Abstract
HSV, a neurotropic virus, travels within neuronal processes by fast axonal transport. During neuronal infection HSV travels retrograde from the sensory nerve terminus to the neuronal cell body, where it replicates or enters latency. During replication HSV travels anterograde from the cell body to the nerve terminus. Postmortem studies find a high frequency of HSV DNA in the trigeminal ganglia as well as the brain. Studies correlating HSV with Alzheimer’s disease (AD) have been controversial. Here we review clinical evidence supporting such a link. Furthermore, the author describes experimental data showing physical interactions between nascent HSV particles and host transport machinery implicated in AD. The author concludes that the complexity of this relationship has been insufficiently explored, although the relative ease and nontoxicity of a potential anti-HSV treatment for AD demands further study.
Keywords: Aβ, Alzheimer’s disease, amyloid precursor protein, APP, cerebrospinal fluid, CNS, encephalitis, fast axonal transport, HSV, senile plaques, trigeminal ganglion, trigeminal nucleus
HSV-1 and HSV-2 initially infect epithelial cells in mucous membranes and secondarily enter local sensory nerve terminals. In the case of HSV-1, initial infection typically occurs in the lip, but can also happen in the mucous membranes of the eye and, rarely, in the nose. HSV-2 typically infects the mucosa of the reproductive tract and also secondarily enters local nerve termini. Although HSV-1 and -2 have tendencies for different anatomic locations, this appears to be a tendency and not a requirement for infectivity. The two types are so similar that PCR primers and antibodies commonly used to detect them clinically do not distinguish between them, and either may cause significant acute disease. Here we will refer to types where possible. If studies did not use type-specific probes, we will refer to the two types as ‘HSV’.
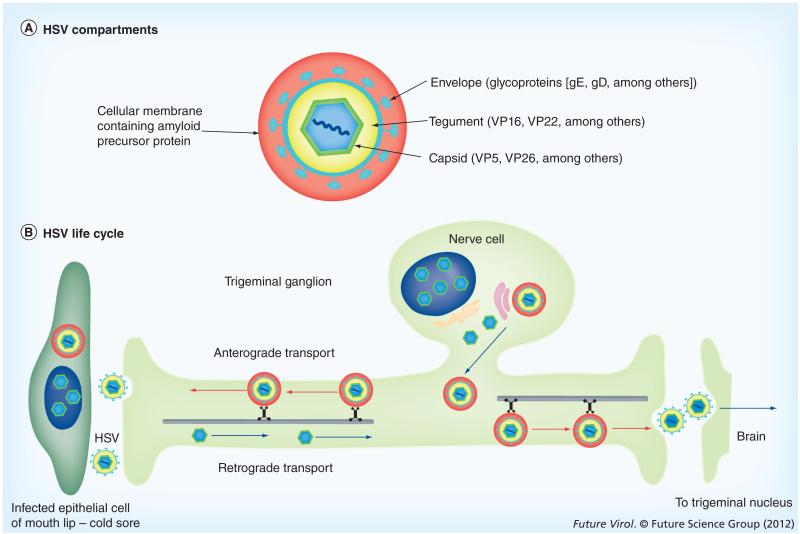
HSV-1 is a dsDNA virus with a genome of 150–152 kb containing 89 open reading frames encoding 87 proteins [1]. Alternative splicing and differing transcriptional start–stop sites may produce more variety in proteins synthesized from the viral genome. The infectious particle is an enveloped virus with four concentric compartments: the DNA core packaged in an icosahedral proteinaceous capsid surrounded by an amorphous tegument, and enveloped in a glycoprotein-rich lipid bilayer (Figure 1) . Both HSV-1 and -2 display neurotropism, with HSV-1 most common in the trigeminal ganglion (TG) containing the cell bodies of the sensory nerves for the lips, eyes, face and meninges, and HSV-2 in the sacral dorsal ganglion containing the cell bodies for the sensory nerves of the perineum and reproductive tract.
Figure 1. HSV structure and life cycle.
(A) HSV compartments: this schematic depicts the four concentric compartments of the infective particle encased in a fifth compartment provided by a cellular membrane system found encircling cytoplasmic viral particles during productive infections and containing amyloid precursor protein. The capsid is approximately 135 nm in diameter and the whole assemblage may be as large as 350–400 nm. (B) HSV life cycle: here we outline the process the virus follows to infect the trigeminal ganglion and progress into the trigeminal nucleus in the brain. Note that the trigeminal ganglion cells project two processes: one to the lip, the other to the brain. Virus traveling in a single neuron can move within these processes to go either to the lip or into the brain. The route from the lip to the brain can be >10 cm in length.
Once inside the sensory process, viral particles travel retrograde towards the neuronal cell’s nucleus in either the TG or sacral dorsal-root ganglia, where virus may become latent or replicate (Figure 1) . Newly synthesized virus travels anterograde within the sensory process to return to the mucosal membrane, thereby causing the recurrent herpes cold sore, or ‘fever blister’. Infection of mucosal epithelial cells results in lysis of the cell, termed a ‘lytic’ infection; whereas infection of neurons most commonly results in latency with periodic reactivations, upon which infective viral particles are produced [2,3]. Viral genomes persist in a nonlinear, episomal, nucleosomal form in infected trigeminal neurons [2].
HSV infection is incurable: the viral genome persists in a latent form as non-integrated episomal dsDNA in the nucleus of the infected neuron for the life of the neuron. Upon reactivation the virus may be shed asymptomatically or may cause acute or chronic disease. Thus, infection may or may not produce disease, and reactivations may produce a range of disease severity. In many infected individuals, blisters on the lip recur at odd intervals, often with a chronicity of more than once a year. Each round of blisters arises from reactivation of the viral genome in the latently infected neuron and probably results in the infection of additional neurons in the ganglion as well. Hence many infections are ‘chronic’ in the sense that reactivations producing acute sores recur over the lifetime of the host. When the eye is involved, repeated herpetic lesions result in blindness [4]. Such chronic, recurrent eruptions represent the third leading cause of infectious blindness worldwide.
Sensory neurons are bipolar in that they project two processes, one to the body surface and the other into the CNS. TG neurons project their second processes into the trigeminal nucleus (TN) located in the brainstem (Figure 1). This second process provides a direct route for HSV into the CNS from infected neurons in the TG, or even during entry from the lip or other facial mucous membranes [5,6]. From the TN in the hindbrain, neuronal projections go to the thalamus and from there to the sensory cortex. Thus, HSV could move throughout the brain and could play pathological roles in various CNS disorders, including Alzheimer’s disease (AD). Recent evidence links HSV-1 at a cellular level to AD. Emerging HSV-1 particles interact with amyloid precursor protein (APP), the parent protein that, when hydrolyzed, produces the Aβ that forms the major component of Alzheimer’s plaques, and to induce maldistribution of APP in HSV-infected cells [7].
Major questions remain as to any patho logical role of HSV in AD, and a cause-and-effect relationship remains controversial. Critical questions are:
-
■
Is HSV DNA, evidence of latency, found in the brain?
-
■
Does asymptomatic, subclinical replication of HSV occur in the CNS, or is CNS infection only acute as in encephalitis, or quiescent as in latency?
-
■
Does subclinical viral replication in the CNS have consequences? In the case of chronic activity, are the pathological effects transient or persistent?
-
■
How might HSV reach the brain?
-
■
How well does the current evidence of HSV and AD support ‘Koch’s postulates’, a traditional method to determine cause–effect relationships of pathologic microorganisms and disease?
Here epidemiologic reports are reviewed, describing some current experimental in vitro evidence, and providing provocative ideas relevant to each of these questions.
HSV is prevalent worldwide
HSV-1 and -2 are endemic in the USA. The prevalence of HSV-1 can be determined by testing serum for antibodies to HSV-1 [8,9], by the detection of HSV-1 DNA in cerebrospinal fluid (CSF) [10], or in TG acquired at autopsy [11]. Such studies have revealed endemic infection rates of 31% in children aged 6–14, rising to 49% in adults aged 14–49, and to a high of 80–90% in the population over 65. In one study of 40 autopsied TGs, HSV-1 sequences were amplified from DNA or RNA extracted from 81% of TGs from demented subjects, and 74% of controls [11]. In another study of younger individuals (average age 38.6 years), HSV-1 or -2 genomes were found by PCR in 53% of TGs [12], and in another study, quantitative PCR detected HSV genomes in 89% of 147 American subjects, with viral load varying from a million copies per sample to almost undetectable [13].
HSV DNA is detected in the brain
HSV-1 and -2 are both found in a high percentage of brains at autopsy in England, Finland and Japan [14,15-18]. In England, 14 out of 14 autopsied brains were positive for HSV-1 tyrosine kinase DNA [17], and 23 out of 36 samples were positive in a subsequent study by the same group [14]. Another English group also found a high prevalence of HSV DNA in brain, with 77 out of 105 subjects (73.3%) found to be positive for HSV using primers based on the HSV-1 gene, gD [18]. Both HSV-1 and -2 may be detected by the tyrosine kinase and gD primers in these studies. In Finland, nested primers specific for HSV-1 were used, which detected 18 out of 114 subjects as being positive for HSV-1 [15], while in Japan specific primers detected HSV-1 in four out of six brains. Thus, HSV-1 DNA, evidence of latent infection, is found in the CNS of a large proportion of the population at death. HSV-2 DNA has also been found in brain autopsy series, but with lower incidence (13–20% positivity) [19].
