation at some loci may be counteracted by decreases at others. Immunohistochemistry techniques present a problem in that they are sensitive to genome-wide changes in average acetylation patterns. The chromatin immunoprecipitation (ChIP) method may be more sensitive to gene-specific changes in acetylation patterns that are not detected in global acetylation assays. Investigations involving ChIP analysis could be considered for future work. It is also possible that increased histone acetylation at H3 or H4 corresponding with increased locomotor stimulation following NaB and MA may be seated in other brain regions such as the nucleus accumbens or prefrontal cortex [6, 42, 43].
4.1 Conclusions
To our knowledge, this is the first study to report an increase in the magnitude of MA-induced locomotor sensitization following chronic, concomitant administration of NaB and MA, and to independently examine effects of an HDAC inhibitor on the acquisition and expression of MA-induced sensitization. We report here that acute NaB administered prior to MA challenge increased the locomotor response to MA regardless of history of previous MA exposure, and repeated treatment with NaB during acquisition of MA-induced sensitization augmented the sensitized response, but not acute response, to 2 mg/kg MA. While there were significant independent effects of NaB and MA exposure, results for histone acetylation at H3 and H4 showed little correspondence with behavioral results. Future studies of MA-induced histone modification should consider chronic drug effects on histone acetylation sites associated with specific genes; such targets could be identified using ChIP. The use of more specific HDAC inhibitors, such as suberoylanilide hydroxamic acid, may also be beneficial. The identification of specific genes that are regulated by associated histone modifications will allow for a meaningful interpretation of the pathways underlying epigenetic effects of MA.
Research Highlights.
Repeated methamphetamine (MA) resulted in a sensitized response to MA challenge.
Acute sodium butyrate (NaB) prior to MA increased the locomotor response to MA.
NaB during acquisition of MA sensitization augmented the response to MA challenge.
Acute MA increased acetylation in the striatum at histone H4K12.
Repeated MA increased acetylation in the striatum at histone H3K14.
Acknowledgments
This work was supported by the Department of Veterans Affairs, and NIDA/NIH grants T32DA07262 and P50DA018165. Thank you to James Stafford for helpful discussions regarding the IHC procedure and interpretation.
Abbreviations
- MA
methamphetamine
- HDAC
histone deacetylase
- NaB
sodium butyrate
- Veh
vehicle
- Sal
saline
- H3K12
histone H3 at lysine K12
- H3K14
histone H3 at lysine K14
- PBS
potassium-buffered saline
- GABA
gamma-aminobutyric acid
- ChIP
chromatin immunoprecipitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
John H. Harkness, Email: harknesj@ohsu.edu.
Robert J. Hitzemann, Email: hitzeman@ohsu.edu.
Stephanie Edmunds, Email: edmundss@ohsu.edu.
References
- 1.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiat. 2001;158:377–82. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 (Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–14. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–46. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–15. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- 6.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–9. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Scibelli AC, McKinnon CS, Reed C, Burkhart-Kasch S, Li N, Baba H, et al. Selective breeding for magnitude of methamphetamine-induced sensitization alters methamphetamine consumption. Psychopharmacology. 2011;214:791–804. doi: 10.1007/s00213-010-2086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes, Brain, and Behavior. 2005;4:110–25. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 9.Giardino WJ, Pastor R, Anacker AM, Spangler E, Cote DM, Li J, et al. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes, Brain, and Behavior. 2011;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brabant C, Tambour S, Quertemont E, Ferrara A, Tirelli E. Do excitatory and inhibitory conditioning processes underlie psychomotor sensitization to amphetamine? An analysis using simple and multiple regressions. Behav Brain Res. 2011;221:227–36. doi: 10.1016/j.bbr.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–65. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Numachi Y, Yoshida S, Yamashita M, Fujiyama K, Naka M, Matsuoka H, et al. Psychostimulant alters expression of DNA methyltransferase mRNA in the rat brain. Ann NY Acad Sci. 2004;1025:102–9. doi: 10.1196/annals.1316.013. [DOI] [PubMed] [Google Scholar]
