Abstract
ADAMTS-like proteins are related to ADAMTS metalloproteases by their similarity to ADAMTS ancillary domains. Here, we have characterized ADAMTSL5, a novel member of the superfamily with a unique modular organization that includes a single C-terminal netrin-like (NTR) module. Alternative splicing of ADAMTSL5 at its 5′ end generates two transcripts that encode different signal peptides, but the same mature protein. These transcripts differ in their translational efficiency. Recombinant ADAMTSL5 is a secreted, N-glycosylated 60 kDa glycoprotein located in the subcellular matrix, on the cell-surface, and in the medium of transfected cells. RT-PCR and western blot analysis of adult mouse tissues showed broad expression. Western blot analysis suggested proteolytic release of the NTR module in transfected cells as well as in some mouse tissues. Immunostaining during mouse organogenesis identified ADAMTSL5 in musculoskeletal tissues such as skeletal muscle, cartilage and bone, as well as in many epithelia. Affinity-chromatography demonstrated heparin-binding of ADAMTSL5 through its NTR-module. Recombinant ADAMTSL5 bound to both fibrillin-1 and fibrillin-2, and co-localized with fibrillin microfibrils in the extracellular matrix of cultured fibroblasts, but without discernible effect on microfibril assembly. ADAMTSL5 is the first family member shown to bind both fibrillin-1 and fibrillin-2. Like other ADAMTS proteins implicated in microfibril biology through identification of human and animal mutations, ADAMTSL5 could have a role in modulating microfibril functions.
Keywords: ADAMTS, ADAMTS-like, netrin-like module, fibrillin microfibril, heparin, alternative splicing
1. Introduction
The ADAMTS superfamily comprises 19 secreted metalloproteases as well as a set of related molecules termed ADAMTS-like proteins (Apte, 2009). Six ADAMTS-like proteins (ADAMTSL1-4, ADAMTSL6 and papilin) were previously described in the literature (Buchner and Meisler, 2003; Hall et al., 2003; Hirohata et al., 2002; Koo et al., 2007; Kramerova et al., 2000; Tsutsui et al., 2010). In contrast to ADAMTS proteases, ADAMTSLs lack a catalytic domain and thus have no proteolytic activity. They contain characteristic modules present in the ancillary domain of an ADAMTS protease, including an N-terminal thrombospondin-1 type 1 repeat, a cysteine-rich module, and a cysteine-free spacer. These three modules constitute the conserved region of all ADAMTS proteins. Downstream of this conserved region, one or more additional thrombospondin type 1 repeats (TSRs) are present in all ADAMTS proteins except ADAMTS4. Additional C-terminal modules are frequently located downstream of the TSRs. Such C-terminal modules include the protease and lacunin (PLAC) module (e.g., in ADAMTS6, ADAMTS10, ADAMTS7, ADAMTS12, ADAMTS16, ADAMTS18, ADAMTS17, ADAMTS19, and in all ADAMTS-like proteins except ADAMTSL5 and the short splice variant of ADAMTSL1), a Gon-1 domain, present only in ADAMTS9, ADAMTS20 and their worm homolog Gon-1, and a unique domain containing an internal PLAC module in the procollagen processing enzymes, ADAMTS2, ADAMTS3, and ADAMTS14 (Apte, 2009). The von Willebrand factor-cleaving protease, ADAMTS13, is the only member to have C-terminal CUB domains (Zheng et al., 2001). These variable C-termini are presumably related to ADAMTS functions in distinct biological contexts, specialized intermolecular interactions or tissue localization. Comparison of modular organization and primary structure of ADAMTSLs suggests that ADAMTSL1 and ADAMTSL3, as well as ADAMTSL4 and ADAMTSL6, comprise highly homologous pairs (Apte, 2009). In contrast, ADAMTSL2 and papilin, and as described here, ADAMTSL5, each has a unique domain composition (Apte, 2009).
Recently, genetic and experimental work identified a role for ADAMTS-like proteins in formation of fibrillin microfibrils and thus potentially, in regulation of growth factors of the TGFβ superfamily (reviewed recently (Hubmacher and Apte, 2011)), a key function of microfibrils. ADAMTSL2 mutations cause human geleophysic dysplasia and canine Musladin-Lueke syndrome (Bader et al., 2010; Le Goff et al., 2008). Geleophysic dysplasia appears to be a consequence of TGFβ dysregulation (Le Goff et al., 2008). This may result from loss of ADAMTSL2 interactions with latent TGFβ-binding protein-1 (LTBP-1) and fibrillin-1 (Le Goff et al., 2011; Le Goff et al., 2008), which are required for extracellular sequestration and regulated activation of TGFβ (Ramirez and Rifkin, 2009). ADAMTSL4 mutations cause recessive isolated ectopia lentis (Ahram et al., 2009), in which assembly or integrity of the fibrillin-1 rich zonule (also known as suspensory ligament of the lens) is impaired. ADAMTSL2, ADAMTSL4, and ADAMTSL6 each bind fibrillin-1 (Gabriel et al., 2011; Le Goff et al., 2011; Tsutsui et al., 2010) and ADAMTSL2, ADAMTSL4 and ADAMTSL6 were shown to accelerate biogenesis of fibrillin-1 microfibrils (Gabriel et al., 2011; Kutz et al., 2011; Tsutsui et al., 2010). Here, a new member of the family, ADAMTSL5, is characterized, and its relationship to fibrillins was investigated. ADAMTSL5 is the first family member shown to bind not only fibrillin-1, but also fibrillin-2.
2. Results
2.1. The unique domain structure of ADAMTSL5 includes a c-terminal netrin-like module
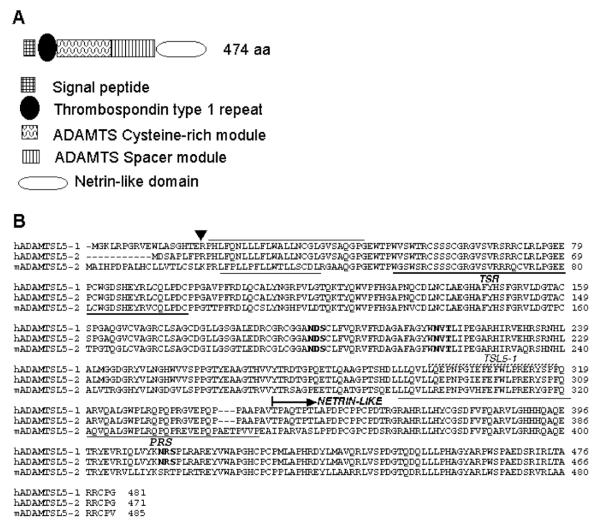
ADAMTSL5 is a secreted protein with a unique domain composition, comprising an N-terminal TSR, a cysteine-rich module, a spacer module, and a C-terminal NTR module, which is connected to the spacer by a proline-rich segment (Fig. 1A,B). The TSR in both human and mouse ADAMTSL5 contains a consensus sequence for O-fucosylation (Cys-Ser-Ser-Ser-Cys, potential modified residue underlined) and C-mannosylation (Trp-Thr-Pro-Trp-Val-Ser-Trp-Thr-Arg-Cys; potential modified residues are underlined) (Fig. 1B) similar to other ADAMTS proteins (Wang et al., 2007; Wang et al., 2009). Human and mouse ADAMTSL5 are highly conserved, with amino acid identity of 82%; with inclusion of functionally conserved amino acids, there is an overall similarity of 88% (Fig. 1B). Three consensus N-linked glycosylation sites are completely conserved in human and mouse ADAMTSL5 (Fig. 1B). The ADAMTSL5 NTR-module contains several conserved residues that are characteristic of this domain (Banyai and Patthy, 1999), specifically six Cys residues and several hydrophobic amino acids. The highest homology of 36% identity and 46% conserved residues was found with the NTR-module of netrin 5, a netrin of unknown function. Lower homology was seen with the NTR modules of netrin 4 (22% identity/43% conserved) and netrin 1 (23% identity/39% conserved).
Figure 1. Domain organization and annotated primary structure of ADAMTSL5.
A. Domain organization (not drawn to scale) The key to the modules is shown at the bottom. B. Primary structure of human and mouse ADAMTSL5 (GenBank accession numbers AK302020 (hADAMTSL5-1), AK131571 (hADAMTSL5-2) and NM_001113548.1 (mADAMTSL5-2): The sequences of alternatively spliced forms of human ADAMTSL5 and of mouse ADAMTSL5 splice variant 2 were aligned using the MegAlign program (Lasergene software). The arrowhead indicates the location upstream of which the primary structures diverge and the adjacent lines indicate the hydrophobic cores of human and mouse signal peptides. The single TSR is underlined with the bold line. N-glycosylation sequons are in bold type. The proline-rich segment (PRS) is indicated and underlined. The peptide immunogen of the rabbit polyclonal antibody (TSL5-1) is indicated by the stippled line. The netrin-like module is indicated.