Presence of HSV has been correlated with several diseases of the CNS, including AD [20], epilepsy [21] and multiple sclerosis [22]. Viral DNA was detected in the senile plaques of AD brains [20], virus has also been found to be present in epileptic seizure foci [21], and can be cultured from the plaques of multiple sclerosis [22].
Statistically significant correlations between HSV and AD were noted by one group [14,17,23]. By contrast, Hemling et al., studying Finnish subjects (n = 34 with AD and 40 controls), reported lower overall rates of brain HSV infection than in England and failed to find statistically significant correlation between AD and detection of HSV [15]. A study from Japan of three familial and two sporadic post-mortem brains of subjects with AD, and six with non-Alzheimer’s supports the Itzhaki-Jamieson reports, demonstrating HSV in plaques and brains in 100% of both types of AD, but in only one out of five brains lacking Aβ pathology [16]. A question raised by this Japanese report is whether AD may itself induce HSV reactivation, which could result in a downward spiral.
The high prevalence of HSV latent infections in the brain and the lower incidence of AD raises the major question of what other factors may lead to AD in an HSV-infected host (see Box 1). The most likely variables influencing the effect of infection are the viral load and the frequency of reactivations with viral production. In addition, the effect of HSV reactivations on the brain and consequent symptomatology may correlate with the anatomical location of infected neurons, their connections and function, and the individual immune response of the patient. Several susceptibility genes for HSV have recently been reported, including TLR3 for both type 1 and 2 [24]; MHC class I allotypes (B*18, C*15 and the group of alleles encoding A19), the high-affinity receptor/ligand pair KIR2DL2/HLA-C1, and the CD16A-158V/F dimorphism for HSV-1 [25]; SNPs in GRIN2b (encoding the NMDA receptor isoform) for HSV-2 [26]; polymorphisms in Fas, the cell death receptor, for women in South Africa with HSV-2 [27]; and two SNPs within chromosome 21 open reading frame 91 for HSV-1 [28].
Box 1. Factors and variables affecting HSV–Alzheimer’s disease correlations.
Host factors
-
■
Age of primary infection
-
■
Location of primary infection
-
■
Location of infected neurons in the brain
-
■
Host immune function and local brain inflammatory response
-
■
Host genotype (ApoE allele, TLR3 SNPs, MHC haplotype and so on)
-
■
Environmental exposures (i.e., aspirin use or other drugs)
-
■
Gut microbiome and its metabolites
Viral factors
-
■
Viral load and distribution
-
■
Frequency and timing of reactivations
-
■
Presence of other viruses (i.e., JC virus, CMV and human herpes virus 6–8)
-
■
Genotype of the HSV type and strains
-
■
Role of HSV-2 compared with HSV-1
-
■
Superinfections with more than one HSV type or strain
Confounding experimental variables
-
■
Small number of subjects in study
-
■
Prevalence of endemic infections (high background)
-
■
Sampling error – small amount of total brain tested
-
■
Reliability of PCR primers for genomically unstable viral genes
-
■
Limited availability of normal brains for age-matched control
One of the most thoroughly studied host genotypic factors hypothesized to link HSV with AD is allelic variation of the ApoE gene. ApoE is a main component of the chylomicron, a serum lipoprotein that binds to specific receptors on liver and other cells, and is essential for the normal catabolism of triglyceride-rich lipoprotein constituents. Several papers report a correlation between HSV-1, ApoE4 genotype and AD among English subjects (AD, n = 39; non-AD, n = 36), aged 54–96 years [14,23], while other studies, also of a small number of subjects (33 AD brains vs 72 non-AD), found significant amounts of HSV-1 but did not confirm a statistically significant association with ApoE4 [29]. While both of these studies demonstrated the presence of HSV DNA, representing latent virus, in the CNS of a significant number of subjects with or without AD, the correlation with ApoE4 genotype had a high odds ratio in one study [23] and was not found to be significant by the χ2 test in another study examining HSV-1 [29]. In female mice deleted for mouse ApoE, HSV-1 viral load was decreased 43-times below that of wildtype, ApoE+/+ mice [30]. However, no correlation has been found between ApoE4 and HSV encephalitis in humans [31]. While female mice are more susceptible to HSV, differences in gender infectivity in humans have been difficult to determine due to differences in male and female exposure rates. Difficulties in determining correlations between HSV-1 and ApoE include the high background of endemic viral infection, the over representation of ApoE4 alleles among the AD subjects, and the necessity of studying outcome at a single time point: the time of death.
The presence of other pathogens in the brain may also contribute to HSV sequelae (see Box 1). Other viral occupants of the human brain in addition to HSV-1 and -2 include other herpes viruses (Varicella zoster [32-34], Human herpes virus 6 [15] and EBV [35,36]; Human herpes virus 7 and 8 [37] and CMV [19]) and other viral species [38], such as the polyoma JC virus (reviewed in [39]). Infection with prokaryotes, including treponemal spirochetes causing syphilis or Lyme disease, may also persist in the brain [40,41].
Detection of HSV DNA in the brain at autopsy by PCR and/or immunohisto chemistry probably leads to under estimation of the incidence of CNS HSV infection owing to sampling error, since only small amounts of this large organ are typically tested: the human brain weighs on average approximately 1500 g, with a volume of approximately 1500 cm3. Both the location in the brain and the amount of tissue sampled will result in false negatives, and may thus lead to a failure to find statistically significant correlations. For example, Hemling et al. tested only 15–61 mg of brain from the hippocampus, the frontal cortex, the temporal cortex and the anterior cingulate gyrus [15]. Only one out of 30 AD brains had detectible HSV, while ten out of 30 controls were positive for virus in this Finnish cohort.
Primer design may also lead to false negatives. Although the HSV genome is relatively stable, small nucleotide differences do occur in different strains [42,43] and can arise even in the same individual. Such base-pair changes can affect PCR efficiency or even give false-negative results, especially when nested primers are targeted against one of the more variable HSV-1 genes, gD. Many of the early studies cited above used different primers for the amplification of different viral genes, which could underlie some of the inter-study variability.
Immunocytochemistry has also been used in the past in attempts to find HSV-1 and -2 in the brain. This approach greatly underestimates viral presence and load, as all viral proteins are only synthesized during viral reactivation, which could quite likely not be occurring at the time of death. Thus, viral DNA detection is a vastly superior method for ascertaining viral presence and load, as CNS HSV persists in latent form for the lifetime of the host. US FDA-approved primers now exist for PCR-based tests that are routinely applied clinically for diagnosis based on the highly conserved HSV glycoprotein G gene, which is rarely mutated and provides the most reliable results [44-46].
An autopsy series using clinically approved reliable primers and exhaustive sampling of the TN, the most likely site of viral entry into the CNS from the TG, of a large cohort of subjects would be the minimum required before a linkage between HSV and AD could be ruled out through application of these DNA detection techniques.
Asymptomatic replication of HSV occurs in both the CNS & the peripheral nervous system
Post-mortem PCR-based studies detect latent virus but are not informative about viral activity. What is the evidence that HSV is not just latent in the brain and nervous system, but also replicates?
Detection of viral DNA in the CSF demonstrates that, even during clinically silent infections, virus replicates in the CNS [10]. Although sensory innervation of the meninges emanates from the TG and superior cervical ganglia, these sensory nerve endings occur in the outer meningeal surface with little direct access to the CSF. Random testing of 3200 CSF samples revealed HSV-1 DNA in 26 and HSV-2 DNA in 36 samples from subjects without symptoms of HSV activity. Finding virus in CSF in the absence of clinical signs or symptoms is not surprising, since HSV-1 can also frequently be detected in saliva and tears of asymptomatic subjects [47]. Varicella zoster, another human alphaherpes virus and the cause of chickenpox and shingles, persists like HSV in a latent form and is shed in saliva of asymptomatic hosts [48,49]. Thus, HSV reactivates in the CNS, producing viral particles, over the lifetime of the host.
Subclinical viral replication in the CNS has consequences, & pathological effects persist
Asymptomatic replication of HSV in the CNS may produce pathologic consequences that are not yet recognized. This has become most apparent in children surviving neonatal herpes encephalitis, but may also pertain to adult CNS latency and reactivations.
In neonates, passage through the birth canal may result in perinatal infection, most commonly with HSV-2, leading to herpetic encephalitis. Neonatal mortality from HSV encephalitis has plummeted since the discovery of acyclovir (9-(2-hydroxyethoxymethyl)guanine), which inhibits viral replication through virally encoded tyrosine kinase activity [50-52], the first anti viral agent, for which Gertude Elion received the Nobel Prize in Medicine and Physiology in 1988 [53]. This success arose from earlier discoveries that purine nucleoside analogs inhibited viral replication with low host toxicity due to a unique virally encoded deoxy-thymidine kinase [54].
