Abstract
The nature of progesterone (P4)’s neuroprotective effects is of interest. We investigated effects of P4 when administered prior to, or following, kainic acid, which produces ictal activity and damage to the hippocampus, to mediate effects on spatial performance. The hypothesis was that P4, compared to vehicle, would reduce decrements in Morris Water Maze performance induced by kainic acid. Experiment 1: We examined the effects of kainic acid on plasma stress hormone, corticosterone, and progestogen (P4 and its metabolites) levels in plasma and the hippocampus following subcutaneous (s.c.) P4 administration to ovariectomized rats. Rats administered kainic acid had the highest corticosterone levels immediately following injection. P4 is 5α-reduced to dihydroprogesterone (DHP) and subsequently metabolized to 5α-pregnan-3α-ol-20-one (3α,5α-THP) by 3α-hydroxysteroid dehydrogenase. The regimen of P4 utilized produced circulating and hippocampal levels of P4, DHP, and 3α,5α-THP within a physiological range, which decline at 14 hours post-injection, and were not altered by kainic acid. Experiment 2: The physiological P4 regimen was administered to rats before, or following, kainic acid-induced seizures, and later effects on water maze performance were compared to that of rats administered vehicle. Rats administered kainic acid had significantly poorer performance in the water maze (i.e. increased latencies and distances to the hidden platform) than did rats administered vehicle. Administration of P4 before, but not after, kainic acid prevented these performance deficits. Thus, these data suggest that a physiological regimen of P4 can prevent some of the deficits in water maze performance produced by kainic acid.
Keywords: dihydroprogesterone, allopregnanolone, neurosteroid, learning, ictal activity, hippocampus, neuroprotection
INTRODUCTION
Progesterone (P4), a steroid hormone secreted by the ovaries, adrenals, placenta, and brain, exerts profound trophic effects on peripheral and central tissues. In the brain, P4 has actions to regulate reproductive and neuroendocrine events. Progesterone exerts trophic actions on neurons and glia, promotes growth of nerve processes, and modulates synaptic plasticity (Reyna-Neyra et al., 2002; Ghoumari et al., 2003; Schumacher et al., 2004; Foy, Akopian and Thompson, 2008). Thus, the brain is a source and target for P4.
Progesterone has actions in the brain to influence cognitive performance. Pregnant rats, compared to non-pregnant rats, perform better in the water maze task midgestation, and in object placement late in gestation, when they have elevated levels of estrogen and P4 (Galea et al., 2000; Frye, Duffy, and Walf, 2007). Without estrogen, P4 enhances the consolidation of memory of young adult, ovariectomized mice to improve performance in prefrontal (T-maze; object recognition) and hippocampal tasks (object placement, water maze, inhibitory avoidance, contextual fear conditioning (Sandstrom and Williams, 2001; Harburger, Bennett and Frick, 2007; Frye and Walf, 2008a). In general, acute administration of P4 post-training, and/or testing when hormone levels were still high, can improve cognitive performance of rodents (Asbury et al., 1998; Galea et al., 2000; Wood, Beylin and Shors, 2001; Walf, Rhodes and Frye, 2006; Frye, Duffy and Walf; 2007; Frye and Walf, 2008a,b; Paris and Frye, 2008). Long-term treatment with P4 influences the consolidation of learning and memory for Pavlovian training experiences and can modulate memory in part through influences on the hippocampus (Moralí et al., 2005; Robertson et al., 2006; Brinton et al., 2008). Chronic administration of P4 can also forestall cognitive decline in mice with an Alzheimer’s Disease phenotype (Frye and Walf, 2008c). Thus, some of P4’s effects on cognitive performance may be related to its capacity to prevent brain damage or decline.