2.2. Alternative splicing of the 5′ end of ADAMTSL5
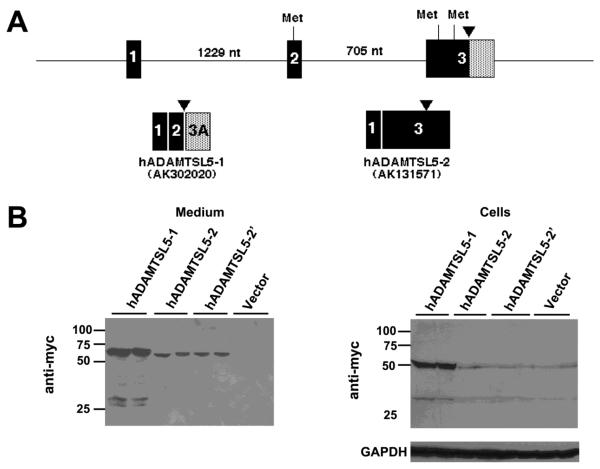
Two human ADAMTSL5 cDNA transcripts were found in GenBank with accession numbers AK302020 (designated hADAMTSL5-1) and AK131571 (designated ADAMTSL5-2). The predicted hADAMTSL5-1 and hADAMTSL5-2 ORFs diverge only in regard to residues upstream of the hydrophobic core of the signal peptide, so that the predicted signal peptidase-processed mature proteins derived from the variants are identical (Fig. 1B). Comparison of these cDNA sequences with ADAMTSL5 genomic sequence determined that alternative splicing at the 5′ end of the gene was the basis for their divergence (Fig. 2A). In hADAMTSL5-1, exon 2 is spliced to a cryptic splice site in exon 3 (Fig. 2A). The hADAMTSL5-1 ORF utilizes a start codon in exon 2. The hADAMTSL5-2 ORF is derived from splicing of exon 1 to exon 3, with skipping of exon 2, eliminating the exon 2-derived start codon present in hADAMTSL5-1. Instead, two putative start codons in different reading frames are present in exon 3; the downstream start codon is in the ORF of ADAMTSL5, whereas the upstream start codon is out of frame.
Figure 2. Alternative splicing at the 5′ end of ADAMTSL5.
A. Genomic region of the 5′ end of ADAMTSL5 (human gene) illustrating exons 1-3 and the intervening introns (size in nucleotides (nt)). The arrowhead indicates the location upstream of which the primary structures diverge (see Fig. 1B for sequences). Putative methionine codons (Met) within an acceptable Kozak consensus sequence are shown at the top of the figure. The pattern of exon splicing to generate the two alternatively spliced transcripts is shown below. Note that there are two potential exon 3 encoded start codons in the ADAMTSL5-2 transcript (Accession no. AK131571). B. Western blots of medium and lysate of HEK293F cells transfected with myc-His6 tagged ADAMTSL5-1 and ADAMTSL5-2 constructs. Lower levels of the expected 60 kDa protein species were obtained with ADAMTSL5-2 constructs in both cell lysate and medium. Abrogation of the upstream start codon in ADAMTSL5-2 (construct hADAMTSL5-2′) did not alter the protein level in cells or medium. The western blots show duplicate transfections for each construct.
The two ADAMTSL5 variant ORFs were each cloned into an expression vector and transfected into HEK-293F cells for identification of expressed proteins by western blot. A consistently lower level of expressed protein was detected using anti-myc in media of cells transfected with ADAMTSL5-2 compared to ADAMTSL5-1, (Fig 2B) and a similar difference was seen the cell lysate. To determine if this was a consequence of preferred utilization of the upstream, out of frame ATG in the hADAMTSL5-2 transcript, we mutated it. Levels of ADAMTSL5-2 and the ATG-mutant (ADAMTSL5-2′) protein in the medium were similar (Fig. 2B) and low levels of both proteins were seen in cell lysates. These results suggest that the ADAMTSL5-2 variant was poorly translated. Subsequent protein analysis therefore primarily used the ADAMTSL5-1 variant.
2.3.ADAMTSL5 is a secreted N-glycosylated protein that binds to the cell-surface and extracellular matrix
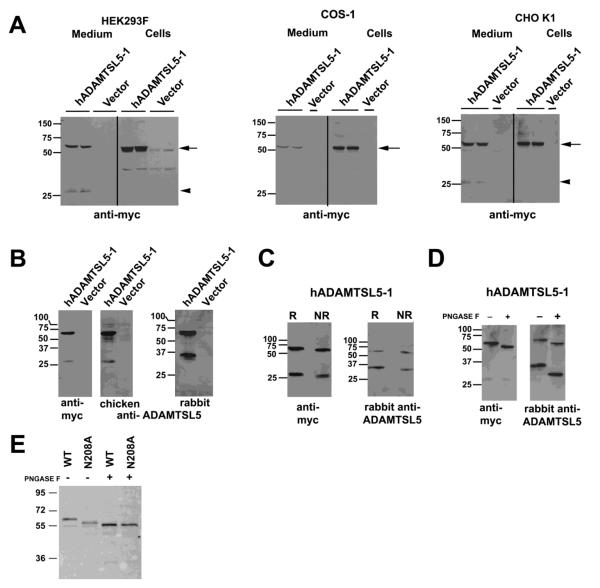
Recombinant ADAMTSL5-1 protein with a C-terminal myc-His6 tag was generated by transfection of HEK293F, COS-1 and CHO-K1 cells. Western blotting of cell lysate demonstrated a major species of 55 kDa in each cell type (Fig. 3A). In the conditioned media, a major species of 55-60 kDa was seen; COS-1 and CHO-K1 cells contained a secreted protein of the same size as in cell lysate (55 kDa), but that in HEK293F medium was ~5 kDa bigger (60 kda). This difference could potentially arise from differences in glycosylation by these different cell types (see below). HEK293F and CHO-K1 media additionally contained a 27 kDa species reactive with anti-myc (Fig. 3A) that potentially represents an ADAMTSL5 fragment arising from proteolysis on the N-terminal side of the NTR-module (see below). Faint anti-myc reactive bands of 60 kDa and 35 kDa seen in HEK293F cells transfected with empty vector (Fig. 3A) could be cross-reacting or non-specific immunoreactivity, since these were not seen in COS-1 and CHO-K1 lysate, nor in western blotting with ADAMTSL5 antibodies (Fig. 3B).
Figure 3. ADAMTSL5 undergoes proteolysis and is N-glycosylated.
A. Cells were transiently transfected with myc-His6 tagged hADAMTSL5-1. The panels show western blots from the lysate and medium of HEK293F cells, COS-1 cells and CHO-K1 cells as indicated above each blot, with anti-myc antibody. The arrow points to intact ADAMTSL-5, and the arrowhead shows a 27 kDa fragment in the medium. B. Characterization of rabbit and chicken polyclonal antibodies by western blot and comparison with anti-myc. Note that the chicken antibody, raised against a C-terminal peptide, gives essentially similar results as anti-myc. The rabbit polyclonal identified a 60 kDa band corresponding to intact ADAMTSL5, and a 33 kDa species corresponding to the N-terminal fragment resulting from proteolysis. C. Comparison of medium of transfected HEK293F cells immunoblotted after reducing (R) or non-reducing (NR) electrophoresis using anti-myc or rabbit polyclonal antibody to ADAMTSL5. Both antibodies react with same 60 kDa band representing intact ADAMTSL5. In contrast to anti-myc, which detects a 27 kDa ADAMTSL5 fragment, the rabbit polyclonal antibody reacts with a 33 kDa species. There is enhanced migration of each detected species under non-reducing conditions. D. Enzymatic deglycosylation of ADAMTSL5 in conditioned medium of stably transfected HEK293F cells. Increased electrophoretic mobility of anti-ADAMTSL5 reactive species is seen in the presence of PNGAse F (+) compared to the untreated control (−). Digestion with PNGAse F results in more rapid migration of the 60 kDa and 33 kDa (anti-ADAMTSL5) species, whereas migration of the 27 kDa myc-reactive band is unaffected. E. ADAMTSL5-N208A migrates more rapidly than wild-type (WT) protein. Deglycosylation of ADAMTSL5-N208A using PNGase F further enhances migration, suggestive of at least one other N-glycosylation site. Molecular weight markers (in kDa) are shown at left of each gel.
For initial validation of anti-ADAMTSL5 rabbit and chicken polyclonal antibodies, we compared them with anti-myc on western blots. As described in detail in the Experimental Procedures section, we generated the rabbit polyclonal antibody to an epitope on the N-terminal side of the NTR module, whereas the commercial chicken antibody was generated to a C-terminal peptide. Under reducing conditions, all three antibodies detected a 60 kDa species in medium of transfected HEK293F cells (Fig. 3B). Whereas anti-myc and the chicken polyclonal detected the additional 27 kDa species described above (consistent with their epitopes being located at the C-terminus), rabbit anti-ADAMTSL5 detected a 33 kDa species (Fig. 3B). Visualization of both the 33 kDa and 27 kDa fragments under non-reducing conditions (Fig. 3C) indicated that proteolytic cleavage of ADAMTSL5 occurred between disulfide-bonded modules, and not internally within a module. Based on the size of the observed fragments, we propose that proteolysis likely occurs within the proline-rich segment that separates the NTR-module from the N-terminal 2/3 of ADAMTSL5 (Figs. 1B, 3C).
Peptide N-glycanase F (PNGase F) treatment of conditioned medium led to more rapid migration of the 60 kDa species, but no change in migration of the 27 kDa fragment (Fig. 3D). Although there is an Asn-Arg-Ser sequon in this fragment (Fig. 1B), the presence of Pro residue immediately succeeding it diminishes the probability of N-glcyosylation (Roitsch and Lehle, 1989). In contrast, the 33 kDa species detected by the rabbit anti-ADAMTSL5 antibody had considerable enhancement of its mobility upon PNGaseF treatment, since the N-terminal fragment contains 2-linkage sites. Indeed, mutation of Asn208 led to accelerated protein mobility, which was further enhanced upon PNGase F digestion (Fig. 3E). Together, these findings indicate that ADAMTSL5 is an N-linked glycoprotein modified at two sites.