Treatment of acute neonatal HSV encephalitis began in the 1980s, first with vidarabine (adenine arabinoside) and more recently, acyclovir [55]. While suppressing viral replication during HSV encephalitis in the perinatal period vastly improves survival, such treatment does not eliminate latent virus, which may reactivate over the lifetime of the host. Perinatal infections with HSV-2 may not always produce acute disease, as studies in the elderly born before the advent of antivirals in the 1980s show that a significant number have HSV-2 latent DNA in the brain (13–20%) [56]. New drugs targeting other essential viral functions are urgently needed as acyclovir resistant strains emerge, and some are already in clinical trials [Ruebsamen-Schaeff H, Hill J, Pers. Comm.].
In our work, we followed an HSV-2-infected immunocompetent child from ages 8 to 12 years [57]. Her history was remarkable for a brief treatment for 2 days with acyclovir during the perinatal period for an episode of clonic seizures and fever. She presented at 8 years of age after a fall, when large asymptomatic granulomatous lesions were found incidentally in her brain on radiologic studies performed to rule out skull fracture. Biopsy of these lesions to rule out tumor revealed actively replicating HSV-2, similar in type to a known maternal infection at the time of pregnancy. Her neurological development had been unremarkable, meeting standard milestones. On presentation at 8 years of age she had no clinical, neurological or overt cognitive deficits. Yet over the 4 years of follow-up, her chronic HSV-2 encephalitis displayed a slow but relentless course, with recurrent viral reactivations and replication, producing lesions of fluctuating mass in the brain, and progressive neurological symptoms, primarily manifesting as attention deficit and learning disabilities. Pharmacologic control of the brain lesions required both antiviral therapy and immune suppression. This child was asymptomatic for >8 years, yet had large brain lesions and replicating HSV-2 in the brain. The lesions persisted despite chronic acyclovir treatment, and progressive deficits have appeared.
While this child’s case may be extreme, and perhaps unique, the finding of HSV DNA in randomly sampled CSF from asymptomatic individuals suggests that chronic replication at low levels occurs frequently in many infected individuals. Subclinical symptomatology of such chronic viral replication has not yet been systematically analyzed, although we and others have proposed hypothetical roles in AD, multiple sclerosis and epilepsy as discussed above. Recent reports show that perinatal HSV infections require longer therapies with acyclovir for those surviving children to attain normal developmental milestones [58], demonstrating that subclinical viral replication produces learning and developmental deficits in children, and pathologic effects are persistent and long-lasting, causing progressive deficits. In a transgenic mouse model of familial AD carrying multiple copies of the human APP gene with both Indiana and Swedish mutation (APPInd/Swe) under control of the Tet-off promoter, suppression of the mutant APP did not result in loss of plaques that had already formed [59].
Thus, the long-term effects of chronic subclinical HSV reactivations in the CNS of children who survive neonatal HSV encephalitis or acquire virus in the perinatal period and appear asymptomatic, require urgent attention.
How HSV-1 gains entry into the CNS: transport through interaction with cellular transport machinery
HSV-1 and 2 are both known to be neurotropic and to engage in active transport within neuronal processes. Because HSV enters sensory ganglia (TG or sacral dorsal root nerves) that also project a second process to the CNS, this transport is the more likely route of entry into the brain.
The author’s laboratory took the novel approach of using HSV-1 as a tool to investigate the cellular mechanisms that govern intra cellular transport [7,60-62]. A major challenge was to distinguish between incoming (infecting) viral particles and outgoing, newly synthesized (infective) particles. Indeed the long-standing controversy concerning the composition of outgoing particles may be a consequence of the experimental challenge of distinguishing in-coming from out-going viral particles. While some laboratories show out-going particles lacking detectable membrane components, others report that virus travels outwards within membrane compartments, leading to the ‘single’ versus ‘married’ hypotheses for viral egress [63-71]. Data supporting both types of transport have been obtained by electronmicroscopy [72-80]. In vitro reconstitution of transport on purified microtubules in the presence of cytosol will allow a dissection of the key molecules involved [81].
To study retrograde transport, the author reconstituted viral particle transport in the squid giant axon by injecting biochemically treated viral particles [60,62]. Green fluorescent protein-labeled viral particles lacking membranes following detergent treatment traveled uniquely retrograde at fast axonal transport rates (2.2 μm/sec) [60]. By contrast, membranated particles traveled anterograde in the giant axon, also at fast transport rates (0.8 μm/sec). Thus, we reasoned that the membranes associated with viral particles contain the receptor that mediates interactions with axonal anterograde transport machinery. Biochemical analysis of this viral-associated membrane revealed the presence of cellular APP, which also colocalized with purified viral particles prior to injection into the axon [62].
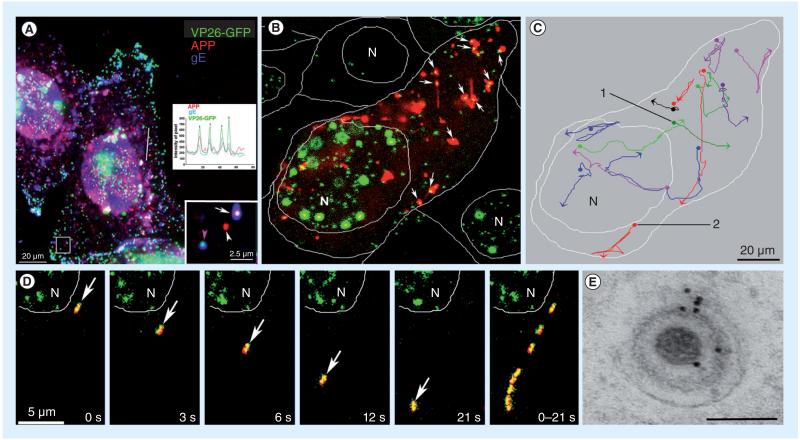
We had a breakthrough that allowed us to analyze the composition of outgoing viral particles in the cytoplasm of a productively infected cell. To do this we developed a culture system in which viral infection is so tightly synchronized that >97% of all cytoplasmic viral particles 6 h postinfection are outgoing [3]. Our data demonstrate that >80% of outgoing viral particles inside infected cell cytoplasm are associated with cellular APP, as identified by immunofluorescence, by cotransfection/infection with fluorescent viral proteins and labeled APP, and by immunogold electronmicroscopy (Figure 2). Intracellular nascent viral particles travel more frequently when associated with APP than without, yet may also travel, albeit rarely, independent of detectible APP (Figures 2 & 3) [7]. Productive infection increased the expression of APP and changed its subcellular location. In addition, productive infection with HSV-1 induced the breakdown and reorganization of the microtubule network and reorganization of cellular organelles (Figure 3). This breakdown allows nascent viral particles to travel in either anterograde (kinesin-based) or retrograde (dynein-based) directions on individual microtubules and yet still reach the cell surface (Figure 3).
Figure 2. HSV-1 interacts with cellular amyloid precursor protein during egress.
(A) VP26-GFP HSV-1 (green) particles colocalize with APP (red) during egress by immunofluorescence after synchronous infection with VP26-GFP HSV-1 (green) and fixation at 7 h postinfection. Examples of two infected cells also stained by immunofluorescence for gE (blue). Lower right inset shows a higher magnification of the boxed region. In this inset, one VP26-GFP-labeled particle displays all three labels (gE, APP and VP26), another has only APP (white arrowhead) or only gE (pink arrowhead). Such single labels demonstrate that colocalization is not due to bleed-through from other fluorescent channels. The inset graph shows intensity profiles along the line drawn across the right-hand cell. Superimposed peaks in the graph for the different colors are indicated by vertical arrows. (B) The first frame of a video sequence showing the initial positions of HSV capsids (VP26-GFP, green) and cellular APP-labeled with red fluorescent protein (APP-monomeric red fluorescent protein, red) in an infected cell at 7–9 h postinfection from a 3-s time-lapse 900-s (15 min) video. Double-labeled particles appear yellow (arrows). Many capsids (64%) colocalize with APP compartments in this frame. Capsids travel with APP vesicles, and sometimes join and separate from them. Positions of the N and of the cell boundaries are delineated by white lines. (C) The tracks of selected VP26-GFP HSV particles that move with the APP-monomeric red fluorescent protein label. Each trace has been assigned a different pseudocolor. Beginnings and ends of movements are indicated by dots and arrowheads, respectively. (D) An example of a double-labeled HSV-APP particle that moves away from the nucleus (arrow). In the last panel eight frames are superimposed to demonstrate the particle’s trajectory. (E) Immunogold-labeled electron micrograph of an HSV-1 particle inside an infected cell cytoplasm probed with anti-C-APP followed by protein-A labeled with 10 nm gold. Gold particles decorate both cellular and viral membranes surrounding capsids.
Scale bar = 100 nm.
APP: Amyloid precursor protein; GFP: Green fluorescent protein; N: Nuclei.
Modified with permission from [7]. Supporting videos for these images can be found on the PLoS One journal website linked to the online publication.