Progesterone can have remarkable neuroprotective effects. In animal models, and among people, high levels of P4 can attenuate seizure-related cognitive or neural deficits; however, effects in lower dosage ranges in animals or in people are more variable (Frye, 2008; Herzog, 2009; Herzog and Frye, 2003). After traumatic brain injury, gonadally-intact female rats have a smaller lesion volume than do ovariectomized, age-matched females or male controls (Bramlett and Dietrich, 2001; Jones et al., 2005). “Wobbler” mice with motoneuron degeneration treated with P4 have reduced neuropathology and more myelination of their Schwann cells and oligodendrocytes than do their vehicle-administered counterparts (Schumacher et al., 2004). In cerebral ischemia, acute or chronic P4 attenuates brain lipid peroxidation and induces survival promoting protein kinases (Sugino et al., 1996; Reyna-Neyra et al., 2002; Balasubramanian et al., 2008; Cai et al., 2008). Improvements in cognitive functioning after experimental traumatic brain injury (TBI) have also previously been observed after P4 treatment (Goss, Hoffman and Stein, 2003; Shear, Galani, Hoffman and Stein, 2002). This improved performance could be due to reduced neuronal death, as P4 has been previously shown to promote neuronal survival after TBI (Asbury et al., 1998) and global ischemia (Cervantes et al., 2002). Thus, P4 can have protective effects against various insults, but how these effects relate to function are of interest.
The water maze is commonly used to assay spatial cognition, or learning and memory, in experimental rodent models. In the water maze, rats are trained to navigate to a platform located below the water’s surface. Spatial learning is then typically assessed a day later by determining the latencies and distances to find the hidden platform. Using this model, we have compared in the present study, the effect of P4 when administered prior to, or following, a regimen of kainic acid, that causes damage to the hippocampus (Buckmaster and Dudek, 1997; Ciriza, Azcoita, and Garcia-Segura, 2004; Ciriza, Carrero, Frye, and Garcia-Segura, 2006), to mediate effects on performance in the water maze. We hypothesized that P4 prior to kainic acid would result in better performance in the water maze.
METHODS
Animal subjects
Adult, female, Long-Evans rats from our in-house colony, originally-derived from stock from Taconic Inc. (Germantown, NY), were kept in a 12:12 h reversed dark: light cycle (lights off at 08:00) and received rodent chow and tap water ad libitum. As adults, approximately 55 days of age, rats were ovariectomized under xylazine (12 mg/kg, intraperitoneal- i.p.) and ketamine (80 mg/kg, i.p.) anesthesia. Rats recovered from ovariectomy for one week before inclusion in the study. Special care was taken to minimize animal suffering and to reduce the number of animals used in this study. Animal use was per NIH guidelines and protocols approved by The University at Albany’s Institutional Animal Care and Use and Committee.
Procedure- Experiment 1
The first experiment was designed to assess the nature and timeframe of progestogen (P4 and its metabolites, dihydroprogesterone (DHP) and 5α-pregnan-3α-ol-20-one (3α,5α-THP)) levels produced in plasma and the hippocampus following subcutaneous (s.c.) P4 administration. A second question was whether kainic acid administration altered these levels. As well, to determine the extent to which stress hormone levels were altered with these manipulations, corticosterone levels in plasma were determined. Rats received one s.c. injection of P4 (4 mg/Kg b.w., Steraloids, Newport, RI; in vegetable oil vehicle). This dosing was based upon the regimen that we have used in previous studies to investigate the effects of P4 to ovariectomized rats for neuroprotection from kainic acid-induced seizures (Ciriza et al., 2006; Frye and Scalise, 2000), and enhance cognitive performance of ovariectomized rats (Frye et al., 2007; Walf et al., 2006). Four hours later, animals received one i.p. injection of kainic acid (7 mg/Kg b.w., Sigma, St. Louis, MO) or vehicle (0.01M phosphate buffered saline). As we have previously reported, all rats that were administered kainic acid showed mild behavioral symptoms, including low activity followed by periods of hyperactivity, head nodding, myoclonic jerks of forelimbs and jaws, wet dog shaking, and/or modest salivation (Ciriza et al., 2006). Although the size of the lesion in the present study was not assessed, a thorough examination of the neural insult produced by this dosing of kainic acid has been described (Ciriza et al., 2004; 2006). In these studies, the number of hilar neurons in the hippocampus, and vimentin expression as a marker of neural damage, were assessed as primary indices. Kainic acid reduced the number of hilar neurons, as well produced damage in CA1-3 pyramidal layers, as well as increased vimentin expression in astrocytes (Ciriza et al., 2004; 2006). As such, this dosing of kainic acid was utilized in the present study.
