Abstract
A new dimethacrylate chelating monomer containing a BisGMA-like backbone structure and a bis(carboxymethyl)-L-lysine chelating group and its ternary zirconium-fluoride complex (antibacterial fluoride-releasing monomer) have been synthesized. The monomer structures were confirmed by 1H-NMR, 13C-NMR, and ES-MS analysis. Several experimental fluoride-releasing dental composites containing different quantities of the new antibacterial fluoride-releasing monomer were formulated and tested for fluoride release, fluoride recharge, compressive and flexural strengths, water sorption and solubility. These composites displayed high fluoride release and recharge capabilities, as well as good physical and mechanical properties.
1. Introduction
Dental caries is still a major health problem worldwide. Resin-based dental composites (RBC) have been widely used to restore carious teeth but they have limited service life (5–7 years). The leading cause for failure of RBCs is secondary (recurrent) caries [1–5]. Fluoride is well documented as the most effective and widely used anticariogenic agent. Fluoride-releasing dental materials may reduce secondary caries at the restoration’s margins [6–9]. Current dental composites have high strength, good wear resistance, and excellent esthetics [10–12], but their fluoride release rates and fluoride recharge capabilities are quite low [13]. The major challenge for the development of fluoride-releasing dental composites is to increase their fluoride-releasing and recharging capabilities while maintaining their physical and mechanical properties.
In recent years, our group has developed several novel fluoride-releasing dental monomers containing ternary zirconium-fluoride chelates and related dental composites [14–18]. In the early studies, dimethacrylate chelating monomers containing amino- or amido- diacetic acids and their ternary zirconium-fluoride chelates were synthesized. The experimental composites containing these novel fluoride-releasing monomers displayed significantly higher fluoride-release and recharge capabilities than commercial fluoride-releasing composites, but their physical and mechanical properties were significantly lower than commercial composites due to high water sorption and solubility [15,16]. Recently, the formulation of the fluoride-releasing composite was optimized to improve the physical and mechanical properties but its fluoride release was significantly reduced, though they were significantly higher than the conventional fluoride releasing dental composites [17]. Therefore, the major challenge for development of fluoride-releasing dental composites remains to increase their fluoride-releasing and recharging capabilities while maintaining their physical and mechanical properties.
Here we report the synthesis of a new dimethacrylate chelating monomer containing bis(carboxymethyl)-L-lysine and its ternary zirconium-fluoride complex, the synthesis of the antibacterial fluoride-releasing monomer (AF), and the characterization of the experimental antibacterial fluoride-releasing dental composite containing such a monomer.
2. Experimental Methods
2.1 Reagents and instruments
2-Bromoethyl methacrylate was purchased from Pfaltz & Bauer. Nα,Nα-bis(carboxymethyl)-L-lysine hydrate was purchased from Fluka. Bisphenol A dimethacrylate (Bis-GMA) was purchased from Polysciences. Ethoxylated bisphenol A dimethacrylate (EBPADMA) and 1,6-hexanediol dimethacrylate (HDDMA) were provided by Esstech. 4,4-bis(4-hydroyphenyl)valeric acid, 2-chloro-1-methyl-pyridinium iodide, camphorquinone (CQ), ethyl 4-dimethylaminobenzoate (4E), and phenyl bis(2,4,6-trimethyl benzoyl)phosphine oxide (PO), and solvents were purchased from Aldrich. Silanized F-releasing glass filler was provided by Caulk/Dentsply. The antibacterial monomer 3 was synthesized using a previously reported method [19]. High resolution mass spectrometry using electrospray ionization (HRMS (ESI)) was carried out on Synapt HDMS (Waters). NMR spectra were acquired on Inova-400 (1H, 13C) NMR spectrometer, 1H at 400MHz, 13C at 125 MHz in CD3OD.
2.2 Monomer Synthesis
Synthesis of 4, 4-Bis [4-(2- methacryloyloxy)ethoxyphenyl] pentanoic acid (1)
A 100 mL round-bottomed flask, equipped with a magnetic stirring bar was charged with 4,4-bis(4-hydroyphenyl) valeric acid (5.72g, 20 mmol), K2CO3 (40 mmol) and DMF (60 mL). The mixture is stirred for 30 min. Bromoethyl methacrylate (9.57g, 50 mmol) was added through one septum via a syringe over 10 min. The reaction mixture was stirred at bath temperature 40 °C for 48 h, and then additional bromoethyl methacrylate (10 mmol) was added at 100 °C and stirred for 24 h. After the reaction was completed, the reaction mixture was quenched by adding water (100 mL) and pH was adjusted to 3 using HCl. The mixture was extracted with diethyl ether (3 × 50 mL). The combined organic extracts were washed sequentially with brine (2 × 50 mL), dried over anhydrous MgSO4, filtered and concentrated under reduced pressure by a rotary evaporator. The crude product was purified on silica gel column with CH3COOC2H5: hexane (3:2) as mobile phase to give (5.11g, 50%) of compound 1.