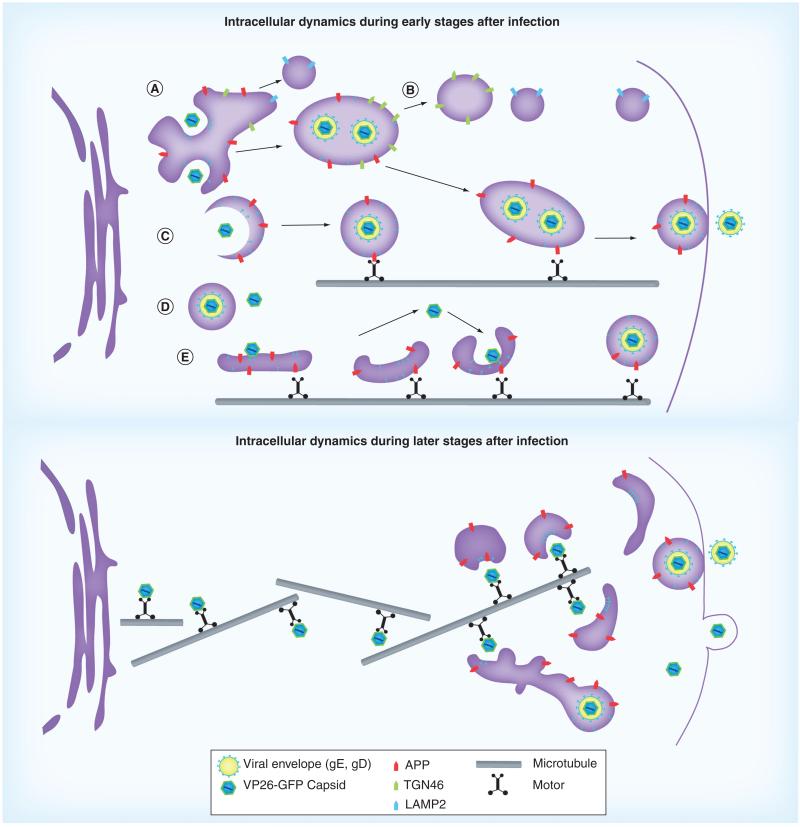
Figure 3. Schematics showing intracellular interactions between cellular amyloid precursor protein and HSV particles at an early stage (top) and a later stage (bottom) of productive infection in epithelial cells.
Intracellular dynamics at early stages during viral replication (top panel): (A) in the perinuclear region, viral particles move around and within the large membranous compartments near the nucleus, and also frequently colocalize with the viral envelope glycoproteins, gE and gD, and with cellular transmembrane organelle proteins in this area of the cell, such as: LAMP2, a lysosomal membrane protein; TGN46, associated with the trans-Golgi network; and APP, whose physiologic function is unknown. In the mid-cytoplasmic area, the LAMP2 compartments are separate from this apparent Golgi network, and rarely colocalize with any viral or Golgi components at the periphery. (B) In the outer cytoplasm neither LAMP2 nor TGN46-positive organelles colocalize with viral particles, while APP particles remain associated both with viral capsids and with viral envelope glycoproteins, gE and gD, probably on their way to the cell surface. (C) Capsids entering smaller post-Golgi compartments that stain uniquely for APP undergo fast transport towards the cell surface.
APP: Amyloid precursor protein; GFP: Green fluorescent protein.
Adapted from [7], with permission as modified from [61].
(D) After leaving the nucleus, viral particles are also found without detectible APP or the other cellular membrane proteins studied here. These particles could be inside some other unlabeled membrane system, or be free in the cytoplasm. These solo particles will be transported only rarely. (E) Capsids, possibly with tegument, may ride on the cytoplasmic surface of APP-labeled membrane systems, attach and detach from these membranes, or bud into them. Any particular capsid may employ all of these mechanisms during transit in the cytoplasm. In each case, we hypothesize that microtubule motors, such as one or more of the kinesin family, are recruited to the capsid-containing membrane-bound particles, possibly via APP or another cellular motor receptor. Dimensions are not to scale. Intracellular dynamics at later stages (bottom panel): as the microtubule network breaks down and reorganizes during productive infection, capsids must travel further to reach the cellular membrane synthesis and packaging centers in the Golgi apparatus, which drifts out during productive infections. In this case capsids travel directly on microtubules, possibly recruiting retrograde machinery as for infective entry, and yet reach the cell surface due to the disorganization of the microtubule network. Upon arrival at the cortex, where the Golgi has drifted, viral envelopment takes place. Dimensions are not to scale.
APP: Amyloid precursor protein; GFP: Green fluorescent protein.
Subsequent experiments in the author’s laboratory also demonstrated that a peptide derived from the cytoplasmic domain of APP was uniquely sufficient to mediate transport of inert plastic nanospheres [82]. Hence we reasoned that virus recruits APP during packaging and uses it to recruit cytoplasmic motors for travel from the perinuclear region to the distant nerve terminal. Such transport would lead to dissemination of virus throughout the arborization of neuronal projections, and thus would be a mechanism by which virus could inhabit multiple sites in the brain, depending upon the projections of each productively infected neuron.
Several virus-encoded proteins are thought to be generally involved in the anterograde transport of various alphaherpesviruses. Glycoprotein E, which forms a heterodimer with glycoprotein I, and is also an Fc receptor for immunoglobulin, travels with viral capsids moving in the anterograde direction [64,83-90]. Virus deleted or mutated for the gE gene fails to enter the optic nerve after retinal inoculation [86]. Another viral protein, US9, also a transmembrane envelope protein, travels with viral capsids of either pseudorabies virus or HSV-1 [64,80,83,91-93]. Interestingly, US9 is one of the genes altered in the H129 strain of HSV-1, which displays especially active anterograde transport [43]. Recent evidence implicates kinesin 3 (Kif1a) in US9-based HSV transport [Kramer T et al. Pers. Comm.].
Intracellular interactions between HSV-1 and APP have been reproduced by others in neurons [94-97]. In cultured human neurons HSV-1 induces Aβ formation [94,95], probably in autophagic vacuoles. Transport to distant sites of Aβ formed abnormally in the neuronal cell body is suggested to be a mechanism of seeding the cortex with Aβ fibrils that act as a nidus for the deposition of normally processed Aβ in the cortex [98,99]. Recent work by Braak et al. demonstrates that similar abnormal processing of Aβ occurs in the hindbrain and brainstem in early AD – in the dorsal vagus area, locus ceruleus and substantia nigra; regions that project neuronal processes throughout the cortex and hippocampus, and are anatomically close to the TN [100,101]. Interactions between HSV-1 and other proteins involved in neurodegeneration have been reviewed extensively by Carter [102]. Most exciting is the recent finding that acyclovir treatment of HSV-1-infected cultured epithelial cells prevents Aβ accumulation even at early stages after inoculation [103].
Evidence for HSV reactivation in cognitive decline
Perhaps the most convincing and powerful evidence linking HSV with dementia comes from a prospective study of 512 cognitively normal elderly subjects in a multicenter European cohort who were followed for 14 years for anti-HSV IgG and IgM antibodies and cognitive function. This study did not distinguish between HSV-1 and -2. In the follow-up, 77 new cases of cognitive impairment consistent with AD were found. Subjects with elevated HSV IgM levels had a significantly higher risk of developing HSV (Cox hazard ratio = 2.55; p = 0.003), while risk in those with IgG was slightly elevated but not statistically significant (hazard ratio = 1.67; p = 0.21). IgM elevations indicate reactivation of HSV, whereas IgG indicates that infection has occurred but the virus is latent. A high proportion of subjects were HSV IgG positive (81%), whereas only a few (7%) had IgM elevations detected over the 14 years of the study. Both AD-like and non-AD dementias occurred at 90% frequency in the HSV-positive subjects with or without the IgM rise. Since IgM would be transitory, it is likely that failure to detect IgM in all subjects who progress to dementia could be due to the timing of blood draw relative to HSV reactivation, or failure of low-grade reactivations to produce a measureable IgM response. In a subsequent study by this group, elevated IgM levels in 1222 subjects (73.9 years, mean age) correlated inversely with plasma Aβ40-42 levels [104]. This was interpreted as indicating that HSV reactivation influences APP processing and CNS deposition, consistent with in vitro work described above in which nascent newly synthesized viral particles interact with APP during packaging, transport and egress [7,94].
Recent evidence implicates Aβ as an antimicrobial peptide that may be an adaptive (innate) immune response to protect the brain from invading organisms [105]. Antimicrobial action was demonstrated for yeast (Candida albicans) as well as for bacteria: both cocci (i.e., Staphylococcus and Streptococcus) and rods (i.e., Listeria and Pseudomonas, Escherichia coli, Enterococcus faecalis), with microbicidal activity higher than other well-recognized antimicrobial peptides and even in some cases antibiotics.
Aβ plaques are also a long-recognized hall-mark of neurosyphilis and may also occur in Lyme disease with involvement of the CNS. In 1913 Noguchi and Moore demonstrated the presence of spirochetes of Trepanema pallidum in the brains of cases of general paresis, a dementia with paralysis previously diagnosed as a mental disorder. Subsequently spirochetes were found to induce Aβ-containing plaques in the brain (reviewed in [40,106]). Other spirochetes also have this consequence, such as Borrelia burgdorferi, the causative agent of Lyme disease [41]. Many other spirochete species exist, often commensal in the mouth and gut of humans, whose presence in brain has not been explored.