Rats were euthanized by rapid decapitation immediately following kainic acid or vehicle injection, or 2, 6, or 10 hours later so that levels of corticosterone and progestogens could be assessed 4, 6, 10, and 14 hours after P4-administration. Following rapid decapitation, trunk blood and whole brains were collected. Trunk blood was centrifuged at 3,000 × g at 4°C so that plasma could be aliquoted into separate tubes for storage with whole brains at −80°C until used for radioimmunoassay. There were 3 samples per condition.
Progestogen Radioimmunoassay- Experiment 1
The levels of P4, DHP, and 3α,5α-THP in plasma and hippocampus were measured by radioimmunoassay according to previously published methods and are briefly described as follows (Frye and Bayon, 1999). Chilled test tubes containing aliquots of plasma were incubated with distilled water, sodium hydroxide, and tritiated steroids, and then steroids were extracted by adding ether to the tubes and snap-freezing and then drying down in a savant. Following gentle thawing on ice, the hippocampus was dissected out of the whole brains and weighed. The hippocampus was homogenized with 6 strokes of a glass/glass homogenizer in 50% methanol, 1% acetic acid, and tritiated steroid, and then centrifuged at 3,000 × g at 4°C. The supernatant was chromatographed on Sep-pak cartridges, first with 50% methanol, 1% acetic acid, and then with 50% methanol alone. The final elutes, collected with 100% methanol, were evaporated in a savant. Samples were then reconstituted with phosphate assay buffer to the original volume before the radioimmunoassays were set up. Standard curves, in duplicate, ranged from 5-4,000 pg for each assay. The standards were added to phosphate assay buffer, followed by addition of progestogen-specific antibodies and tritiated progestogens. The antibodies used were: P4 (P#337 from Dr G D Niswender, Colorado State University; 1:30,000 dilution), DHP (X-947; 1:5000) and 3α,5α-THP (#921412-5, from Dr Robert Purdy, Veterans Medical Affairs,). The tritiated steroids, from Perkin–Elmer (Boston, MA, USA), were: P4 (NET-208: specific activity = 47.5 Ci/mmol), and 3α,5α-THP (for DHP and 3α,5α-THP assays; NET-1047: specific activity = 65.0 Ci/mmol). The total assay volumes for P4, DHP, and 3α,5α-THP were 800, 950, and 1250 μl, respectively. Assays were incubated overnight at 4°C, and then separation of bound and free progestogens was done by rapidly adding ice cold dextran-coated charcoal to the tubes. Tubes were incubated with charcoal for 15 minutes on ice and then centrifuged at 1,200 × g at 4°C for 10 minutes and the supernatant was poured into a glass scintillation vial with 6 ml Scintiverse cocktail (Fisher Scientific). Unknowns were interpolated from counts determined by the scintillation counter and the standard curve using Assay Zap. Concentrations of progestogens were calculated as per Rodbard and Hutt’s (Rodbard, 1974) logit-log method, interpolation of the standards, and correction for recovery. Corrections were done for each sample based upon the amount of plasma and wet weight of the dissected hippocampus that were assayed, such that means are expressed as ng/ml for progestogens in plasma and ng/g for progestogens in hippocampus tissue. The intra-assay and inter-assay, coefficients of variance were: P4 (0.09 and 0.10), DHP (0.12 and 0.14), and 3α,5α-THP (0.14 and 0.14), respectively.
Corticosterone Radioimmunoassay- Experiment 1
Corticosterone was measured in 10 μl of each sample collected with radioimmunoassay methods as described (Frye and Bayon, 1999). Corticosterone was extracted from plasma by heating tubes with samples at 60-70°C for 30 min. Following this, samples were incubated at room temperature for 60 min with tritiated corticosterone and antibody. As described above, charcoal was utilized to separate bound and free steroid and samples were spun and decanted into scintillation vials with Scintiverse and counted and analyzed. Corrections were done for each sample based upon the amount of plasma that was assayed, such that means are expressed as ng/dl for corticosterone in plasma. The inter- and intra-assay reliability coefficients were 0.05 and 0.08, respectively
Procedure- Experiment 2
The second experiment was designed to assess the functional effects of kainic acid-induced ictal activity for water maze performance, and the extent to which physiological P4 regimen may abrogate behavioral deficits in this task. Rats received one s.c. injection of P4 (4 mg/Kg b.w., Steraloids, Newport, RI) or vehicle (vegetable oil vehicle) either 4 hours before, or 2 hours after, i.p. kainic acid (7 mg/Kg b.w., Sigma, St. Louis, MO) or vehicle (0.01M phosphate buffered saline) injections. Two and a half weeks later, rats were trained and tested in the water maze.