Analysis: 1H NMR (CD3OD, 400MHz) δ:1.54(s,3H), 1.93(m, 6H), 2.13(m,2H), 2.38(q,2H), 4.17(t, 2H), 4.30(m, 4H), 2.47(t, 2H), 5.58(m, 2H), 6.12(m, 2H), 6.73(t, 2H), 6.80(t, 2H), 7.02(q,2H), 7.08(q, 2H); 13C NMR (CD3OD, 125 MHz) δ: 18.47, 27.96, 30.42, 36.75, 44.70, 63.48, 66.11,66.63, 114.39, 115.15, 126.47, 128.53, 136.11, 140.66, 156.74, 167.21,174.25. HRMS (ESI) analysis (in MeOH/CH2Cl2, negative ion mode): m/z = 509.2207 ([M-H]−, calculated: 509.2181 for C29H45O8).
Synthesis of (S)-2,2′-(5-(4,4-bis(4-(2-(methacryloyloxy)ethoxy)phenl)pentanamido)-1-carboxypentylazanediyl)diacetic acid (2)
A 250 mL round-bottomed flask equipped with a magnetic stirring bar was charged with 2-chloro-1-methyl-pyridinium iodide (2.81 g, 11 mmol) and DMF (100 mL). After it was dissolved, compound 1 (5.09 g, 10 mmol) was added. The reaction mixture was stirred at 100 °C for 2 h. Nα,Nα-bis(carboxymethyl)-L-lysine hydrate (2.88g, 11 mmol) was added. The reaction mixture was stirred vigorously for 18 h and quenched by adding water (100 mL). The pH value of the aqueous layer was adjusted to 3 and the solution was extracted with diethyl ether (3 × 50 mL). The combined organic extracts were washed with brine (2 × 150 mL), dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure by a rotary evaporator. The crude product was purified on silica gel column with CH3COOC2H5: CH3OH (1:4) as mobile phase to give (3.56g, 47.2%) of compound 2.
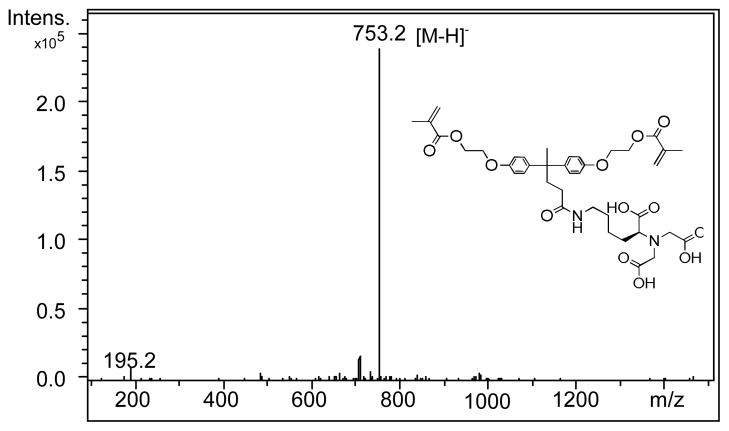
Analysis: 1HNMR (CD3OD, 400 MHz) δ: 1.29(m,2H): 1.43–1.62(m, 4H), 1.89(s, 3H), 1.96(s,6H), 2.13–2.35(m, 4H), 2.98–3.10(m, 6H), 3.85(m, 1H), 4.19–4.56(m, 8H), 5.58(m, 2H), 6.16 (m, 2H), 6.73(t, 2H), 6.80(t, 2H), 7.02(q, 2H), 7.08(q, 2H), 8.01(s, NH). 13C NMR (CD3OD, 125 MHz) δ: 18.48, 27.97, 30.40, 31.81, 36.80, 44.66, 55.31, 62.42, 63.64, 66.64, 114.35, 126.47, 128.53, 136.15, 139.33, 140.60, 140.85, 154.34, 167.67, 174.11, 174.22. HRMS (ESI) (negative ion mode): for C39H49N2O13 [M-H]−, found m/z = 753.3303, Calculated: 753.3240.