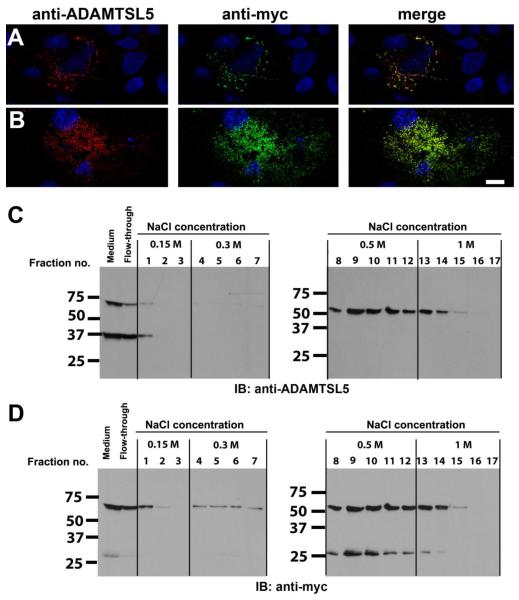
To determine the localization of ADAMTSL5-1 in regard to the cell-matrix interface, we used conventional (post-fixation) as well as immunostaining of transiently transfected non-permeabilized COS-1 cells using the rabbit polyclonal ADAMTSL5 antibody or anti-myc antibody. Staining with both antibodies gave a similar pattern and co-staining with these antibodies demonstrated co-localization of the signals, indicating that the immunoreactivity derived from intact, full-length ADAMTSL5. (Fig. 4 A,B). Permeabilized cells showed strong staining of protein within the secretory pathway whereas empty vector transfected cells did not show a signal (data not shown). After staining non-permeabilized cells, only extracellular proteins were visualized in optical sections through the cell and cell-substratum using confocal microscopy (supplemental movie). Upon non-permeabilized staining ADAMTSL5 had a punctate staining pattern, which was associated with the surface of cells as well as the substratum (ECM underlying the cells) (Fig. 4A,B). Indeed, where cells had detached from the substratum, exposed cellular substratum (“footprints” associated with remnant cytoskeletal filaments, but without an intact cell or nucleus overlying the footprint) were observed (data not shown). Confocal microscopy reconstructions revealed that in cells in which ADAMTSL5 was cell-surface associated, staining frequently extended only to the middle of the cell, indicating a baso-lateral distribution, and did not extend to the apical surface. These observations strongly suggest that ADAMTSL5 is associated with the peri-cellular and sub-cellular ECM of cells, a similar distribution to that previously demonstrated for ADAMTSL1, ADAMTSL2, ADAMTSL3 and ADAMTSL4 (Gabriel et al., 2011; Hall et al., 2003; Hirohata et al., 2002; Koo et al., 2007).
Figure 4. ADAMTSL5 is located at the cell-surface and in the ECM of transiently transfected COS-1 cells and binds heparin.
A,B. Staining of non-permeabilized cells with anti-myc and anti-ADAMTSL5 antibodies gave identical staining patterns. Cos-1 cells were transiently transfected with myc-tagged hADAMTSL5-1 and were co-stained with rabbit anti-ADAMTSL5 (red) and anti-myc (green) antibody with the non-permeabilized staining technique. Nuclei were stained with DAPI and appear blue. Representative confocal images showing cell surface association (A, optical plane through mid-cell region) and ECM association (B, subcellular optical plane) are shown (see supplemental movie). Note that the staining pattern obtained with the two antibodies coincides, indicating that the signal is derived from full-length ADAMTSL5. Scale bar=10 μm. C,D. Heparin-agarose beads were incubated with conditioned medium from HEK293 cells stably transfected with hADAMTSL5-1. After incubation, the beads were washed and eluted with increasing salt concentrations as indicated. ADAMTSL5 was detected in the fractions by western blot using the rabbit anti-ADAMTSL5 antibody (C). After stripping, the blots were reprobed with a myc-antibody (D). Full-length ADAMTSL5 was eluted with 0.5–1 M NaCl, indicating strong binding to heparin. Furthermore, the C-terminal NTR-module containing 27 kDa fragment, which is myc-tagged, bound to the heparin-agarose with similar affinity as the full-length protein, whereas the N-terminal cleavage product of ADAMTSL5 (37 kDa fragment in G) was not retained by the heparin column (compare C and D). This suggests that the NTR-module mediated heparin binding.
Since NTR-modules are known to bind heparin (Bekhouche et al., 2010; Geisbrecht et al., 2003; Kappler et al., 2000), and because cell-surface localization could result from binding to heparan-sulfate proteoglycans, medium containing ADAMTSL5 was incubated with heparin-agarose, followed by sequential washing and elution from the matrix with increasing concentrations of salt. The fractions were assayed by western blotting using rabbit anti-ADAMTSL5 (Fig. 4C) or anti-myc (Fig. 4D). ADAMTSL5 binding to heparin-agarose was indicated by decreased protein in medium after incubation with the affinity matrix (Fig. 4C,D, lanes indicating flow-through). Washes with 0.15 or 0.3 M NaCl did not lead to significant elution. However 0.5 and 1M NaCl substantially eluted the bound proteins, indicative of an ionic interaction between ADAMTSL5 and heparin (Fig. 4C,D). Of the two fragments of ADAMTSL5 detected in conditioned medium of HEK293F cells, only the C-terminal fragment containing the NTR-module was retained by the heparin matrix (Fig. 4D), suggesting that it contained the binding site(s). In contrast, the 33 kDa N-terminal fragment did not bind robustly to the heparin matrix (see lane labeled flow-through in Fig. 4C) and consequently, eluted from the matrix in the first wash.
2.4. ADAMTSL5 mRNA and protein are located in epithelial and connective tissues during mouse embryonic development
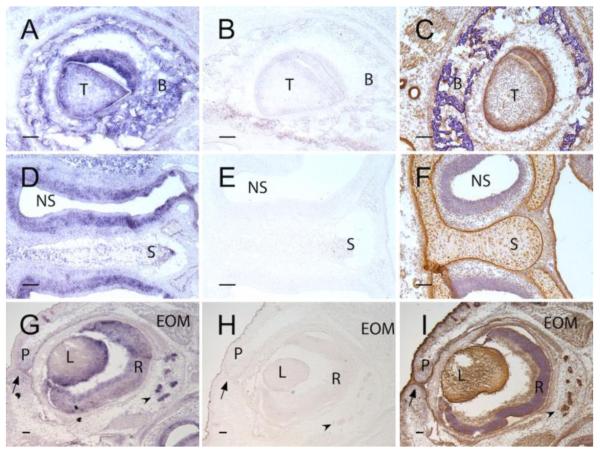
To validate the rabbit polyclonal antibody for determination of tissue distribution of ADAMTSL5, in situ hybridization was first done to establish concordance between mRNA and protein distribution in E16.5 mouse heads. The antisense probe hybridized to developing teeth, alveolar bone, developing nasal structures, such as nasal sinus epithelium and cartilage, and in the developing eye, the retina, lacrimal gland epithelium, lens, extra-ocular muscles and epithelium of the palpebrae (Fig. 5 A,D,G). In contrast, no signal was obtained with the sense probe (Fig. 5B,E,H), indicative of specific hybridization signal from the anti-sense probe. ADAMTSL5 immunostaining showed a similar protein distribution (Fig. 5C,F,I).
Figure 5. In situ hybridization (ISH) and immunohistochemistry identify ADAMTSL5 in specific developing mouse craniofacial structures.
Panels A,D and G show ISH using an antisense Adamtsl5 cRNA probe (purple stain), panels B,E,H, show ISH with the corresponding sense (control) cRNA probe. Panels C, F, I show immunostaining (brown signal) with rabbit anti-ADAMTSL5 polyclonal antibody. A-C. Developing molar tooth. Note specific signal in tooth (T) and surrounding alveolar bone (B). Panel C shows the corresponding immunostaining image, with signal additionally seen in epithelium of salivary gland ducts at the left-hand edge of the panel. D-F. Nasal septum and nasal sinus. In D, note ISH signal in nasal epithelium lining the sinus (NS) and in the cartilage of the nasal septum (S). In F, ADAMTSL5 immunostaining is seen in nasal septal cartilage (S), but there is relatively weak signal in epithelium lining the nasal septum (NS). In contrast, strong immunostaining was noted in the perichondrium of nasal septal cartilage (S). Panels G-I show a developing eye. ISH signal is seen in the lens (L), retina (R), epithelium of the fused palpebrae (P, arrow shows fusion seam) and in lacrimal gland epithelium (arrowhead). Note the similar distribution of ADAMTSL5 protein in panel I.