Morris Water Maze testing- Experiment 2
The water maze testing procedure was utilized to assess spatial memory as per Rhodes and Frye (2006), and modified from (Frye, 1995b; Morris, 1984). The water maze consisted of a blue tank (555 cm circumference, 71 cm deep) filled with 24–27°C water in a brightly-lit testing room with several extra-maze distal cues (shelving, door, faucet, experimenter at computer recording the data, videocamera mounted on ceiling). The water was made opaque with powdered milk so that the clear Plexiglas platform (5.3 × 5.3 × 33.5 cm), placed 2.5 cm below the surface of the water and 60 cm from the tank’s side, could not be seen. On Day 1, rats were habituated to swimming in the pool for 120 s. Habituation consisted of a free swim, with no platform in the tank. On Day 2, rats were trained to find the hidden platform over two consecutive trials. During these training trials, rats had 120 s to find the hidden platform, starting from each of two different positions on the edge of the pool furthest from the hidden platform. Rats that did not find the platform during training were guided to it by the experimenter. All rats remained on the platform for 45 s after each training trial. On Day 3, rats had four consecutive testing trials, in which the start positions were randomized across trials. Three start positions, one in each but the quadrant containing the hidden platform, were utilized to minimize large differences in distances from the start position to the platform (Vorhees and Williams, 2006). The latency to reach the platform, and the distances swam before reaching the platform, were assessed during the training and testing trials as indices of performance in this task. These measures were also utilized to measure swim speeds. As such, the latencies, distances, and swim speeds in each trial were highly correlated (0.82-1.0). Data were collected simultaneously by an experimenter and the Any-maze video tracking system (Stoelting Co., Wood Dale, IL).
Statistical Analyses
In Experiment 1, a Levene’s test demonstrated that there was heterogeneity of variance. As such, the non-parametric Kruskal Wallis test was used to assess differences in steroid levels. In Experiment 2, behavioral data were analyzed using two-way ANOVAs with kainic acid (vehicle or kainic acid) and P4 regimen (vehicle or P4 before kainic acid or P4 after kainic acid) as between-subjects variables. Fisher’s Least Significant Difference post-hoc tests were used to determine group differences. The alpha level for statistical significance was p ≤ 0.05, and a statistical trend was noted when p ≤ 0.10. Data are expressed as mean ± SEM.
RESULTS
In Experiment 1, the levels of corticosterone and progestogens in plasma and progestogens in the hippocampus were measured to verify that the dosing regimen of P4 utilized was effective in producing physiological levels of P4, DHP, and 3α,5α-THP, and determine if kainic acid altered corticosterone or progestogen levels. Although the differences did not reach statistical significance, kainic acid increased corticosterone levels in plasma (Table 1). As compared to typical values among ovariectomized rats for plasma corticosterone (~5.0 ng/dl ± 0.9 SEM; Walf and Frye, 2005), the present data demonstrate that kainic acid, but not vehicle, injections increase plasma corticosterone levels, with the highest levels produced immediately after injection.
Table 1.
Plasma levels of corticosterone (B), and plasma and hippocampus levels of progesterone (P4), dihydroprogesterone (DHP), and 5α-pregnan-3α-ol-20-one (3α,5α-THP) in rats administered vehicle or kainic acid 4 hours post-P4 administration. Tissues were collected 4, 6, 10, or 14 hours post-P4 administration (n=3).