That Aβ may be protective is further supported by the results of clinical trials with drugs intended to clear Aβ from the brain. Two different approaches, anti-Aβ antibodies (i.e., bap-ineuzumab [107]) or inhibition of gamma secratase (i.e., semagacestat [72]) failed to improve cognition, while effectively removing plaque. These clinical drug trials have been halted. Conversely, a recent report from Iceland suggests that mutations that decrease but do not eliminate Aβ production have a strong protective effect against AD [108]. The Aβ hypothesis for AD is now under review.
Fulfillment of Koch’s postulates & proof of causation
Whether infectious diseases promote or accelerate AD remains controversial, in part because Koch’s postulates have not been fulfilled. Robert Koch was a 19th century physician who applied a series of logical steps to identify the causative organisms for anthrax and tuberculosis. These steps were later codified as ‘postulates’ and have been re-applied to identify the causative organisms in many other diseases, although in some they have had limited power (see Table 1).
Table 1.
Koch’s postulates revisited.
| Postulates | Caveats |
|---|---|
| The agent must be found in abundance in all individuals suffering from the disease but not in healthy individuals (Koch later abandoned the universal requirement for this postulate) |
Healthy individuals may harbor cholera and typhoid and not be affected but become carriers; polio only causes paralysis in a small subset of cases; asymptomatic or subclinical infections occur for many viruses, such as HIV and hepatitis C as well as HSV For HSV, many variables influence disease (see Box 1) |
| The microorganism must be isolated from a diseased individual and grown in culture |
Prions have never been grown in culture, and many other difficult- or impossible-to-culture infectious disease-causing organisms exist that are now identified by nucleic acid assays and confirmed by consensus as disease-causing For HSV, this postulate has been fulfilled for mucosal blisters and acute encephalitis. DNA has been found in the CNS in many studies |
| The cultured microorganism should cause disease when introduced into a healthy organism |
Infection may depend on host factors as well, as in the resistance to malaria of individuals carrying the sickle-cell gene. Koch himself used the word 'should' instead of 'must' to indicate that this is not universal. This postulate cannot be ethically satisfied for fatal diseases with no animal model, such as sporadic Alzheimer's disease For HSV, this postulate has been fulfilled for cold sores, encephalitis and teratology |
| The microorganism must be re-isolated from the inoculated diseased individual |
Re-isolation requires that the microorganism can be inoculated For HSV, this postulate has been fulfilled for cold sores, encephalitis and teratology |
As methodology to identify infectious disease-causing organisms progresses, revisions of Koch’s postulates have been proposed and successfully applied to other organisms, first by AB Hill as presented and discussed at the British Royal Society of Medicine in 1965 [109], and then by Fredericks and Relman in 1996 [110], whose proposal replaces culture with nucleic acid techniques. For HSV, DNA has been isolated from AD brains, although HSV infection is not limited to AD in autopsy series; many non-AD harbor HSV; and many who have HSV do not have AD. The most powerful correlations comes from the European longitudinal prospective study matching serological evidence of HSV reactivations with the onset of cognitive impairment [111].
As for incurable fatal diseases, of which AD is an example, infecting a healthy person with the isolated organism is not an option. For AD there are no validated animal models other than transgenic mice carrying various human genes found in familial AD. These often display aspects of AD without the cognitive components, which are difficult to determine in mice. There are also no animal models that replicate human HSV infections in all their aspects, especially latency and the reactivations that appear to be very important in the hypothesized HSV–AD relationship. Hence, Koch’s antiquated postulates cannot be ethically resolved for HSV and AD in the manner originally proposed. However, if the logic behind each postulate is extracted, it may be possible to establish a causal relationship between HSV and AD by developing a new set of 21st century postulates. For example, one such might be, in spontaneously occurring HSV encephalitis resulting in death, does the brain display Aβ plaques such as those pathognomic for AD?
Conclusion
Given the failure of Aβ-targeted therapies to improve cognition, alternative etiologies for AD pathology are currently needed. Infectious and inflammatory agents such as HSV may prove integral in our understanding of the pathogenesis and treatment of the disease.
Mounting evidence for a relationship between HSV activity and CNS injury demands further study, with clinical trials to determine if HSV suppression in the elderly protects cognition and prevents cognitive impairment. Urgent attention must be directed to survivors of perinatal HSV encephalitis, with long-term outcome studies and alternative drugs as acyclovir resistance is emerging. Satisfaction of postulates for proof of a causal relationship will require a creative approach that respects 21st century ethical considerations and exploits 21st century methodologies not available to Koch.
Future perspective
Viral pathogens lurk in the human brain, often in a quiescent state. Even for HSV, perhaps one of the most vigorously studied, little is known about incidence, prevalence, location or viral load in the brain. While studies around the world using PCR approaches detect HSV DNA in brain, a sign of latent infection, few studies have focused on its reactivation from latency. Detection of HSV DNA in CSF and the presence of anti-HSV IgM in the serum of apparently healthy subjects demonstrates that low levels of reactivation without overt symptoms occurs. Aβ deposits have been also found in apparently healthy, cognitively normal adults. Aβ plaques have long been known to appear in tertiary syphilis and have recently been reported in Lyme disease. What is now needed is to determine if the microbiome of the brain induces Aβ as part of an inflammatory innate immune response. The physical inter action within the cell between nascent virus and APP, the parent protein for Aβ, together with the prevalence of HSV infection in the general population, demand a closer look at causal relationships between HSV and AD, where the plaques are associated with tau-based neurofibrillary tangles. In the absence of animal models and with a potentially fatal outcome, (HSV encephalitis and AD), satisfaction of Koch’s postulates in the traditional manner in humans would be unethical. Determination of any causal link between HSV reactivation and Alzheimer’s will thus require alternative, yet equally convincing, postulates.
Executive summary.
HSV is prevalent worldwide & HSV DNA is found in the brain
-
■
Endemic infection rates for HSV in the US are immense: 31% of children aged 6–14 years, rising to 49% in adults aged 14–49 years, to a high of 89% of the population over 65 years.
-
■
HSV-1 DNA is commonly detected by PCR in post-mortem examination of brains in England (73.3%), Finland (16%), Japan (44%) and the USA (81%).
Asymptomatic replication of HSV in both brain & the peripheral nervous system
-
■
Chronic HSV replication occurs in the peripheral and CNS, with HSV-1 DNA found in saliva and HSV-1 and -2 found in the cerebral spinal fluid of asymptomatic subjects.
-
■
Cognitive impairment correlates with serologic evidence of HSV reactivation in cognitively normal elderly subjects.
HSV-1 interacts with amyloid precursor protein, the substrate of the major protein, Aβ, in Alzheimer’s plaques
-
■
Nascent HSV-1 particles travel with amyloid precursor protein inside cells, resulting in abnormal amyloid precursor protein distribution.
-
■
HSV-1 infection of neurons in culture leads to increased Aβ production.
Conclusion
-
■
Evidence is accumulating that HSV plays a role in Alzheimer’s disease. To satisfy a causal link between HSV and Alzheimer’s disease, an approach that takes 21st century ethical considerations and methodologies into account must be developed.
Future perspective
-
■
Monitoring cognition in HSV-positive subjects with mild cognitive impairment during long-term treatment with acyclovir, US FDA-approved for herpes virus therapy, will provide support for a causal relationship while also developing a novel treatment modality.
-
■
If HSV replication in the brain in vivo results in AD-like pathology, than a causal link satisfying Koch’s postulates will be obtained.
Acknowledgements
The author is grateful to RD O’Connor for discussion and editing of this review, and to present and former laboratory members including: K Kilpatrick, A Gonzales, GJ Ziomek, P Ferland, S Cheng, K MacLaughlin, A Rasin and A Novikov, as well as to the collaborator, P Webster of House Ear Institute, for their comments and questions over the years. The author also thanks the NIH for funding this research into cytoskeletal dynamics, the pathogenic role of transport defects, and HSV as a tool and a pathogen in neurodegenerative diseases.
Footnotes
Financial & competing interests disclosure
Research supported in the author’s laboratory was supported by NIGMS P5OGM08273, GM47368; NINDS NS046810 and NS062184. The author is an endowed Professor with funds from the Harvey Family Endowment that supported RD O’Connor and K MacLaughlin in part. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
- 1.Roizman B, Knipe D. Herpes simplex viruses and their replication. In: Knipe D, Howley P, editors. Fields Virology. Lippincott Williams & Wilkins; PA, USA: 2001. pp. 2399–2461. [Google Scholar]
- 2.Su YH, Moxley MJ, Ng AK, et al. Stability and circularization of herpes simplex virus type 1 genomes in quiescently infected PC12 cultures. J. Gen. Virol. 2002;83(Pt 12):2943–2950. doi: 10.1099/0022-1317-83-12-2943. [DOI] [PubMed] [Google Scholar]
- 3.Mellerick DM, Fraser NW. Physical state of the latent herpes simplex virus genome in a mouse model system: evidence suggesting an episomal state. Virology. 1987;158(2):265–275. doi: 10.1016/0042-6822(87)90198-x. [DOI] [PubMed] [Google Scholar]
- 4.Farooq AV, Shukla D. Corneal latency and transmission of herpes simplex virus-1. Future Virol. 2011;6(1):101–108. doi: 10.2217/fvl.10.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearer EL. Perspectives on herpes-APP interactions. Aging Cell. 2004;3(2):81–84. doi: 10.1111/j.1474-9728.2004.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itzhaki RF, Dobson CB, Wozniak MA, et al. Commentary on ‘Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of Alzheimer’s disease’ by Prasanna Satpute-Krishnan. Aging Cell. 2003;2(6):305–318. doi: 10.1046/j.1474-9728.2003.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Aging Cell. 2004;3(2):79–80. doi: 10.1111/j.1474-9728.2004.00088.x. [DOI] [PubMed] [Google Scholar]
- 7.Cheng SB, Ferland P, Webster P, Bearer EL. Herpes simplex virus dances with amyloid precursor protein while exiting the cell. PLoS One. 2011;6(3):e17966. doi: 10.1371/journal.pone.0017966. Live video microscopy of green fluorescent protein (GFP)-labeled HSV capsids interacting with red fluorescent protein-labeled amyloid precursor protein (APP) inside synchronously infected cells. Infection results in upregulation and redistribution of APP. Immunogold electron microscopy confirms that endogenous APP associates with HSV particles in the cytoplasm. Statistical analysis demonstrates that the association of APP improves viral transport frequency. Supporting videos linked to the online version of this paper are posted on the PLoS One website.