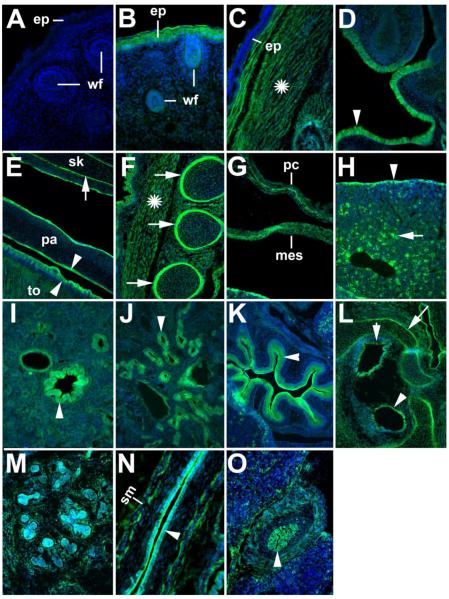
Immunofluorescence using the rabbit ADAMTSL5 polyclonal antibody was done in mouse embryo sections at E14.5 and E16.5 to evaluate the distribution of ADAMTSL5 during organogenesis. At both developmental stages, a similar expression pattern was found, with expression in skin (strong in some regions such as head (Fig. 6B), ventral epidermis, and around the genital tubercle (data not shown), and in skin appendages such as whisker follicles (Fig.6B), but absent in dorsal skin (Fig. 6C)), nasal and oral epithelium (Fig. 6D,E), muscle and perichondrium of ribs (Fig. 6F), pericardium, pleural mesothelium and peritoneum (Fig. 6G), bronchial epithelium (Fig. 6I), renal tubular epithelium (Fig. 6J), stomach lining (Fig. 6K), neuroepithelium of inner ear (Fig. 6L), salivary gland (Fig. 6M), esophageal lining (Fig. 6N), and bile duct epithelium (Fig. 6O). Isolated cells in liver also stained with the antibody (Fig. 6H). Strong expression was seen in skeletal muscle, including that of the tongue (Fig. 6C,E,F), Smooth muscle cells of the esophagus and bile duct (Fig. 6N,O) and stromal cells in the salivary gland and in craniofacial mesenchyme were also positive (Fig. 6L,M). In skeletal tissues obtained from E17.5 mouse embryos, strong immunohistochemical staining was obtained in cartilage ECM after pre-treatment of sections with hyaluronidase (Fig. 7A-C), and signal was also present in bone cells and bone matrix in the vertebral column (Fig. 7A). Cartilage of the ribs (Fig. 7B) and long bones (Fig. 7C) also had strong ADAMTSL5 signal. Negative controls done by omission of primary antibody (Fig. 6A) or preincubation of this antibody with the immunogen peptide had no corresponding signal (Fig. 7D-F).
Figure 6. Detection of ADAMTSL5 by immunofluorescence in cryosections of 14.5 and 16.5 day-old mouse embryos.
The expression pattern was essentially identical at both developmental stages. Shown are epifluorescence images of sagittal (A – K, M-O) or transverse (L) cryosections of E16.5 embryos (A – L) or E14.5 embryos (M – O). The sections were stained with rabbit anti-ADAMTSL5 (green) and nuclei were stained with DAPI (blue). Sections stained with secondary antibody only (A) did not give any signal. ADAMTSL5 was detected in the follicles of the developing whiskers (wf) (B), and in the epidermis (ep) throughout most of the body, e.g. head (B),but not in the epidermis of the back (C). ADAMTSL5 was also detected in the skeletal muscle throughout the body, including the skeletal muscle of the back (C, asterisk). ADAMTSL5 signal was observed in the nasal mucosa (D, arrowhead), the tongue musculature (E), and the skeletal muscle overlying the ribs (F). Oral epithelium of the palate (pa) (E) and tongue (E, to) were strongly stained; arrowheads indicate epithelial staining in D and E. ADAMTSL5 antibody stained the perichondrium of diverse skeletal elements such as skull bones (sk, basophenoid bone, E), ribs (F) and the bones of the inner ear (L); arrows indicate perichondrial staining. Note that these sections were not hyaluronidase treated, a procedure necessary to detect ADAMTSL5 in cartilage. In the thorax and abdomen, pericardium (pc) and mesothelium (mes) were also stained (G). In the liver, ADAMTSL5 antibody stained the liver capsule (arrowhead, H) and interspersed cells in the liver mesenchyme (arrow, H). In the lung, ADAMTSL5 was expressed in the bronchial eptihelium (arrowhead, I), and in the kidney, ADAMTSL5 was detected in the medullary tubular epithelium(J, arrowhead). The epithelium of the stomach was strongly positive (arrowhead, K). ADAMTSL5 was also detected in the sensory epithelium of the developing inner ear (L, arrowheads). ADAMTSL5 antibody stained both the epithelium and the stroma of the E14.5 salivary gland (M). Epithelial linings of the oesophagus (N), and bile duct (arrowhead in O) were stained. Note that anti-ADAMTSL5 stained both epithelium and smooth muscle layer (sm) of the esophagus (N), and bile duct (O). Smooth muscle staining in oesophagus and lung was confirmed by co-staining with a-smooth muscle actin (data not shown). Scale bars indicate 100 μm (C – H, J-M, O) or 50 μm (A, B, I, N).
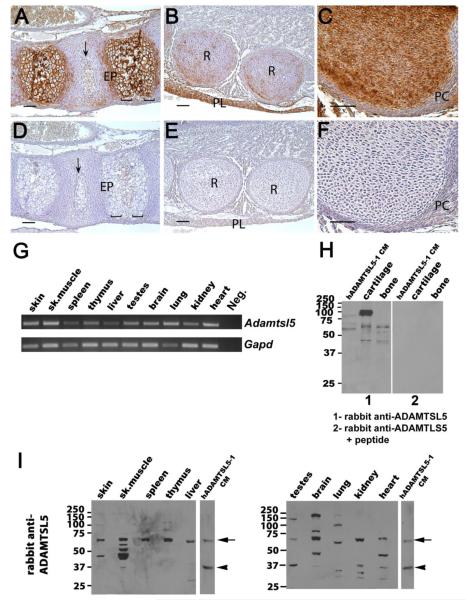
Figure 7. ADAMTSL5 mRNA and protein are widely distributed in mouse tissues.
A-F. Localization of ADAMTSL5 in E17.5 mouse cartilage, bone and intervertebral disc (IVD). Panels A-C show immunostaining in the spine (A), ribs (B) and hip (C). Panels D-F show corresponding sections stained with anti-ADAMTSL5 pre-incubated with the immunogenic peptide as a control. In the spine (A), antibody reactivity (brown color) is seen in extracellular matrix around hypertrophic chondrocytes of the endplate (EP) as well as in vertebral bone and IVD (arrow). In panel B, staining is seen in ECM of the ribs (R), as well as in the pleura (PL) lining the thoracic cavity. In panel C, note the strong staining in cartilage extracellular matrix and weaker staining in perichondrium (PC). No signal is seen in controls (D-F). Scale bars indicate 50 μm. G. Using RT-PCR, Adamtsl5 mRNA is detected in all tissues analyzed. As a negative control, PCR was performed without template (neg.), and as a positive control, PCR with Gapd primers was performed. The identity of the Adamtsl5 PCR product was confirmed by sequencing. H. Panel 1 shows western blot of adult mouse cartilage and bone extracts using recombinant ADAMTSL5 from conditioned medium (CM) of HEK293F cells as a control. Panel 2 show that pre-incubation of the rabbit polyclonal antibody with the peptide immunogen leads to loss of all immunoreactivity. I. Western blots of adult mouse tissue extracts using rabbit polyclonal anti-ADAMTSL5 antibody. Conditioned medium (CM) from stably transfected HEK293 cells was used as a positive control; vertical white lines separate lanes that were run on the same gel and regrouped for presentation. Full-length recombinant ADAMTSL5 (arrow) migrated at approximately 60 kDa with minor differences attributable to variable N-glycosylation (see also differences in the relative size of ADAMTSL5 expressed in HEK293F, COS-1 and CHO-K1 cells in Fig. 3A). The arrowhead indicates a 37 kDa fragment. Bands of similar molecular weight as the observed fragment with the rabbit antibody were present in testes, skeletal muscle, kidney and heart. Additional lower molecular weight bands, which likely correspond to different proteolytic products, are present in some tissues. In some extracts, bands with a higher molecular weight than expected are also observed, i.e., in the brain (70 kDa and 160 kDa) and in lung and testes, (90 kDa or 140 kDa). The origin of these bands is presently unknown.
2.5. ADAMTSL5 mRNA and Protein are Widely Distributed in Mouse Tissues
RT-PCR of RNA extracted from adult mouse organs demonstrated widespread Adamtsl5 mRNA expression (Fig. 7G). Western blotting of mouse tissue extracts using the rabbit polyclonal antibody supported this by detection of the expected 60 kDa species in several mouse tissues (e.g. cartilage, bone, skin, skeletal muscle, spleen, thymus, liver, testis, brain, kidney and heart, Fig. 7H,I). Although spleen and thymus primarily had the intact, 60 kDa band, additional smaller species were seen in other tissues (e.g. heart, kidney, lung, liver) Only skeletal muscle, testis, kidney and heart included a 33 kDa species (Fig. 7I) corresponding to that seen in conditioned medium of transfected cells. Unexpectedly, thymus, testes, brain and lung, cartilage and bone (Fig. 7H,I) had larger than expected species. These large species are unlikely to result from extensive glycosylation since they migrate as sharp rather than blurred bands. It is possible that these represent as yet undiscovered longer, alternatively spliced forms of ADAMTSL5. We observed loss of all reactive bands in cartilage, bone, and HEK293F-derived ADAMTSL5 upon pre-incubation of the antibody with immunogenic peptide (Fig. 7H), suggesting that these larger bands are immunologically related to ADAMTSL5. The molecular species detected by the chicken antibody were similar to those obtained with the rabbit polyclonal (data not shown), with the expected differences in the smaller reactive bands arising from the different locations of their epitopes.