| Conditions | Plasma levels | Hippocampus levels | ||||||
|---|---|---|---|---|---|---|---|---|
| Kainic acid condition | Time post-P4 | B (ng/dl) | P4 (ng/ml) | DHP (ng/ml) | 3α,5α-THP (ng/ml) | P4 (ng/g) | DHP (ng/g) | 3α,5α-THP (ng/g) |
| Vehicle | 4 hours | 0.2 ± 0.1 | 78.8 ± 0.3 | 38.1 ± 1.0 | 28.3 ± 10.3 | 34.3 ± 11.1 | 24.6 ± 17.3 | 15.6 ± 8.3 |
| 6 hours | 2.3 ± 1.1 | 55.4 ± 23.6 | 36.5 ± 2.7 | 15.2 ± 11.7 | 37.5 ± 30.2 | 8.2 ± 7.4 | 17.1 ± 6.0 | |
| 10 hours | 0.6 ± 0.2 | 38.9 ± 20.1 | 38.1 ± 1.0 | 19.8 ± 9.4 | 42.4 ± 18.1 | 12.4 ± 11.6 | 13.4 ± 5.0 | |
| 14 hours | 0.5 ± 0.1 | 7.4 ± 0.6 | 23.9 ± 7.7 | 16.4 ± 11.3 | 14.3 ± 2.8 | 9.8 ± 3.1 | 5.8 ± 2.1 | |
| Kainic acid | 4 hours | 19.8 ± 9.6 | 67.3 ± 11.8 | 38.6 ± 0.6 | 29.7 ± 8.8 | 24.1 ± 9.2 | 34.9 ± 24.9 | 13.6 ± 6.6 |
| 6 hours | 0.9 ± 0.3 | 79.0 ± 0.1 | 39.1 ± 0.1 | 6.6 ± 3.0 | 43.0 ± 16.1 | 12.0 ± 4.7 | 24.3 ± 7.9 | |
| 10 hours | 10.2 ± 9.6 | 53.5 ± 25.5 | 31.0 ± 8.1 | 19.4 ± 9.6 | 31.9 ± 7.5 | 7.5 ± 6.2 | 10.2 ± 3.3 | |
| 14 hours | 0.9 ± 0.3 | 10.7 ± 5.9 | 28.8 ± 5.4 | 4.6 ± 1.2 | 11.9 ± 3.7 | 7.8 ± 6.1 | 7.5 ± 0.4 | |
Although there were no statistically significant effects of kainic acid condition, or timing, for progestogen levels in plasma or the hippocampus, the data demonstrate that the P4 regimen utilized produced levels within a physiological range between 4 and 10 hours, which were low by 14 hours, post-P4 administration (Table 1). Indeed, ovariectomized rats typically have very low levels of P4 (plasma: ~7.0 ng/ml ± 5.0 SEM; hippocampus: ~2.0 ng/g ± 0.5 SEM), DHP (plasma: ~4.0 ng/ml ± 0.7 SEM; hippocampus: ~3.6 ng/g ± 0.4 SEM), and 3α,5α-THP (plasma: ~1.5 ng/ml ± 0.5 SEM; hippocampus: ~1.8 ng/g ± 0.2 SEM; Ciriza et al., 2006). The present results demonstrate that P4 regimen utilized produces physiological levels of progestogens in plasma and hippocampus.
In Experiment 2, the effects of P4 administration before or after kainic acid-induced seizures for water maze performance were determined. There were no significant differences between groups for mean latencies or distances swam to reach the platform over two training trials (Table 2). However, there were main effects of kainic acid and P4 condition for mean latencies and distances to the hidden platform during testing trials. Kainic acid, compared to vehicle, significantly increased latencies (F(1,49)=10.18, p < 0.01) and distances swam (F(1,49)=5.09, p < 0.02) to the hidden platform (Figure 1, Table 2). The main effects of P4 condition for latencies (F(2,49)=6.50, p < 0.01) and distances (F(2,49)=6.04, p < 0.01) demonstrated that rats administered P4 before kainic acid seizures had shorter latencies and distances swam to the platform compared to rats that were administered vehicle or P4 after the kainic acid-induced seizure (Figure 1, Table 2).
Table 2.