Synthesis of fluoride-releasing monomer (4) and antibacterial fluoride-releasing monomer (5)
Compound 2 (2 mmol) and ZrF4/MeOH (2.2 mmol) were added to a 100 mL round-bottomed flask, equipped with a magnetic stirring bar. The reaction mixture was stirred at room temperature for a half hour and the solvent was removed by rotary evaporation to produce the fluoride-releasing monomer (ternary zirconium fluoride complex) 4. When antibacterial monomer 3 (2 mmol) was added to the above reaction mixture and under the same condition, the product is the antibacterial fluoride-releasing (AF) monomer 5.
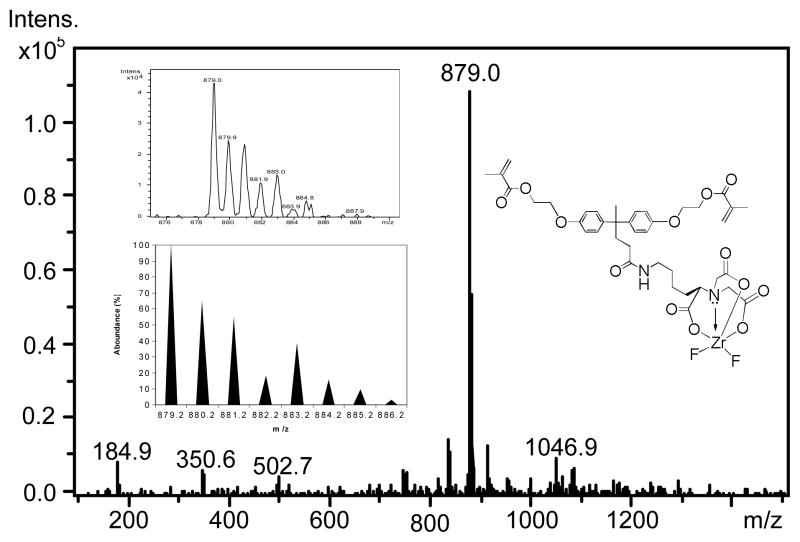
Analysis: HRMS (ESI) Monomer 4: (negative ion mode, in MeOH) m/z = 879.0 ([M-H]−, Calculated: 879.2099 for C39H47F2N2O13Zr); Monomer 5: (negative ion mode) m/z = 879.0; (positive ion mode) m/z = 374.3051 ([M3-Br]+, Calculated: 374.3054 for C24H40NO2+).
2.3 Formulation of Experimental Composites
The compositions of experimental and control dental composites are shown in Table 1. Exp20, Exp25 and Exp30 contain 20%, 25% and 39% of AF monomer 5 in the total monomer mixture. The monomers of control contain Bis-GMA:EBPADMA:HDDMA (40:40:20). The composites were formulated by blending the monomers 29%, silanized F-releasing glass filler 70%, and photo initiators 1% in a Speed Mixer (Model CDAC 150FVZ, FlackTek, Inc.). The composite specimens were photopolymerized using an Optilux 501 dental curing light (Kerr Corp. Orange, CA) for 40 s on each surface.
Table 1.
Compositions of experimental and Control Composites (Wt %)
| Components | Control | Exp20 | Exp25 | Exp30 |
|---|---|---|---|---|
| Bis GMA | 11.6 | 8.7 | 6.525 | 6.09 |
| EBPADMA | 11.6 | 8.7 | 6.525 | 6.09 |
| HDDMA | 5.8 | 5.8 | 4.35 | 4.06 |
| AF Monomer | 0 | 5.8 | 7.25 | 8.7 |
| F-releasing Filler | 70 | 70 | 70 | 70 |
| CQ | 0.14 | 0.14 | 0.14 | 0.14 |
| 4E | 0.58 | 0.58 | 0.58 | 0.58 |
| PO | 0.29 | 0.29 | 0.29 | 0.29 |
2.4 Fluoride Release and Recharge
Fluoride release of both materials from cylindrical samples (4 × 9 mm (diameter × height), n = 5) in 3 mL de-ionized water was analyzed daily for 30 days and then weekly for 65 days using ion-selective electrode. Then the specimens were recharged by “60 Second Taste Gel” (containing 1.23 w/w% fluoride ion) for 1 min and rinsed with running deionized water for 30 seconds. Fluoride release after recharge was measured daily for 5 days and the recharge cycles were repeated 3 times.