2.6. ADAMTSL5 binds recombinant fibrillin-1 and fibrillin-2 and colocalizes with fibrillin microfibrils assembled by cultured fibroblasts
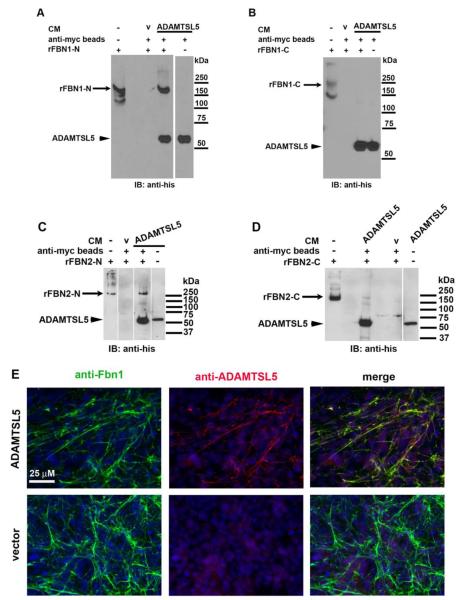
We investigated the binding of ADAMTSL5 to recombinant polypeptides representing the N- and C-terminal halves of fibrillin-1 and fibrillin-2. By affinity isolation of myc-tagged ADAMTSL5 using anti-myc beads, the N-terminal, but not the C-terminal fragment of fibrillin-1, was also isolated (Fig. 8A,B). In the absence of ADAMTSL5, neither fibrillin-1 fragment bound to the beads, suggesting that co-isolation resulted specifically from binding of ADAMTSL5 to fibrillin-1 (Fig. 8A,B, see lanes marked V (medium from cells transfected with empty vector)). In contrast, both the N- and C-terminal halves of fibrillin-2 bound to ADAMTSL5; however, the binding of the N-terminal half appeared to be much stronger than binding of the C-terminal half (Fig. 8C,D). Additional evidence for binding to fibrillins was obtained using in vitro microfibril biogenesis. In cultured fBNL cells, fibrillin microfibrils, which are heteropolymeric assemblies containing both fibrillin-1 and fibrillin-2, are deposited after several days in culture at high density. When fBNL cell culture was done in the presence of ADAMTSL5, the exogenous protein co-localized with fibrillin-1 in microfibrils (Fig. 8E). Similar co-localization was seen when the cultures were exposed to the primary antibodies without prior fixation (non-permeabilized staining, data not shown), suggesting that the stained structures were extracellular and corresponded to fibrillin-1 containing microfibrils in ECM. In cultured fBNL cells that were incubated with HEK293F cells transfected with the empty vector (i.e., a control not expressing ADAMTSL5), no signal was seen with anti-ADAMTSL5 antibody (Fig. 8E). Taken together, the findings are consistent with specific binding of ADAMTSL5 to fibrillin-1 and fibrillin-2 and to their macromolecular assemblies, i.e., fibrillin microfibrils. However, comparison of microfibril density in fBNL cell cultures grown in the presence of ADAMTSL5 or vector conditioned medium, did not identify a consistent difference (data not shown).
Figure 8. ADAMTSL5 binds to fibrillin-1 and fibrillin-2, and to fibrillin microfibrils assembled by fibroblast cultures.
A-D. Affinity pull-down with anti-myc agarose beads was done using myc-tagged ADAMTSL5 conditioned medium from HEK293F cells mixed with, or without polypeptides corresponding to fibrillin-1 N-terminal fragment rFBN1-N (panel A), fibrillin-1 C-terminal fragment rFBN1-C (panel B), fibrillin-2 N-terminal fragment (panel C; rFBN2-N) and fibrillin-2 C-terminal fragment (panel D; rFBN2-C) as indicated. Anti-His6 monoclonal antibody was used for detection by immunoblotting, since all the recombinant proteins used had C-terminal His6 tags. The N-terminal half of fibrillin-1, but not its C-terminal half, bound specifically to ADAMTSL5 (panels A and B). The N-terminal half of fibrillin-2 bound more robustly than the C-terminal half (panels C and D). Molecular weight markers are shown to the right of each panel. Vertical white lines on gels indicate removal of intervening lanes from the gels to bring together the lanes of interest. CM, conditioned medium; v, vector control (e.g. CM from vector-transfected cells E. Co-localization of ADAMTSL5 with fibrillin-1 containing microfibrils formed in fBNL cells co-cultured with ADAMTSL5 transfected or empty pcDNA vector-transfected HEK293F cells (V) for 6 days. Co-localization is evident from the yellow signal seen in merged image (top right).
Because microfibril assembly depends on, and is initiated in association with a fibronectin fibril network, such that fibrillin microfibrils co-localize extensively with fibronectin (Kinsey et al., 2008; Sabatier et al., 2009), we asked whether ADAMTSL5 bound to fibronectin. However, direct binding to fibronectin was not supported by an affinity co-isolation assay similar to that employed for ADAMTSL5-fibrillin interactions (data not shown).
3. Discussion
ADAMTSLs have emerged as an interesting class of proteins owing to the recent functional implication of ADAMTSL2, ADAMTSL4 and ADAMTSL6 in microfibril biogenesis (Gabriel et al., 2011; Hubmacher and Apte, 2011; Le Goff et al., 2011; Sengle et al., 2012; Tsutsui et al., 2010) and recent work on a nematode ADAMTSL, named muscle arm defective development-4 (MADD-4), whose closest mammalian homologs are ADAMTSL1 and ADAMTSL3. MADD-4 is thought to be a guidance protein that functionally interacts with UNC-4/DCC4, a netrin receptor, via an undefined mechanism. ADAMTSL5 is related to netrins in containing the NTR module, but it likely belongs to the first category of ADAMTSLs because of its affinity for both fibrillin-1 and fibrillin-2. Indeed, ADAMTSL5 is the first member of the ADAMTS superfamily shown to interact with fibrillin-2. There is considerable interest in microfibrils, not just as structural entities, but also as a crucial mechanism of growth factor regulation in extracellular matrix (Ramirez and Sakai, 2010). Thus, the binding of ADAMTSL5 to fibrillins is potentially significant in these contexts.
Recent genetic discoveries identified critical roles for ADAMTS-like proteins in regard to fibrillin-1 microfibrils (reviewed in (Hubmacher and Apte, 2011)). Geleophysic dysplasia and isolated ectopia lentis, which are human genetic disorders caused by loss of ADAMTSL2 and ADAMTSL4 functions respectively, are also mimicked by fibrillin-1 mutations, and these proteins, like ADAMTSL6, were shown to bind fibrillin-1. ADAMTSL4 was shown to accelerate microfibril biogenesis in vitro, and ADAMTSL6 to do so in vivo (Gabriel et al., 2011; Tsutsui et al., 2010). Although ADAMTSL5 bound to fibrillin-1, it does not appear to accelerate microfibril biogenesis by fibroblasts. The precise binding sites of ADAMTSL5 and other ADAMTSLs on fibrillin-1 have not been finely mapped, but it is possible that ADAMTSL5 might modify the action of these proteins on microfibril biogenesis via a competitive mechanism. ADAMTSL5 has two novel structural features, i.e., the absence of C-terminal TSRs, and an NTR module. NTR-modules contain 6 cysteines that typically form disulfide bonds with the following pattern: C1-C4; C2-C5; C3-C6 as well as conserved hydrophobic/basic motifs (Banyai and Patthy, 1999; Chong et al. 2002). These residues are conserved in the ADAMTSL5 NTR module, predicting a similar structure. Other than this conservation, overall sequence homology between NTR modules is weak (often only 20% or lower), although they share a similar tertiary structure. NTR modules are found in netrins, secreted frizzled related proteins (SFRPs), complement factors C3, C4, C5, pro-collagen C-proteinase enhancer proteins (PCOLCEs) and tissue inhibitors of metalloproteases (TIMPs). Netrin 1 has a well-documented role in chemoattraction or repulsion in axon guidance during neural development, whereas Netrin-4 is a component of basement membranes and has been reported to regulate neuronal outgrowth and lymphangiogenesis (Koch et al., 2000; Larrieu-Lahargue et al., 2010). SFRPs inhibit Wnt signaling by binding to Wnt-ligands, and PCOLCEs enhance the activity of the collagen type I C-proteinase BMP-1.
Notably, these functions are not mediated directly by the NTR-module in netrins, SFRPs and PCOLCEs, which is postulated to be an ancillary/helper domain (Banyai and Patthy, 1999; Weiss et al., 2010). This also suggests that the presence of the NTR-module in ADAMTSL5 is not functionally relevant to netrin/DCC signaling.
Homology between the ADAMTSL5 and TIMP-1 NTR-modules was so weak that it was undetectable by the BLAST search. The NTR-module of TIMP-1 is located at its N-terminus. It functions as a metalloprotease inhibitor, and its N-terminal cysteine is crucial for this function. The ADAMTSL5 NTR is located at the C-terminus and it not yet known if the cleaved NTR-module bears an N-terminal cysteine. We did not observe an inhibition of processing of versican (an ADAMTS5 substrate) by ADAMTS5 in the presence of ADAMTSL5 (data not shown).
The NTR modules of netrin-1 and PCOLCE 1 bind to heparin and are implicated in their association with cell surface or matrix heparan sulfate proteoglycans (HSPG) (Bekhouche et al., 2010; Kappler et al., 2000). ADAMTSL5 resembles other ADAMTS proteins in being found both within the conditioned medium as well as in pericellular matrix. The latter localization may result at least in part from the heparin-binding property of the ADAMTSL5 NTR-module, since cell-surface HSPGs provide a means of binding secreted proteins. In the case of netrin-1, a C-terminal sequence motif rich in positively charged residues was shown to mediate heparin binding (Kappler et al., 2000). The corresponding sequence in ADAMTSL5, i.e., RIRLTARR, is also polybasic and could have a similar binding property. C-terminal processing as we observed, is likely to significantly alter the localization of ADAMTSL5 by separating its heparin-binding NTR module.