- 8.Xu F, Lee FK, Morrow RA, et al. Seroprevalence of herpes simplex virus type 1 in children in the United States. J. Pediatr. 2007;151(4):374–377. doi: 10.1016/j.jpeds.2007.04.065. [DOI] [PubMed] [Google Scholar]
- 9.Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296(8):964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- 10.Peter JB, Sevall JS. Review of 3200 serially received CSF samples submitted for type-specific HSV detection by PCR in the reference laboratory setting. Mol. Cell. Probes. 2001;15(3):177–182. doi: 10.1006/mcpr.2001.0356. [DOI] [PubMed] [Google Scholar]
- 11.Ball MJ, Mathews R, Steiner I, et al. Latent HSV 1 virus in trigeminal ganglia: the optimal site for linking prevention of Alzheimer’s disease to vaccination. Neurobiol. Aging. 2001;22(5):705–709. doi: 10.1016/s0197-4580(01)00253-6. discussion 717-719. [DOI] [PubMed] [Google Scholar]
- 12.Pevenstein SR, Williams RK, McChesney D, Mont EK, Smialek JE, Straus SE. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J. Virol. 1999;73(12):10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill JM, Ball MJ, Neumann DM, et al. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J. Virol. 2008;82(16):8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamieson GA, Maitland NJ, Wilcock GK, Yates CM, Itzhaki RF. Herpes simplex virus type 1 DNA is present in specific regions of brain from aged people with and without senile dementia of the Alzheimer type. J. Pathol. 1992;167(4):365–368. doi: 10.1002/path.1711670403. [DOI] [PubMed] [Google Scholar]
- 15.Hemling N, Roytta M, Rinne J, et al. Herpesviruses in brains in Alzheimer’s and Parkinson’s diseases. Ann. Neurol. 2003;54(2):267–271. doi: 10.1002/ana.10662. [DOI] [PubMed] [Google Scholar]
- 16.Mori I, Kimura Y, Naiki H, et al. Reactivation of HSV-1 in the brain of patients with familial Alzheimer’s disease. J. Med. Virol. 2004;73(4):605–611. doi: 10.1002/jmv.20133. [DOI] [PubMed] [Google Scholar]
- 17.Jamieson GA, Maitland NJ, Wilcock GK, Craske J, Itzhaki RF. Latent herpes simplex virus type 1 in normal and Alzheimer’s disease brains. J. Med. Virol. 1991;33(4):224–227. doi: 10.1002/jmv.1890330403. The first demonstration of the high prevalence of HSV in the brains of English subjects at autopsy. PCR primers for the HSV thymidine kinase were used to detect HSV in DNA isolated from 100-300 mg of brain selected from the temporal, frontal and hippocampal areas. Postive controls for the PCR reaction included HSV DNA extracted from virus propagated in Vero cells. All eight Alzheimer’s disease (AD) subjects tested were positive for HSV DNA as were six non-AD subjects.
- 18.Bertrand P, Guillaume D, Hellauer K, et al. Distribution of herpes simplex virus type 1 DNA in selected areas of normal and Alzheimer’s disease brains: a PCR study. Neurodegeneration. 1993;2:201–208. [Google Scholar]
- 19.Lin WR, Wozniak MA, Wilcock GK, Itzhaki RF. Cytomegalovirus is present in a very high proportion of brains from vascular dementia patients. Neurobiol. Dis. 2002;9(1):82–87. doi: 10.1006/nbdi.2001.0465. [DOI] [PubMed] [Google Scholar]
- 20.Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer’s disease amyloid plaques. J. Pathol. 2009;217(1):131–138. doi: 10.1002/path.2449. [DOI] [PubMed] [Google Scholar]
- 21.Sanders VJ, Felisan SL, Waddell AE, et al. Presence of herpes simplex DNA in surgical tissue from human epileptic seizure foci detected by polymerase chain reaction: preliminary study. Arch. Neurol. 1997;54(8):954–960. doi: 10.1001/archneur.1997.00550200020005. [DOI] [PubMed] [Google Scholar]
- 22.Sanders VJ, Felisan S, Waddell A, Tourtellotte WW. Detection of herpesviridae in postmortem multiple sclerosis brain tissue and controls by polymerase chain reaction. J. Neurovirol. 1996;2(4):249–258. doi: 10.3109/13550289609146888. [DOI] [PubMed] [Google Scholar]
- 23.Itzhaki RF, Lin WR, Shang D, Wilcock GK, Faragher B, Jamieson GA. Herpes simplex virus type 1 in brain and risk of Alzheimer’s disease. Lancet. 1997;349(9047):241–244. doi: 10.1016/S0140-6736(96)10149-5. An early report of the high prevalence of HSV in post-mortem brains, and an attempt to correlate with ApoE4 allele for AD in a group of 84 English autopsy specimens. In this cohort, logistical regression analysis demonstrated that ApoE4 frequency was significantly higher in subjects with both AD and HSV than in the HSV-negative AD group, with a CI of 95%. Although this cohort demonstrated correlations, other subsequent studies, by this group and others (see [14]), have not reproduced the correlation with the ApoE4 allele, although the prevalence of HSV in the brain is now well established.
- 24.Svensson A, Tunback P, Nordstrom I, Padyukov L, Eriksson K. Polymorphisms in TLR3 confers natural resistance to HSV-2 infection. J. Gen. Virol. 2012;93(Pt 8):1717–1724. doi: 10.1099/vir.0.042572-0. [DOI] [PubMed] [Google Scholar]
- 25.Moraru M, Cisneros E, Gomez-Lozano N, et al. Host genetic factors in susceptibility to herpes simplex type 1 virus infection: contribution of polymorphic genes at the interface of innate and adaptive immunity. J. Immunol. 2012;188(9):4412–4420. doi: 10.4049/jimmunol.1103434. [DOI] [PubMed] [Google Scholar]
- 26.Demontis D, Nyegaard M, Buttenschon HN, et al. Association of GRIN1 and GRIN2A-D with schizophrenia and genetic interaction with maternal herpes simplex virus-2 infection affecting disease risk. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2011;156B(8):913–922. doi: 10.1002/ajmg.b.31234. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee K, Dandara C, Gyllensten U, et al. A Fas gene polymorphism influences herpes simplex virus type 2 infection in South African women. J. Med. Virol. 2010;82(12):2082–2086. doi: 10.1002/jmv.21926. [DOI] [PubMed] [Google Scholar]
- 28.Kriesel JD, Jones BB, Matsunami N, et al. C21orf91 genotypes correlate with herpes simplex labialis (cold sore) frequency: description of a cold sore susceptibility gene. J. Infect. Dis. 2011;204(11):1654–1662. doi: 10.1093/infdis/jir633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beffert U, Bertrand P, Champagne D, Gauthier S, Poirier J. HSV-1 in brain and risk of Alzheimer’s disease. Lancet. 1998;351(9112):1330–1331. doi: 10.1016/S0140-6736(05)79057-7. [DOI] [PubMed] [Google Scholar]
- 30.Guzman-Sanchez F, Valdivieso F, Burgos JS. Aging-related neurostructural, neuropathological, and behavioral changes associated with herpes simplex virus type 1 brain infection in mice. J. Alzheimers Dis. 2012;30(4):779–790. doi: 10.3233/JAD-2012-120070. [DOI] [PubMed] [Google Scholar]
- 31.Lin W-R, Wozniak MA, Esiri MM, Klenerman P, Itzhaki RF. Herpes simplex encephalitis: involvement of apolipoprotein E. J. Neurol. Neurosurg. Psychiatry. 2001;70:117–119. doi: 10.1136/jnnp.70.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eshleman E, Shahzad A, Cohrs RJ. Varicella zoster virus latency. Future Virol. 2011;6(3):341–355. doi: 10.2217/fvl.10.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilden DH, Kleinschmidt-Demasters BK, Laguardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 2000;342(9):635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- 34.Gilden DH, Mahalingam R, Cohrs RJ, Tyler KL. Herpesvirus infections of the nervous system. Nat. Clin. Pract. Neurol. 3(2):82–94. doi: 10.1038/ncpneuro0401. [DOI] [PubMed] [Google Scholar]
- 35.Itzhaki RF, Wozniak MA. Herpes simplex virus type 1, apolipoprotein E, and cholesterol: a dangerous liaison in Alzheimer’s disease and other disorders. Prog. Lipid Res. 2006;45(1):73–90. doi: 10.1016/j.plipres.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Wozniak MA, Shipley SJ, Dobson CB, et al. Does apolipoprotein E determine outcome of infection by varicella zoster virus and by Epstein Barr virus? Eur. J. Hum. Genet. 2007;15(6):672–678. doi: 10.1038/sj.ejhg.5201812. [DOI] [PubMed] [Google Scholar]
- 37.Opsahl ML, Kennedy PG. Investigating the presence of human herpesvirus 7 and 8 in multiple sclerosis and normal control brain tissue. J. Neurol. Sci. 2006;240(1-2):37–44. doi: 10.1016/j.jns.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Honjo K, Van Reekum R, Verhoeff NP. Alzheimer’s disease and infection: do infectious agents contribute to progression of Alzheimer’s disease? Alzheimers Dement. 2009;5(4):348–360. doi: 10.1016/j.jalz.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Tavazzi E, White MK, Khalili K. Progressive multifocal leukoencephalopathy: clinical and molecular aspects. Rev. Med. Virol. 2012;22(1):18–32. doi: 10.1002/rmv.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miklossy J. Alzheimer’s disease - a neurospirochetosis. Analysis of the evidence following Koch’s and Hill’s criteria. J. Neuroinflammation. 2011;8:90. doi: 10.1186/1742-2094-8-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miklossy J, Khalili K, Gern L, et al. Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease. J. Alzheimers Dis. 2004;6(6):639–649. doi: 10.3233/jad-2004-6608. discussion 673-681. Follows on from previous reports that spirochetes in the brain induce neuropathologic changes of AD and identifies the Lyme disease agent Borrelia burgdorferei strain as sensu stricto based on genetic and molecular analyses. Borrelia have also been cultured from AD plaques, and 14 AD patients tested for spirochetes were positive, with 0 out of 13 controls found positive. This study and others cited in it demonstrate that neurospirochetosis results in Aβ deposition and the pathologic changes of AD.