2.5 Measurement of Physical and Mechanical Properties
Cylindrical comprehensive strength specimens (4 × 9 mm (D × H), n = 10) and rectangular flexure strength specimens (25 × 2 × 2 mm, n = 10) were prepared, immersed in de-ionized water, stored 37 °C for 24 h, and tested on the Instron 5566 testing machine with crosshead speed of 1 mm/min. The water sorption and solubility test were conducted using disk specimens (15 × 2 mm (D × H), n = 5) according to ISO standard 4049.
2.6 Data Analysis
The experimental data were analyzed using ANOVA and post hoc Tukey test with the significance level set at 95% (α = 0.05).
2.7 Cytotoxicity Test
Human gingival fibroblasts were obtained from extracted molars from patients with healthy gingiva following informed consent as prescribed in an approved IRB protocol. Gingival fibroblasts were maintained in MEMα containing 10% fetal calf serum (FCS) and 200 units/ml penicillin and 200 mu;g/ml streptomycin. Cells were grown in 48-well plates for 24 hours prior to exposure to the monomers. The growth media containing 0.1%DMSO were supplemented with 10−4M, 10−5M, 10−6M, and 10−7M of synthesized monomer 2 and 4 were as added to cells for 24 hrs. BisGMA served as a positive control for cytotoxicity. Cell survival was quantified using a BioTek Synergy 2 fluorescent multi-well plate reader.
3. Results and Discussion
The new dimethacrylate chelating monomer 2 has been designed to have a BisGMA-like backbone structure and a tetra-dentate chelating group (three carboxylic acids and one amino group) (Scheme 1). Therefore, the resulting ternary zirconium-fluoride complex has higher stability than the monomers containing amino- or amido-diacedic acids previously reported [15, 16]. The potential increase of hydrophilicity (water sorption) of this chelating group has been compensated by removal of the two pendent hydroxyl groups from the backbone structure. The calculated water-octanol partition coefficient CLogP = 4.43 indicates the monomer is rather hydrophobic, which helps reduce water sorption of the composite.
Scheme 1.
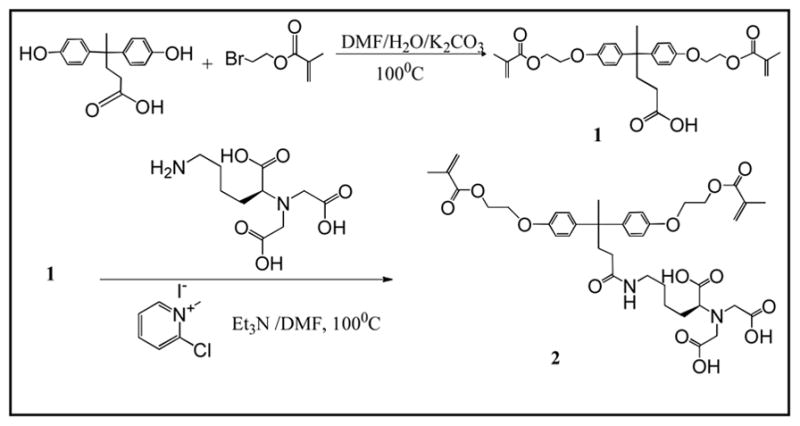
Synthesis of chelating monomer 2 and antibacterial fluoride-releasing monomer 5.
The dimethacrylate chelating monomer 2 can be readily synthesized by two steps (Scheme 1). The mono-substitution was a side reaction in first step. This directly affected the yield of intermediate 1. By increasing the reaction temperature (100°C) and increasing the amount of 2-bromoethyl methacrylate, the yield of intermediate 1 was increased to ca. 20%.
Chelating monomer 2 can react with ZF4/methanol solution and 3 to form a ternary zirconium-fluoride complex 4 and antibacterial fluoride-releasing (AF) monomer 5. The negative ion ES-MS spectra of monomers 2 and 5 are shown in Figure 1 and 2, respectively. The negative ion ES-MS spectrum of monomer 5 (Figure 2) shows high abundance of m/z = 879.0 and the unique isotope distribution pattern which matches that of the anion part of the monomer [LZrF2]−. The AF monomer 5 includes a long-chain monomer [N-benzyl-11-(methacryloyloxy])-N, N-dimethylundecan-1-amminium] as the counter ion. The positive ion ES-MS spectrum (not shown) of monomer 5 shows a single peak at m/z = 374.3051 (calculated: 374.3054), which is the cation part of monomer 3. It has antimicrobial effect as reported in our previous study [19].