ADAMTSL5 is present in several mammalian species, with high conservation of domain structure and amino acid sequence. A hypothetical protein designated BRAFLDRAFT_105750 from Branchiostoma floridae (lancelet or amphioxus) contains a C-terminal NTR-module connected to a typical ADAMTS CRD and spacer, via a proline-rich segment, but this protein does not apparently contain an N-terminal TSR. The cephalochordate amphioxus is regarded as one of the closest invertebrate relatives of the vertebrates, possessing a vertebrate-like body plan. The apparent absence of a gene encoding the TSR, CRD, and NTR-module in invertebrate model organism genomes (C. elegans, D. Melanogaster), suggests relatively recent evolution of ADAMTSL5 and a specialized function in vertebrates. Indeed, the fibrillins are not present in worms or fruit flies either (Piha-Gossack et al., 2012), further suggesting a “modern” function for ADAMTSL5
ADAMTSL5 appears to undergo both transcriptional and post-translational regulation. Alternative splicing at the 5′ end of ADAMTSL5 does not generate a protein with an alternative structure, but an mRNA that has low translational efficiency, and/or a signal peptide that does not efficiently translocate into the ER. Subsequent to translation, ADAMTSL5 undergoes N-glycosylation, although this occurred to a different extent in different cell types. Furthermore, after secretion, ADAMTSL5 can be processed to release a fragment containing the NTR-module. Indeed, western blotting of tissue extracts suggested substantial molecular diversity, with both larger than expected forms whose origins are unclear, and smaller species attributable to proteolytic cleavage. The spatial distribution of ADAMTSL5 during late mouse embryogenesis suggested that it might be present in both the epithelial and connective tissue components of organs. Intense cellular staining was consistent with in vitro analysis suggesting localization to pericellular matrix, although like many ECM proteins, its detection may be masked by other ECM components. Indeed, hyaluronidase treatment of cartilage, which removes the abundant hyaluronan present in its ECM exposed matrix staining of ADAMTSL5. Mice with targeted inactivation of Adamtsl5 are currently unavailable to assess the function of this unique and intriguing molecule.
4. Experimental Procedures
Unless specified, all reagents were from Sigma-Aldrich St. Louis, MO.
4.1.ADAMTSL5 expression plasmids, site-directed mutagenesis, transfection and stable cell lines
Human ADAMTSL5 cDNAs FLJ57655 (TESTI2022478, GenBank accession no. AK302020, designated here as ADAMTSL5-1), and FLJ16825 (UTERU2036507, GenBank accession no. AK131571, designated here as ADAMTSL5-2) representing distinct ADAMTSL5 transcripts in the vector pME18SFL3, were purchased from the National Institute of Technology and Evaluation Biological Resource Center (Chiba, Japan). Using site-directed mutagenesis (QuikChange site-directed mutagenesis kit, Stratagene, La Jolla, CA), an Eco RI recognition site was introduced at the 5′ end of these clones, and the stop codon was replaced with a Sal I recognition site. The insert of each clone was excised by digestion with Eco RI and Sal I endonucleases (New England Biolabs, Ipswich, MA) and ligated into Eco RI + Xho I digested pcDNA3.1/myc-His A (Invitrogen, Thousand Oaks, CA) for expression with C-terminal myc+His6 tags.
For generation of V5-tagged ADAMTSL5, full length human ADAMTSL5-1 cDNA lacking the stop codon was amplified from bone cDNA using primers incorporating an Eco RI restriction site at the 5′ end and an Xba I restriction site at the 3′ end. The PCR product was cloned into pCR2.1 and verified by sequencing prior to cloning into the expression vector pcDNA6/V5-HIS (Stratagene) using Eco RI and Xba I. Site-directed mutagenesis was used to mutate Asn208 to Ala in this construct.
The 5′ untranslated region of hADAMTSL5-2 (accession no. AK131571) contained an additional upstream ATG codon which was not in the ADAMTSL5 open reading frame (ORF). To determine if translation at this ATG (nucleotides 59-61 of AK131571, …ccgcgcagccgATGacattctgag…) inhibited translation of the cognate ADAMTSL5 ORF, it was mutated to ATA. Primers used are available on request.
ADAMTSL5 expression plasmids were transiently transfected into HEK293F cells or HEK293H cells (for the V5-tagged construct) ATCC, Manassas, VA) using FuGENE 6 (Roche Applied Science, Indianapolis, IN) or Lipofectamine 2000 (Invitrogen, Thousand Oaks, CA) respectively. After transfection, cells were cultured in serum-free (SF) 293 Medium II (Gibco) containing 1% antibiotics and 4 mM L-Glutamine. Transfection with the empty pcDNA3.1/myc-His A vector was used as a control for protein expression. HEK293F cells stably expressing ADAMTSL5 were obtained using selection pressure of 1 mg/ml G418.
4.2. RT-PCR analysis of Adamtsl5 expression in mouse tissues
For RNA extraction, 50–100 mg mouse tissue was homogenized in 1 ml TRIZOL reagent for RNA extraction (Invitrogen, Thousand Oaks, CA) using the Ultra-Turax T25 (IKA Works, Inc., Wilmington, NC). 2 μg RNA was reversed transcribed using the SuperScriptTM First Strand Synthesis Kit (Invitrogen, Thousand Oaks, CA) according to manufacturer’s instructions. Adamtsl5 RT-PCR was performed with 0.5 μl cDNA using the following oligonucleotide primers: forward primer, 5′ ACT TGG ACG TAT TGC CTT GGG 3′; reverse primer, 5′ TCC GGG AAG TCG GAC ACA TT 3′. These primers spanned exon junctions to eliminate potential amplification of contaminating genomic DNA. PCR products were analyzed by agarose gel electrophoresis and correct amplification of the target sequence was confirmed by nucleotide sequencing. As a control for the amount of template cDNA in each PCR reaction, Gapd primers – forward primer, 5′ TCT GAG GGC CCA CTG AAG 3′ and reverse primer, 5′ AGG GTT TCT TAC TCC TTG GAG G 3′ were used. The negative control PCR utilized distilled water instead of the reverse-transcribed RNA.
4.3. RNA in situ hybridization
An Adamtsl5 cDNA ((BC034843 - Open Biosystems, Thermo-Fisher Scientific) was digested with EcoR I and Xho I to release a 638 bp fragment that was cloned into the corresponding sites of pBluescript (−) (Stratagene, Thousand Oaks, CA). T7 and T3 RNA polymerases were used to generate digoxigenin-UTP labeled antisense and sense probes by in vitro transcription using the plasmid linearized with EcoR I or Xho I in conjunction with RNA polymerases T7 and T3 respectively (reagents from Roche Applied Science, Indianopolis, IN). Transverse cryosections through the heads of embryos of 16.5 days gestational age (E16.5) were used for hybridization to the transcribed probes essentially as previously described (Holmes and Niswander, 2001), except that sections were treated with 10mg/ml Proteinase K (Roche Applied Science) for 1 minute prior to hybridization.
4.4. Antibodies
Rabbit polyclonal antibody was generated to an ADAMTSL5 peptide NH2-QEPNPGIEFEFWLPRERYSPFQARV-COOH (residues 299-322 of human ADAMTSL5-1) with an N-terminal Cys residue added for conjugation to keyhole limpet hemocyanin prior to immunization (Alpha Diagnostics International, San Antonio, TX). Immune sera were affinity-purified against the immobilized immunogen peptide. Chicken polyclonal ADAMTSL5 antibody raised to a 17-amino acid peptide near the C-terminus of ADAMTSL5 was purchased from ProSci Incorporated (Poway, CA; catalog no. 5687). Mouse monoclonal antibody to the myc tag (clone 9E10) was from the Lerner Research Institute Hybridoma Core. Monoclonal antibody to human fibrillin-1 (clone 11C1.3) was purchased from Millipore (E. Billerica, MA) and anti-V5 epitope from ABD Serotec (Raleigh, NC). Secondary antibodies (AlexaTM 488, AlexaTM 568, CyTM3 or peroxidase-tagged) were purchased from Invitrogen (Thousand Oaks, CA) or Jackson ImmunoResearch Laboratories (West Grove, PA).
4.5. Analysis of mouse tissue extracts
For protein extraction, tissues obtained from adult mice were homogenized with the Ultra-Turax T25 (Ika-Works, Inc., Wilmington, NC) in 5-volumes of ice-cold lysis buffer (Tris-buffered saline, 1% Triton X-100, 1x EDTA-free complete protease inhibitor (Roche Applied Science, Indianapolis, IN), 2 mM EDTA pH 7.4). After centrifuging at 14,000 rpm for 30 min at 4°C, the pellet was discarded, and the supernatant was stored at −80°C. 15 μl protein extract was mixed with Laemmli sample buffer, reconstituted to 5% (v/v) 2-mercaptoethanol and boiled for 5 min. Protein electrophoresis in 10% SDS-polyacrylamide gels was followed by western blotting to polyvinylfluoridine (PVDF) membrane using rabbit anti-ADAMTSL5 at 1:500-1:2000 dilution (overnight at 4°C) and horseradish peroxidase-conjugated goat-anti-rabbit polyclonal antibody at 1:10,000 (room temperature for 1 hr). Antibody binding was detected using chemiluminescence (HygloTM, Denville Scientific Inc., Metuchen, NJ). The membranes were stripped (Blot Restore Membrane Rejuvenation Kit, Millipore) and re-probed with polyclonal chicken anti-human ADAMTSL5 antibody at 1:1000 dilution.
4.6. Analysis of transfected ADAMTSL5 By immunoblotting and immunofluoresence
Conditioned media or cell lysate were prepared for western blotting with anti-ADAMTSL5 or anti-myc (1:2000 dilution) as described above. For determination of N-glycosylation, conditioned medium or lysate of stably transfected cells was treated with PNGase F (New England Biolabs, Ipswich, MA) prior to electrophoresis as previously described (Hirohata et al., 2002; Somerville et al., 2003). Conditioned medium of transfected HEK293F cells was also analyzed under non-reducing conditions in which sample was prepared without addition of 2-mercaptoethanol.