Training trials mean latencies and distances swam to the hidden platform and mean distances swam during the testing trial of rats administered with i.p. vehicle and s.c. vehicle (n=11), progesterone (P4) pre-vehicle (n=10), or P4 post-vehicle (n=10), or i.p. kainic acid and s.c. vehicle (n=8), P4 pre-kainic acid (n=8), or P4 post-kainic acid (n=8).
| Kainic acid condition | P4 condition | Training latencies (secs ± SEM) | Training distances (m ± SEM) | Testing distances (m ± SEM) |
|---|---|---|---|---|
| Vehicle | Vehicle | 37.3 ± 6.0 | 9.7 ± 1.2 | 6.8 ± 1.0 |
| Kainic acid | 48.3 ± 6.1 | 11.5 ± 1.4 | 11.4 ± 1.6** | |
| Vehicle | P4 before kainic acid | 39.3 ± 6.8 | 9.9 ± 1.6 | 6.2 ± 0.9ˆ |
| Kainic acid | 32.3 ± 4.5 | 7.3 ± 1.7 | 6.1 ± 0.9**ˆ | |
| Vehicle | P4 after kainic acid | 41.4 ± 6.1 | 10.0 ± 1.1 | 8.8 ± 0.9 |
| Kainic acid | 45.9 ± 4.1 | 11.7 ± 0.9 | 10.0 ± 0.6** |
p<0.05 compared to vehicle administration
p<0.05 compared to vehicle or P4 administration after kainic acid
Figure 1.
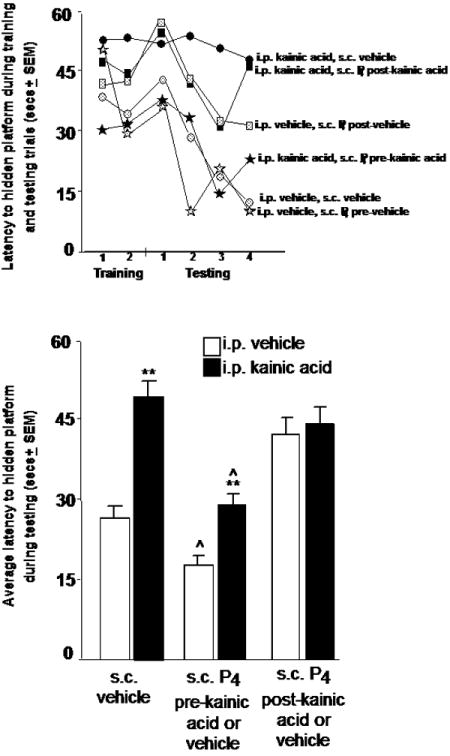
Top: Group means of rats for latencies (in secs) to reach the hidden platform across two training trials and four testing trials. Rats were injected with i.p. vehicle and s.c. vehicle (n=11), progesterone (P4) pre-vehicle (n=10), or P4 post-vehicle (n=10), or i.p. kainic acid and s.c. vehicle (n=8), P4 pre-kainic acid (n=8), or P4 post-kainic acid (n=8). Bottom: Testing trials mean latencies (mean secs ± SEM) to the hidden platform of rats administered vehicle or kainic acid and P4 before or after kainic acid seizure. ** p<0.05 compared to vehicle administration ˆ p<0.05 compared to vehicle or P4 administration after kainic acid
DISCUSSION
The present data supported our hypothesis that P4 prior to, but not after, kainic acid would improve performance in the water maze of rats, suggesting a functional protective effect of P4. In the first experiment, the timeframe and nature of progestogens and stress hormones produced by an s.c. injection of P4 (4 mg/kg) to ovariectomized rats was determined. Circulating and hippocampal levels of progestogens produced by P4 were increased, and within a physiological range, between 4 and 10 hours, but declined by 14 hours, post- administration; albeit, there were no statistically significant group differences. There was no effect of the kainic acid treatment on these levels of progestogens, but rats injected with kainic acid had higher corticosterone levels in plasma acutely after its administration, but this effect did not reach statistical significance. In the second experiment, a physiological P4 regimen was administered to rats before, or following, kainic acid-induced seizures, and effects on water maze performance were compared to rats administered vehicle. Rats administered kainic acid had increased latencies and distances swam to the hidden platform than did rats administered vehicle, indicating poorer performance in this task. Notably, administration of P4 before, but not after, kainic acid reduced these performance deficits. Thus, these data suggest that a physiological regimen of P4 can prevent some of the water maze performance deficits observed following kainic acid-induced ictal activity.