Figure 1.
Negative ion ES-MS spectrum of chelating monomer 2.
Figure 2.
Negative ES-MS spectrum of the anion part of antibacterial fluoride releasing monomer 5. The inserts are measured and calculated isotope distribution patterns of the peak m/z = 879.0.
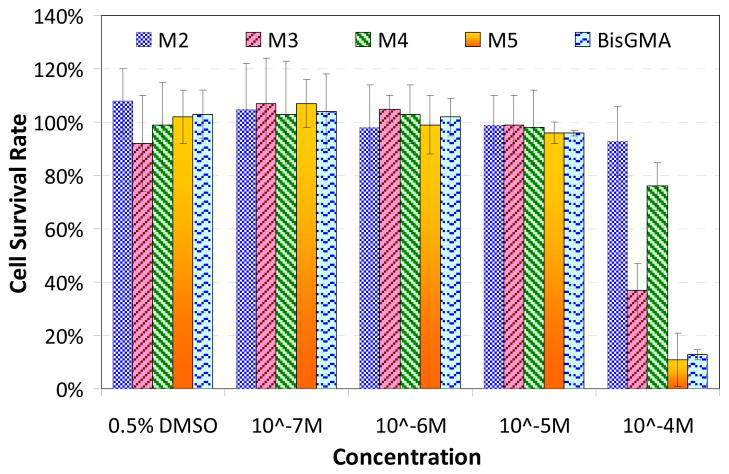
Figure 3 shows the results of cytotoxicity of synthesized monomer and BisGMA different concentrations. At 10−5M, all monomers show no cytotoxicity. At 10−4M, chelating monomer 2 had 93% cell survival rate, which indicates good biocompatibility. Monomer 4 had 73% cell survival rate, indicating slight cytotoxcity, which may have been caused by fluoride. Monomer 5 displayed some cytotoxicity (11% cell survival), but it is very similar to that of BisGMA (13%). Therefore, the synthesized monomers are acceptable for dental applications.
Figure 3.
Cytotoxicity test – cell survival rate under different concentration of monomers (M2, M3, M4, and M5 represents monomers 2, 3, 4 and 5 respectively).
The experimental and control dental composites were formulated and their compositions are listed in Table 1. The flexural strength of the composites was tested after storage in deionized water for 24 h and 3 months. Water sorption and solubility of the composites were also tested according ISO 4049-2000. The results are listed in Table 2. The experimental composites containing up to 25% antibacterial fluoride-releasing (AF) monomer 5 in total monomers have similar physical and mechanical properties to the control composite (p = 0.244). When the amount of AF monomer 5 is increased to 30% of the total monomers, the water sorption increases significantly and the mechanical properties decrease significantly.
Table 2.
Physical and mechanical properties of experimental and control composites.
| Materials | Flexure Strength, 24 hrs (MPa) | Flexure Strength, 3 months (MPa) | Water Sorption (μg/mm3) | Solubility (μg/mm3) |
|---|---|---|---|---|
| Control | 108.9±9.7A | 96.1±19.2A | 13.6±1.2a | 1.48±0.9a |
| Exp20 | 113.6±15.9A | 80.8±8.6A,B | 13.75±1.1a | 1.84±1.5a |
| Exp25 | 95.0±11.9A,B | 80.6±12.0A,B | 14.5±2.1a,b | 2.0±0.8a |
| Exp30 | 85.1±9.5B | 73.8±7.9B | 20.5±1.9c | 3.3±0.4b |
The groups with the same superscript letters in the same column have no significant difference (p>0.05).