For immunofluorescence to ascertain extracellular distribution, COS-1 cells (ATCC, Manassas, VA) were transiently transfected as described above followed by staining of non-permeabilized, unfixed cells, essentially as previously described (Hirohata et al., 2002) with the following differences/ modifications: COS-1 cells were seeded into 12 wells on 15 mm round coverslips (Thermo Fisher Scientific, Pittsburgh, PA) pretreated with poly-L-Lysine. Cells were incubated for 15 min on ice in DMEM containing 1% FBS, and subsequently for 1h with anti-myc or anti-ADAMTSL5 antibody diluted in DMEM containing 1% FBS (rabbit anti-ADAMTSL5, 1:200; mouse anti-myc antibody 9E10, 1:200). Secondary antibodies from (Invitrogen, Thousand Oaks, CA) were used at the following dilutions: anti-rabbit IgG antibody conjugated to Alexa 568, 1:500; anti-mouse IgG antibody conjugated to Alexa 488, 1:500. Images were taken with an epifluorescence microscope (Leica Microsystems Inc., Bannockburn, IL) or a confocal microscope (TCS SP2, Leica). In some experiments, the cells were permeabilized by fixation with methanol prior to immunostaining. Staining of cells transfected with the empty vector served as a negative control.
4.7. Section immunohistochemistry and immunofluorescence
Mouse embryos from timed pregnancies, and tissues from adult C57/Bl6 mice were obtained under a protocol approved by the Cleveland Clinic Institutional Animal Care and Use Committee. These were fixed for 45 min-1h in 4% paraformaldehyde prior to paraffin embedding. For immunohistochemistry, E17.5 embryos were decalcified using 14% EDTA in PBS. For cryo-embedding, tissues were fixed and stored at −20°C in methanol until use or were directly frozen. After methanol fixation, tissues were washed several times with PBS, and soaked overnight in 30% sucrose (methanol fixed tissues: 30% sucrose in PBS; tissues not fixed with methanol: 30% sucrose in distilled water) and snap-frozen by immersion into isopentane pre-cooled to −80°C. 10–14 μm thick cryostat sections on SuperfrostR Plus slides (Thermo Fisher) were air-dried and stored at −80°C until use. After incubation for over 30 min at room temperature in blocking solution (PBS with 3% normal sheep serum) sections were incubated 2h at room temperature or overnight at 4°C with rabbit anti-ADAMTSL5 antibody diluted 1:300 in blocking solution. After rinsing sections with PBS (3×5 min), sections were incubated for 45 min with Alexa 488 or Alexa 568 conjugated secondary antibodies (Invitrogen) diluted 1:500 in blocking solution. Sections were rinsed and mounted in Prolong GoldR anti-fading reagent with DAPI (Invitrogen). 7 mm-thick paraffin sections were dewaxed with Histoclear, rehydrated, incubated with 0.3% (v/v) H2O2 in methanol for 30 min at room temperature to block endogenous peroxidases, serum-blocked and heat treated for 30 min at 60°C in 10 mM Na-Citrate, pH 6.0, 0.05% Tween-20 (v/v). They were subsequently treated with 0.2% (w/v) hyaluronidase in PBS at 37°C for 30 min and incubated with the ADAMTSL5 polyclonal antibody as above. Following incubation with the secondary-horseradish peroxidase-conjugated antibody, an indirect immunoperoxidase method was used. Stained slides were viewed using a Leica DMR microscope and photographed using a Q Imaging Retiga EX CCD camera (Surrey, BC, Canada) and ImagePro software.
4.8. Analysis of heparin binding
HEK293 cells stably expressing myc-His6 tagged hADAMTSL5-1 were grown to confluence in DMEM containing 10% FBS and antibiotics, washed 3 times with PBS, and were incubated for 2 days with serum-free SF293 medium II (Gibco) containing 1% antibiotics and 4 mM L-glutamine. The medium was centrifuged for 5 min at 1500 rpm. Complete Protease Inhibitor tablets, Mini, EDTA free (Roche Applied Science) were added to a final concentration of 0.7X, the pH was adjusted to pH 7–7.2 and conditioned medium was stored at −80°C until use. 0.3 ml heparin-agarose beads (0.6 ml of 50% solution, Pierce catalog no. 20207) were equilibrated with 20 mM Tris, 150 mM NaCl, pH 7.4 (wash buffer) by washing thrice, mixed with 25 ml conditioned medium and incubated for 2 hours at 4°C with rotation. After centrifugation (5 min at 1500 rpm), the beads were loaded into a column (Poly-Prep Chromatography column, Bio-Rad catalog no. 731-1550). The column was washed thrice with wash buffer and bound protein was step-eluted with 20 mM Tris, pH 7.4 containing increasing amounts of NaCl (0.3M, 0.5M, 1M and 1.5 M). For each salt concentration, a 5-fold column volume was applied, and eluate was collected in 1 ml fractions. 30 μl of each fraction was analyzed by 10% reducing SDS-PAGE and western blot using anti-myc or rabbit anti-ADAMTSL5 antibody as described above.
4.9. Analysis of the interaction of ADAMTSL5 with fibrillin-1 and fibrillin-2 by immunoaffinity analysis
Conditioned 293 SFM II medium was collected from HEK293F cells stably expressing myc-His6 tagged ADAMTSL5 and cultured at 37 °C in 8% CO2. After adjustment to pH 7.2 and addition of phenylmethanesulfonylfluoride (1mM) and Triton X-100 (0.1% v/v), the medium was incubated overnight with anti-myc conjugated agarose beads at 4 °C. Fibrillin-1 and fibrillin-2 polypeptides were previously described (Jensen et al., 2001; Lin et al., 2002). The beads were washed thrice with 0.1% Triton X-100 in PBS, and mixed with 200 μL of 5 μg/mL His6 tagged fibrillin-1 polypeptides rFBN1-N or rFBN1-C representing the N- and C-terminal halves of human fibrillin-1, in Tris-buffered saline with 0.1% Triton X-100 containing EDTA-free, complete mini-protease inhibitor cocktail (Roche) at RT for three hours. The beads were washed thrice with 0.1% Triton X-100 in PBS and bound material was eluted by mixing the beads with increasing salt concentrations. As a final step, Laemmli sample buffer with 5% 2-mercaptoethanol was added to the beads followed by heating to 100°C for 5 min. The samples were analyzed by 7.5% SDS-PAGE, and western blotting using monoclonal anti-his antibody (R&D) to detect both ADAMTSL5 and fibrillin-1. Medium collected from HEK293F cells stably transfected with the empty vector was processed in parallel as a negative control. An identical method was used to seek interactions between ADAMTSL5 and fibrillin-2 polypeptides rFBN2-N and rFBN2-C representing N- and C-terminal halves of human fibrillin-2.
4.10. In vitro fibrillin microfibril deposition and immunocytochemistry
3.2 ×104 fibroblasts isolated from fetal bovine nuchal ligament (fBNL cells, < 10 passages) and 2.2 ×104 HEK293F cells expressing ADAMTSL5 or pcDNA3.1 vector were co-cultured in 8-chamber slides. The cells were cultured with DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin for six days with a change of medium every 72 h. Cells were fixed for 7 min with cold methanol on ice, air-dried, blocked 60 minutes with 5% normal goat serum in PBS, and incubated with anti-fibrillin-1 monoclonal antibody (clone 11c.3, MAB1919, Millipore; diluted 1:200) overnight at 4 °C to detect fibrillin-1 made by the fBNL cells and in co-localization experiments, with rabbit anti-ADAMSL5 at 1:300. Goat anti-mouse Alexa 488 (Invitrogen) and Alexa 568 goat-anti-rabbit were used as the secondary antibodies at 6mg/mL. Nuclei were stained with DAPI (Prolong Anti-Fading Reagent with DAPI, Invitrogen).
Highlights.
ADAMTSL5 is a novel member of the ADAMTS superfamily of secreted proteins
Alternative splicing at the 5′ end affects translational efficiency, but not the sequence of mature ADAMTSL5 protein.
In transfected cells, ADAMTSL5 localizes to the cell surface and sub-cellular extracellular matrix.
ADAMTSL5 binds to heparin, fibrillin-1, fibrillin-2, and fibrillin microfibrils
Acknowledgements
This work was supported by National Institutes of Health awards AR53890 (to SSA), R01AR055957 (to J.F.) and R01DE01870 (to RA). HB was supported by a post-doctoral fellowship from National Institutes of Health T32HL007914-08 (Training Program in Vascular Biology and Pathology, Principal Investigator: Edward Plow).