- 42.Ushijima Y, Luo C, Goshima F, Yamauchi Y, Kimura H, Nishiyama Y. Determination and analysis of the DNA sequence of highly attenuated herpes simplex virus type 1 mutant HF10, a potential oncolytic virus. Microbes Infect. 2007;9(2):142–149. doi: 10.1016/j.micinf.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Szpara ML, Parsons L, Enquist LW. Sequence variability in clinical and laboratory isolates of herpes simplex virus 1 reveals new mutations. J. Virol. 2010;84(10):5303–5313. doi: 10.1128/JVI.00312-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGeoch DJ, Cunningham C, Mcintyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J. Gen. Virol. 1991;72(Pt 12):3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- 45.Mcgeoch DJ, Dolan A, Donald S, Brauer DH. Complete DNA sequence of the short repeat region in the genome of herpes simplex virus type 1. Nucleic Acids Res. 1986;14(4):1727–1745. doi: 10.1093/nar/14.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aurelius E. Herpes simplex encephalitis. Early diagnosis and immune activation in the acute stage and during long-term follow-up. Scand. J. Infect. Dis. 1993;(Suppl. 89):3–62. [PubMed] [Google Scholar]
- 47.Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest. Ophthalmol. Vis. Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J. Med. Virol. 2008;80(6):1116–1122. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagel MA, Choe A, Cohrs RJ, et al. Persistence of varicella zoster virus DNA in saliva after herpes zoster. J. Infect. Dis. 2011;204(6):820–824. doi: 10.1093/infdis/jir425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elion GB, Furman PA, Fyfe JA, De Miranda P, Beauchamp L, Schaeffer HJ. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc. Natl Acad. Sci. USA. 1977;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fyfe JA, Keller PM, Furman PA, Miller RL, Elion GB. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J. Biol. Chem. 1978;253(24):8721–8727. [PubMed] [Google Scholar]
- 52.Schaeffer HJ, Beauchamp L, De Miranda P, Elion GB, Bauer DJ, Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- 53.Elion GB. Acyclovir: discovery, mechanism of action, and selectivity. J. Med. Virol. 1993;(Suppl. 1):2–6. doi: 10.1002/jmv.1890410503. [DOI] [PubMed] [Google Scholar]
- 54.De Clercq E, Krajewska E, Descamps J, Torrence PF. Anti-herpes activity of deoxythymidine analogues: specific dependence on virus-induced deoxythymidine kinase. Mol. Pharmacol. 1977;13(5):980–984. [PubMed] [Google Scholar]
- 55.Whitley RJ. The use of antiviral drugs during the neonatal period. Clin. Perinatol. 2012;39(1):69–81. doi: 10.1016/j.clp.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lin WR, Wozniak MA, Cooper RJ, Wilcock GK, Itzhaki RF. Herpesviruses in brain and Alzheimer’s disease. J. Pathol. 2002;197(3):395–402. doi: 10.1002/path.1127. [DOI] [PubMed] [Google Scholar]
- 57.Brown WD, Bearer EL, Donahue JE. Chronic active herpes simplex type 2 encephalitis in an asymptomatic immunocompetent child. J. Child Neurol. 2010;25(7):901–908. doi: 10.1177/0883073809353449. A case report of a child presenting with head trauma status postperinatal HSV encephalitis successfully suppressed with acyclovir who continues to have chronic active HSV CNS infection with tumor-like appearance on imaging. Demonstrates subclinical chronicity of HSV replication in brain.
- 58.Kimberlin DW, Whitley RJ, Wan W, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N. Engl. J. Med. 2011;365(14):1284–1292. doi: 10.1056/NEJMoa1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jankowsky JL, Slunt HH, Gonzales V, et al. Persistent amyloidosis following suppression of Abeta production in a transgenic model of Alzheimer disease. PLoS Med. 2005;2(12):e355. doi: 10.1371/journal.pmed.0020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bearer EL, Breakefield XO, Schuback D, Reese TS, Lavail JH. Retrograde axonal transport of herpes simplex virus: evidence for a single mechanism and a role for tegument. Proc. Natl Acad. Sci. USA. 2000;97(14):8146–8150. doi: 10.1073/pnas.97.14.8146. Demonstrates the requirement for membranes for anterograde transport in axons. First report of GFP-labeling of virus, and reconstitution in a heterologous system of retrograde transport imaged live by confocal laser-scanning microscopy in the giant axon of the squid after detergent-stripping of viral/host membranes.
- 61.Bearer EL, Satpute-Krishnan P. The role of the cytoskeleton in the life cycle of viruses and intracellular bacteria: tracks, motors, and polymerization machines. Curr. Drug Targets Infect. Disord. 2002;2(3):247–264. doi: 10.2174/1568005023342407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Satpute-Krishnan P, Degiorgis JA, Bearer EL. Fast anterograde transport of herpes simplex virus: role for the amyloid precursor protein of alzheimer’s disease. Aging Cell. 2003;2(6):305–318. doi: 10.1046/j.1474-9728.2003.00069.x. Identification of host cell APP associated with HSV particles that undergo anterograde transport in the squid axon. Live video of anterograde movements of GFP-labeled HSV in the axon and analysis of transport dynamics with comparison to endogenous organelles, including mitochondrial transport. Negative-stain electron microscopy of isolated virus within a second, cellular-derived, membrane.
- 63.Snyder A, Bruun B, Browne HM, Johnson DC. A herpes simplex virus gD-YFP fusion glycoprotein is transported separately from viral capsids in neuronal axons. J. Virol. 2007;81(15):8337–8340. doi: 10.1128/JVI.00520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Snyder A, Polcicova K, Johnson DC. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. J. Virol. 2008;82(21):10613–10624. doi: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Snyder A, Wisner TW, Johnson DC. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. J. Virol. 2006;80(22):11165–11177. doi: 10.1128/JVI.01107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antinone SE, Zaichick SV, Smith GA. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. J. Virol. 2010;84(24):13019–13030. doi: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Negatsch A, Granzow H, Maresch C, et al. Ultrastructural analysis of virion formation and intraaxonal transport of herpes simplex virus type 1 in primary rat neurons. J. Virol. 2010;84(24):13031–13035. doi: 10.1128/JVI.01784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alconada A, Bauer U, Sodeik B, Hoflack B. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 1999;73:377–387. doi: 10.1128/jvi.73.1.377-387.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ibiricu I, Huiskonen JT, Dohner K, Bradke F, Sodeik B, Grunewald K. Cryo electron tomography of herpes simplex virus during axonal transport and secondary envelopment in primary neurons. PLoS Pathog. 2011;7(12):e1002406. doi: 10.1371/journal.ppat.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Radtke K, Kieneke D, Wolfstein A, et al. Plus- and minus-end directed microtubule motors bind simultaneously to herpes simplex virus capsids using different inner tegument structures. PLoS Pathog. 2010;6(7):e1000991. doi: 10.1371/journal.ppat.1000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turcotte S, Letellier J, Lippe R. Herpes simplex virus type 1 capsids transit by the trans-Golgi network, where viral glycoproteins accumulate independently of capsid egress. J. Virol. 2005;79(14):8847–8860. doi: 10.1128/JVI.79.14.8847-8860.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cunningham AL, Diefenbach RJ, Miranda-Saksena M, et al. The cycle of human herpes simplex virus infection: virus transport and immune control. J. Infect. Dis. 2006;194(Suppl 1):S11–18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 73.Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 2007;18(1):35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 74.Holland DJ, Miranda-Saksena M, Boadle RA, Armati P, Cunningham AL. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 1999;73(10):8503–8511. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miranda-Saksena M, Armati P, Boadle RA, Holland DJ, Cunningham AL. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 2000;74(4):1827–1839. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Penfold ME, Armati P, Cunningham AL. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl Acad. Sci. USA. 1994;91(14):6529–6533. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kristensson K, Lycke E, Roytta M, Svennerholm B, Vahlne A. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine] J. Gen. Virol. 1986;67(Pt 9):2023–2028. doi: 10.1099/0022-1317-67-9-2023. [DOI] [PubMed] [Google Scholar]
- 78.Lycke E, Hamark B, Johansson M, Krotochwil A, Lycke J, Svennerholm B. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch. Virol. 1988;101(1-2):87–104. doi: 10.1007/BF01314654. [DOI] [PubMed] [Google Scholar]
- 79.Lycke E, Kristensson K, Svennerholm B, Vahlne A, Ziegler R. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J. Gen. Virol. 1984;65(Pt 1):55–64. doi: 10.1099/0022-1317-65-1-55. Electron microscopy study that described the process of viral envelopment and transport. Enveloped virions were only found inside a second transport membrane in the cytoplasm and neuritic extensions in human embryonic dorsal root ganglion cells in culture.