- 13.Kalda A, Heidmets LT, Shen HY, Zharkovsky A, Chen JF. Histone deacetylase inhibitors modulates the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behav Brain Res. 2007;181:76–84. doi: 10.1016/j.bbr.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renthal W, Nestler EJ. Histone acetylation in drug addiction. Semin Cell Dev Biol. 2009;20:387–94. doi: 10.1016/j.semcdb.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–14. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 16.Shen HY, Kalda A, Yu L, Ferrara J, Zhu J, Chen JF. Additive effects of histone deacetylase inhibitors and amphetamine on histone H4 acetylation, cAMP responsive element binding protein phosphorylation and DeltaFosB expression in the striatum and locomotor sensitization in mice. Neuroscience. 2008;157:644–55. doi: 10.1016/j.neuroscience.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 2011;12:7–18. doi: 10.1038/nrg2905. [DOI] [PubMed] [Google Scholar]
- 18.Rodriquez M, Aquino M, Bruno I, De Martino G, Taddei M, Gomez-Paloma L. Chemistry and biology of chromatin remodeling agents: state of art and future perspectives of HDAC inhibitors. Curr Med Chem. 2006;13:1119–39. doi: 10.2174/092986706776360905. [DOI] [PubMed] [Google Scholar]
- 19.Szyf M. The dynamic epigenome and its implications in toxicology. Toxicol Sci. 2007;100:7–23. doi: 10.1093/toxsci/kfm177. [DOI] [PubMed] [Google Scholar]
- 20.Malvaez M, Barrett RM, Wood MA, Sanchis-Segura C. Epigenetic mechanisms underlying extinction of memory and drug-seeking behavior. Mamm Genome. 2009;20:612–23. doi: 10.1007/s00335-009-9224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coccurello R, Caprioli A, Ghirardi O, Virmani A. Valproate and acetyl-L-carnitine prevent methamphetamine-induced behavioral sensitization in mice. Ann N Y Acad Sci. 2007;1122:260–75. doi: 10.1196/annals.1403.019. [DOI] [PubMed] [Google Scholar]
- 22.Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2010;67:36–43. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li JX, Han R, Deng YP, Chen SQ, Liang JH. Different effects of valproate on methamphetamine- and cocaine-induced behavioral sensitization in mice. Behav Brain Res. 2005;161:125–32. doi: 10.1016/j.bbr.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 24.Sanchis-Segura C, Lopez-Atalaya JP, Barco A. Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology. 2009;34:2642–54. doi: 10.1038/npp.2009.125. [DOI] [PubMed] [Google Scholar]
- 25.Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- 26.Taber KH, Black DN, Porrino LJ, Hurley RA. Neuroanatomy of dopamine: reward and addiction. J Neuropsychiatry Clin Neurosci. 2012;24:1–4. doi: 10.1176/appi.neuropsych.24.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Egorin MJ, Yuan ZM, Sentz DL, Plaisance K, Eiseman JL. Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemother Pharmacol. 1999;43:445–53. doi: 10.1007/s002800050922. [DOI] [PubMed] [Google Scholar]
- 28.Kamens HM, McKinnon CS, Li N, Helms ML, Belknap JK, Phillips TJ. The alpha 3 subunit gene of the nicotinic acetylcholine receptor is a candidate gene for ethanol stimulation. Genes, brain, and behavior. 2009;8:600–9. doi: 10.1111/j.1601-183X.2008.00444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips TJ, Huson MG, McKinnon CS. Localization of genes mediating acute and sensitized locomotor responses to cocaine in BXD/Ty recombinant inbred mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:3023–34. doi: 10.1523/JNEUROSCI.18-08-03023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009;43:411–20. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paxinos G, Franklin KBJ. The Mouse Brain in Sterotaxic Coordinates. 2nd. New York: Elsevier Academic Press; 2003. [Google Scholar]
- 32.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell Mol Life Sci. 2007;64:2090–103. doi: 10.1007/s00018-007-7079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S–93S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- 36.Perucca E. An introduction to antiepileptic drugs. Epilepsia. 2005;46 (Suppl 4):31–7. doi: 10.1111/j.1528-1167.2005.463007.x. [DOI] [PubMed] [Google Scholar]
- 37.Rho JM, Sankar R. The pharmacologic basis of antiepileptic drug action. Epilepsia. 1999;40:1471–83. doi: 10.1111/j.1528-1157.1999.tb02029.x. [DOI] [PubMed] [Google Scholar]
- 38.Cott J, Engel J. Suppression by GABAergic drugs of the locomotor stimulation induced by morphine, amphetamine, and apomorphine: evidence for both pre- and post-synaptic inhibition of catecholamine systems. J Neural Transm. 1977;40:253–68. doi: 10.1007/BF01257019. [DOI] [PubMed] [Google Scholar]
- 39.Maze I, Russo SJ. Transcriptional mechanisms: underlying addiction-related structural plasticity. Mol Interv. 2010;10:219–30. doi: 10.1124/mi.10.4.5. [DOI] [PubMed] [Google Scholar]
- 40.Colvis CM, Pollock JD, Goodman RH, Impey S, Dunn J, Mandel G, et al. Epigenetic mechanisms and gene networks in the nervous system. J Neurosci. 2005;25:10379–89. doi: 10.1523/JNEUROSCI.4119-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res. 2004;132:146–54. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, Morgan D, et al. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–17. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]