Abbreviations
- ADAMTSL5
A disintegrin-like and metalloprotease domain with thrombospondin type 1 motifs like 5
- ECM
extracellular matrix
- TSR
thrombospondin type 1 repeat
- NTR module
netrin-like module
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahram D, Sato TS, Kohilan A, Tayeh M, Chen S, Leal S, Al-Salem M, El-Shanti H. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am J Hum Genet. 2009;84:274–278. doi: 10.1016/j.ajhg.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader HL, Ruhe AL, Wang LW, Wong AK, Walsh KF, Packer RA, Mitelman J, Robertson KR, O’Brien DP, Broman KW, Shelton GD, Apte SS, Neff MW. An ADAMTSL2 founder mutation causes Musladin-Lueke Syndrome, a heritable disorder of beagle dogs, featuring stiff skin and joint contractures. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyai L, Patthy L. The NTR module: domains of netrins, secreted frizzled related proteins, and type I procollagen C-proteinase enhancer protein are homologous with tissue inhibitors of metalloproteases. Protein Sci. 1999;8:1636–1642. doi: 10.1110/ps.8.8.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhouche M, Kronenberg D, Vadon-Le Goff S, Bijakowski C, Lim NH, Font B, Kessler E, Colige A, Nagase H, Murphy G, Hulmes DJ, Moali C. Role of the netrin-like domain of procollagen C-proteinase enhancer-1 in the control of metalloproteinase activity. J Biol Chem. 2010;285:15950–15959. doi: 10.1074/jbc.M109.086447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner DA, Meisler MH. TSRC1, a widely expressed gene containing seven thrombospondin type I repeats. Gene. 2003;307:23–30. doi: 10.1016/s0378-1119(03)00423-2. [DOI] [PubMed] [Google Scholar]
- Gabriel LA, Wang LW, Bader H, Ho JC, Majors AK, Hollyfield JG, Traboulsi EI, Apte SS. ADAMTSL4, a secreted glycoprotein widely distributed in the eye, binds fibrillin-1 microfibrils and accelerates microfibril biogenesis. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbrecht BV, Dowd KA, Barfield RW, Longo PA, Leahy DJ. Netrin binds discrete subdomains of DCC and UNC5 and mediates interactions between DCC and heparin. J Biol Chem. 2003;278:32561–32568. doi: 10.1074/jbc.M302943200. [DOI] [PubMed] [Google Scholar]
- Hall NG, Klenotic P, Anand-Apte B, Apte SS. ADAMTSL-3/punctin-2, a novel glycoprotein in extracellular matrix related to the ADAMTS family of metalloproteases. Matrix Biol. 2003;22:501–510. doi: 10.1016/s0945-053x(03)00075-1. [DOI] [PubMed] [Google Scholar]
- Hirohata S, Wang LW, Miyagi M, Yan L, Seldin MF, Keene DR, Crabb JW, Apte SS. Punctin, a novel ADAMTS-like molecule (ADAMTSL-1) in extracellular matrix. J Biol Chem. 2002;22:22. doi: 10.1074/jbc.M109665200. [DOI] [PubMed] [Google Scholar]
- Holmes G, Niswander L. Expression of slit-2 and slit-3 during chick development. Dev Dyn. 2001;222:301–307. doi: 10.1002/dvdy.1182. [DOI] [PubMed] [Google Scholar]
- Hubmacher D, Apte SS. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin-1: a novel mechanism influencing microfibril assembly and function. Cell Mol Life Sci. 2011;68:3137–3148. doi: 10.1007/s00018-011-0780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen SA, Reinhardt DP, Gibson MA, Weiss AS. Protein interaction studies of MAGP-1 with tropoelastin and fibrillin-1. J Biol Chem. 2001;276:39661–39666. doi: 10.1074/jbc.M104533200. [DOI] [PubMed] [Google Scholar]
- Kappler J, Franken S, Junghans U, Hoffmann R, Linke T, Muller HW, Koch KW. Glycosaminoglycan-binding properties and secondary structure of the C-terminus of netrin-1. Biochem Biophys Res Commun. 2000;271:287–291. doi: 10.1006/bbrc.2000.2583. [DOI] [PubMed] [Google Scholar]
- Kinsey R, Williamson MR, Chaudhry S, Mellody KT, McGovern A, Takahashi S, Shuttleworth CA, Kielty CM. Fibrillin-1 microfibril deposition is dependent on fibronectin assembly. J Cell Sci. 2008;121:2696–2704. doi: 10.1242/jcs.029819. [DOI] [PubMed] [Google Scholar]
- Koo BH, Le Goff C, Jungers KA, Vasanji A, O’Flaherty J, Weyman CM, Apte SS. ADAMTS-like 2 (ADAMTSL2) is a secreted glycoprotein that is widely expressed during mouse embryogenesis and is regulated during skeletal myogenesis. Matrix Biol. 2007;26:431–441. doi: 10.1016/j.matbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Kramerova IA, Kawaguchi N, Fessler LI, Nelson RE, Chen Y, Kramerov AA, Kusche-Gullberg M, Kramer JM, Ackley BD, Sieron AL, Prockop DJ, Fessler JH. Papilin in development; a pericellular protein with a homology to the ADAMTS metalloproteinases. Development. 2000;127:5475–5485. doi: 10.1242/dev.127.24.5475. [DOI] [PubMed] [Google Scholar]
- Kutz WE, Wang LW, Bader HL, Majors AK, Iwata K, Traboulsi EI, Sakai LY, Keene DR, Apte SS. ADAMTS10 Protein Interacts with Fibrillin-1 and Promotes Its Deposition in Extracellular Matrix of Cultured Fibroblasts. J Biol Chem. 2011;286:17156–17167. doi: 10.1074/jbc.M111.231571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, Brady AF, Cordier MP, Devriendt K, Genevieve D, Kiper PO, Kitoh H, Krakow D, Lynch SA, Le Merrer M, Megarbane A, Mortier G, Odent S, Polak M, Rohrbach M, Sillence D, Stolte-Dijkstra I, Superti-Furga A, Rimoin DL, Topouchian V, Unger S, Zabel B, Bole-Feysot C, Nitschke P, Handford P, Casanova JL, Boileau C, Apte SS, Munnich A, Cormier-Daire V. Mutations in the TGFbeta Binding-Protein-Like Domain 5 of FBN1 Are Responsible for Acromicric and Geleophysic Dysplasias. Am J Hum Genet. 2011;89:7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goff C, Morice-Picard F, Dagoneau N, Wang LW, Perrot C, Crow YJ, Bauer F, Flori E, Prost-Squarcioni C, Krakow D, Ge G, Greenspan DS, Bonnet D, Le Merrer M, Munnich A, Apte SS, Cormier-Daire V. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat Genet. 2008;40:1119–1123. doi: 10.1038/ng.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G, Tiedemann K, Vollbrandt T, Peters H, Batge B, Brinckmann J, Reinhardt DP. Homo- and heterotypic fibrillin-1 and -2 interactions constitute the basis for the assembly of microfibrils. J Biol Chem. 2002;277:50795–50804. doi: 10.1074/jbc.M210611200. [DOI] [PubMed] [Google Scholar]
- Piha-Gossack A, Sossin W, Reinhardt DP. The evolution of extracellular fibrillins and their functional domains. PLoS One. 2012;7:e33560. doi: 10.1371/journal.pone.0033560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Rifkin DB. Extracellular microfibrils: contextual platforms for TGFbeta and BMP signaling. Curr Opin Cell Biol. 2009;21:616–622. doi: 10.1016/j.ceb.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez F, Sakai LY. Biogenesis and function of fibrillin assemblies. Cell Tissue Res. 2010;339:71–82. doi: 10.1007/s00441-009-0822-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, Lehle L. Structural requirements for protein N-glycosylation. Influence of acceptor peptides on cotranslational glycosylation of yeast invertase and site-directed mutagenesis around a sequon sequence. Eur J Biochem. 1989;181:525–529. doi: 10.1111/j.1432-1033.1989.tb14755.x. [DOI] [PubMed] [Google Scholar]
- Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol Biol Cell. 2009;20:846–858. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengle G, Tsutsui K, Keene DR, Tufa SF, Carlson EJ, Charbonneau NL, Ono RN, Sasaki T, Wirtz MK, Samples JR, Fessler LI, Fessler JH, Sekiguchi K, Hayflick SJ, Sakai LY. Microenvironmental regulation by fibrillin-1. PLoS Genet. 2012;8:e1002425. doi: 10.1371/journal.pgen.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville RP, Longpre JM, Jungers KA, Engle JM, Ross M, Evanko S, Wight TN, Leduc R, Apte SS. Characterization of ADAMTS-9 and ADAMTS-20 as a distinct ADAMTS subfamily related to Caenorhabditis elegans GON-1. J Biol Chem. 2003;278:9503–9513. doi: 10.1074/jbc.M211009200. [DOI] [PubMed] [Google Scholar]
- Tsutsui K, Manabe RI, Yamada T, Nakano I, Oguri Y, Keene DR, Sengle G, Sakai LY, Sekiguchi K. A disintegrin and metalloproteinase with thrombospondin motifs-like-6 (ADAMTSL-6) is a novel extracellular matrix protein that binds to fibrillin-1 and promotes fibrillin-1 fibril formation. J Biol Chem. 2010;285:4870–4882. doi: 10.1074/jbc.M109.076919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LW, Dlugosz M, Somerville RP, Raed M, Haltiwanger RS, Apte SS. O-fucosylation of thrombospondin type 1 repeats in ADAMTS-like-1/punctin-1 regulates secretion: implications for the ADAMTS superfamily. J Biol Chem. 2007;282:17024–17031. doi: 10.1074/jbc.M701065200. [DOI] [PubMed] [Google Scholar]
- Wang LW, Leonhard-Melief C, Haltiwanger RS, Apte SS. Post-translational modification of thrombospondin type-1 repeats in ADAMTS-like 1/punctin-1 by C-mannosylation of tryptophan. J Biol Chem. 2009;284:30004–30015. doi: 10.1074/jbc.M109.038059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T, Ricard-Blum S, Moschcovich L, Wineman E, Mesilaty S, Kessler E. Binding of procollagen C-proteinase enhancer-1 (PCPE-1) to heparin/heparan sulfate: properties and role in PCPE-1 interaction with cells. J Biol Chem. 2010;285:33867–33874. doi: 10.1074/jbc.M110.141366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;13:13. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]