- 80.Lavail JH, Tauscher AN, Sucher A, Harrabi O, Brandimarti R. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience. 2007;146(3):974–985. doi: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee GE, Murray JW, Wolkoff AW, Wilson DW. Reconstitution of herpes simplex virus microtubule-dependent trafficking in vitro. J. Virol. 2006;80(9):4264–4275. doi: 10.1128/JVI.80.9.4264-4275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Satpute-Krishnan P, Degiorgis JA, Conley MP, Jang M, Bearer EL. A peptide zipcode sufficient for anterograde transport within amyloid precursor protein. Proc. Natl Acad. Sci. USA. 2006;103(44):16532–16537. doi: 10.1073/pnas.0607527103. First demonstration that the cytoplasmic tail of APP is sufficient to mediate transport of exogenous cargo in a living axon.
- 83.Mcgraw HM, Awasthi S, Wojcechowskyj JA, Friedman HM. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. J. Virol. 2009;83(17):8315–8326. doi: 10.1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O’Regan KJ, Bucks MA, Murphy MA, Wills JW, Courtney RJ. A conserved region of the herpes simplex virus type 1 tegument protein VP22 facilitates interaction with the cytoplasmic tail of glycoprotein E (gE) Virology. 2007;358(1):192–200. doi: 10.1016/j.virol.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 85.Saldanha CE, Lubinski J, Martin C, et al. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 2000;74(15):6712–6719. doi: 10.1128/jvi.74.15.6712-6719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang F, Tang W, McGraw HM, Bennett J, Enquist LW, Friedman HM. Herpes simplex virus type 1 glycoprotein E is required for axonal localization of capsid, tegument, and membrane glycoproteins. J. Virol. 2005;79(21):13362–13372. doi: 10.1128/JVI.79.21.13362-13372.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang F, Zumbrun EE, Huang J, Si H, Makaroun L, Friedman HM. Herpes simplex virus type 2 glycoprotein E is required for efficient virus spread from epithelial cells to neurons and for targeting viral proteins from the neuron cell body into axons. Virology. 2010;405(2):269–279. doi: 10.1016/j.virol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dingwell KS, Doering LC, Johnson DC. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 1995;69(11):7087–7098. doi: 10.1128/jvi.69.11.7087-7098.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farnsworth A, Johnson DC. Herpes simplex virus gE/gI must accumulate in the trans-Golgi network at early times and then redistribute to cell junctions to promote cell-cell spread. J. Virol. 2006;80(7):3167–3179. doi: 10.1128/JVI.80.7.3167-3179.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Farnsworth A, Wisner TW, Johnson DC. Cytoplasmic residues of herpes simplex virus glycoprotein gE required for secondary envelopment and binding of tegument proteins VP22 and UL11 to gE and gD. J. Virol. 2007;81(1):319–331. doi: 10.1128/JVI.01842-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huber MT, Tomazin R, Wisner T, Boname J, Johnson DC. Human cytomegalovirus US7, US8, US9, and US10 are cytoplasmic glycoproteins, not found at cell surfaces, and US9 does not mediate cell-to-cell spread. J. Virol. 2002;76(11):5748–5758. doi: 10.1128/JVI.76.11.5748-5758.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lyman MG, Feierbach B, Curanovic D, Bisher M, Enquist LW. Pseudorabies virus Us9 directs axonal sorting of viral capsids. J. Virol. 2007;81(20):11363–11371. doi: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taylor MP, Kramer T, Lyman MG, Kratchmarov R, Enquist LW. Visualization of an alphaherpesvirus membrane protein that is essential for anterograde axonal spread of infection in neurons. MBio. 2012;3(2):e00063–12. doi: 10.1128/mBio.00063-12. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.De Chiara G, Marcocci ME, Civitelli L, et al. APP processing induced by herpes simplex virus type 1 (HSV-1)yields several APP fragments in human and rat neuronal cells. PLoS One. 2010;5(11):e13989. doi: 10.1371/journal.pone.0013989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Piacentini R, Civitelli L, Ripoli C, et al. HSV-1 promotes Ca2+ -mediated APP phosphorylation and Abeta accumulation in rat cortical neurons. Neurobiol. Aging. 2011;32(12):2323.e13–e26. doi: 10.1016/j.neurobiolaging.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 96.Santana S, Recuero M, Bullido MJ, Valdivieso F, Aldudo J. Herpes simplex virus type I induces the accumulation of intracellular beta-amyloid in autophagic compartments and the inhibition of the non-amyloidogenic pathway in human neuroblastoma cells. Neurobiol. Aging. 2012;33(2):430 e19–e33. doi: 10.1016/j.neurobiolaging.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 97.Wozniak MA, Itzhaki RF, Shipley SJ, Dobson CB. Herpes simplex virus infection causes cellular beta-amyloid accumulation and secretase upregulation. Neurosci. Lett. 2007;429(2-3):95–100. doi: 10.1016/j.neulet.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 98.Muresan V, Muresan Z. Is abnormal axonal transport a cause, a contributing factor or a consequence of the neuronal pathology in Alzheimer’s disease? Future Neurol. 2009;4(6):761–773. doi: 10.2217/fnl.09.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muresan V, Varvel NH, Lamb BT, Muresan Z. The cleavage products of amyloid-beta precursor protein are sorted to distinct carrier vesicles that are independently transported within neurites. J. Neurosci. 2009;29(11):3565–3578. doi: 10.1523/JNEUROSCI.2558-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 101.Braak H, Thal DR, Matschke J, Ghebremedhin E, Del Tredici K. Age-related appearance of dendritic inclusions in catecholaminergic brainstem neurons. Neurobiol. Aging. 2012 doi: 10.1016/j.neurobiolaging.2012.02.031. doi:10.1016/j. neurobiolaging.2012.02.031. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 102.Carter CJ. Interactions between the products of the Herpes simplex genome and Alzheimer’s disease susceptibility genes: relevance to pathological-signalling cascades. Neurochem. Int. 2008;52(6):920–934. doi: 10.1016/j.neuint.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 103.Wozniak MA, Frost AL, Preston CM, Itzhaki RF. Antivirals reduce the formation of key Alzheimer’s disease molecules in cell cultures acutely infected with herpes simplex virus type 1. PLoS One. 2011;6(10):e25152. doi: 10.1371/journal.pone.0025152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Feart C, Helmer C, Fleury H, et al. Association between IgM anti-herpes simplex virus and plasma amyloid-beta levels. PLoS One. 2011;6(12):e29480. doi: 10.1371/journal.pone.0029480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Soscia SJ, Kirby JE, Washicosky KJ, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5(3):e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miklossy J. Emerging roles of pathogens in Alzheimer disease. Expert Rev. Mol. Med. 2011;13:e30. doi: 10.1017/S1462399411002006. [DOI] [PubMed] [Google Scholar]
- 107.Panza F, Frisardi V, Imbimbo BP, Seripa D, Solfrizzi V, Pilotto A. Monoclonal antibodies against beta-amyloid (Abeta) for the treatment of Alzheimer’s disease: the Abeta target at a crossroads. Expert Opin. Biol. Ther. 2011;11(6):679–686. doi: 10.1517/14712598.2011.579099. [DOI] [PubMed] [Google Scholar]
- 108.Jonsson T, Atwal JK, Steinberg S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 109.Hill AB. The environment and disease: association or causation? Proc. R. Soc. Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fredericks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin. Microbiol. Rev. 1996;9(1):18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Letenneur L, Peres K, Fleury H, et al. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer’s disease: a population-based cohort study. PLoS One. 2008;3(11):e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]