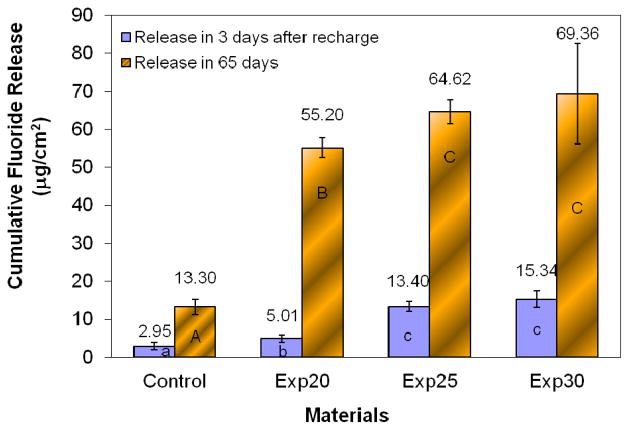
Figure 4 shows the fluoride release and recharge profiles, cumulative fluoride release and recharge of the experimental and control composites. The experimental composite containing the new antibacterial fluoride-releasing monomer has significantly higher cumulative fluoride release and recharge capability than the control composite (p < 0.05). In general, the fluoride release and recharge capabilities of the experimental composites increase with the increasing amount of AF monomer. However, further increase the amount of AF monomer above 25% has little effect on fluoride-release and recharge but it will increase water sorption and reduce mechanical properties of the composite. Therefore, the optimal concentration of AF monomer appears to be around 25% of the total monomers. In clinic application, such a new fluoride-releasing dental composite is expected to have a sustained fluoride release capability and enhanced caries-inhibitive effect. Therefore, it may prolong the service life of the composite restoration and reduce the cost for replacement.
Figure 4.
Cumulative fluoride release and fluoride recharge. The columns with the same letters have no significan difference (p>0.05).
In conclusion, the new dimethacrylate chelating monomer containing bis(carboxymethyl)-L-lysine and its ternary zirconium-fluoride complex (antibacterial fluoride-releasing monomer) has been successfully synthesized. The experimental dental composite containing the new fluoride-releasing monomer displays high fluoride release and recharge capabilities as well as good physical and mechanical properties. The new monomer may be useful in the formulation of a variety of fluoride-releasing dental materials.
Acknowledgments
This study is supported by NIH/NIDCR grant 1R01DE019203A1.
References
- 1.Gordon JC. J Am Dent Assoc. 2005;136:201–203. doi: 10.14219/jada.archive.2005.0142. [DOI] [PubMed] [Google Scholar]
- 2.Ivar A, Mjör O. J Am Dent Assoc. 2005;136:1426–1433. doi: 10.14219/jada.archive.2005.0057. [DOI] [PubMed] [Google Scholar]
- 3.Elderton RJ. J Dentistry. 1976;4:257–262. doi: 10.1016/s0300-5712(76)80002-4. [DOI] [PubMed] [Google Scholar]
- 4.Mjör IA. Oper Dent. 1985;10:88–92. [PubMed] [Google Scholar]
- 5.van Dijken JWV. Acta Odontol Scand. 1986;44:357–367. doi: 10.3109/00016358609094346. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman BF, Rawls HR, Querens AE. J Dent Res. 1984;63:689–692. doi: 10.1177/00220345840630051701. [DOI] [PubMed] [Google Scholar]
- 7.Gomez C, Donly KJ. Quint Int. 1994;25:355–358. [PubMed] [Google Scholar]
- 8.Ripa LW. Adv Dent Res. 1991 Dec;:56–59. doi: 10.1177/08959374910050010801. [DOI] [PubMed] [Google Scholar]
- 9.Wiegand A, Buchalla W, Attin T. Dent Mater. 2003;23:3343–3362. [Google Scholar]
- 10.Ferracane JL. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 11.Bowen RL, Marjenhoff WA. Adv Dent Res. 1992;6:44–49. doi: 10.1177/08959374920060011601. [DOI] [PubMed] [Google Scholar]
- 12.Silikas N, Masouras K, Satterthwaite J, Watts DC. Inter J Nano Biomater. 2007;1:116–127. [Google Scholar]
- 13.Xu X, Burgess JO. Biomaterials. 2003;24:2451–2461. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 14.Xu X, Burgess JO, Ding X, Ling L. US Paten. 2004;6:703, 518. [Google Scholar]
- 15.Xu X, Ling L, Ding X, Burgess JO. J Polym Sci: Part A: Polym Chem. 2004;42:985–958. [Google Scholar]
- 16.Xu X, Ding X, Ling L, Burgess JO. J Polym Sci: Part A: Polym Chem. 2005;43:3153–3166. [Google Scholar]
- 17.Xu X, Ling L, Wang R, Burgess JO. Dent Mater. 2006;22:1014–1023. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 18.Ling L, Xu X, Choi GY, Billodeaux D, Guo G, Diwan RM. J Dent Res. 2009;88:83– 88. doi: 10.1177/0022034508328254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Wang YP, Liao S, Wen ZT, Fan Y. J Biomed Mater Res Part B Appl Mater. 2012;100B:1151– 1162. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]