The present data support previous studies on the neuroprotective effects of progestogens. Progestogens show neuroprotective effects in a variety of insult models (e.g. oxidative stress, excitatory neurotoxicity, ischemia and post-injury cerebral edema; Sugino et al., 1996; Wang et al., 2006; O’Connor et al., 2007). Furthermore, P4 can have effects to reduce damage as a secondary effect. For instance, P4 reduces edema, having subsequent effects to spare neurons from secondary neuronal death and improves cognitive function following injury (Roof et al., 1994, 1996, 1997). Secondary neuronal loss following cortical contusion among male rats can be reduced by P4 (O’Connor et al., 2006; Guo et al., 2006; Djebaili, Hoffman and Stein, 2004). A question is the dosing of progestogens that can have these protective effects. Here, we provide data characterizing the timing and nature of progestogens produced in circulation and the hippocampus following a single s.c. injection of one dosage of P4 to ovariectomized rats. The P4, DHP, and 3α,5α-THP levels produced both in circulation and in the hippocampus by 4 mg/kg s.c. P4 in vegetable oil are similar to those reported in these tissues among proestrous rats that are naturally-receptive (Butcher et al., 1974; Horikoshi and Suzuki, 1974; Shaikh and Shaikh, 1975; Belanger et al., 1981; Purdy et al., 1990; Palumbo et al., 1995; Frye et al., 1998; Frye and Bayon, 1999; Frye et al., 2000). This was important to determine because some of the neuroprotective effects of P4 may be regimen-dependent. As such, we were interested in investigating effects of a physiologically-relevant range of progestogens when kainic acid was administered to rats in Experiment 2. We have previously demonstrated neuroprotective effects of P4 against kainic acid excitotoxicity occurs in the 1 to 4 mg/kg b.w. range, rather than 8 mg/Kg b.w., when administered to ovariectomized rats (Ciriza et al., 2006). Other studies have demonstrated that low doses of P4, which likely produced physiological levels similar to what we have observed here, reduce ictal activity and hippocampal damage following kainic acid administration to ovariectomized rats (Hoffman et al., 2003). However, less hippocampal neuroprotection has been noted following traumatic brain injury with higher dosing of P4, which likely produces circulating P4 levels in the high physiological to supraphysiological range (Robertson et al., 2006). Another consideration to make is the timing of the effects of P4. In the present study, we found that P4 dosing before kainic acid seizures reduced latencies and distances swam to the hidden platform in the water maze. Administration of P4 following kainic acid-induced seizures did not have the same protective effect. Other studies have demonstrated that long-term treatment with P4, with or without co-administration of estradiol, do not have neuroprotective effects against kainic acid-induced cell loss in the hippocampus of young or middle-aged female rats (Rosario et al., 2006; Carroll et al., 2008). It has been proposed that post-injury P4 treatments allow a greater range of dosages of P4 treatments than do pre-injury treatments (Goss et al., 2001). As such, it was important in the present study to determine these circulating and hippocampal levels of progestogens, and relate them to a functional response comparing a post-injury and pre-injury treatment of P4. Of interest in future studies would be examination of the dose-responsive effects of P4 administered before neuronal injury to further understand how P4 can have protective effects to prevent neuronal damage. Indeed, the nature of progestogens’ effects are likely much different for reversal of damage. For example, survival rates following motor vehicle accident traumatic brain injury are increased by sixty percent if a very high dosage of progesterone (450 mg) is administered within 8-24 hr of accident. However, in terms of functional recovery, effects have been modest and large difference in Glasgow Coma scores of persons administered P4 compared to placebo/vehicle are not immediately found, despite increased survival (Stein, 2010). Thus, P4, when administered acutely in dosage that produces physiological levels of P4 and its metabolites, before kainic acid injury, but not after, reduces functional deficits in the water maze of female rats.
An important consideration is whether variations in P4 metabolism may underlie the protective effects of P4 in different animal models of neural insult (Hoffman et al., 2003). In the central nervous system, the neuroprotective effects of P4 (Frye, 1995a; Frank and Sagratella, 2000; Frye and Scalise, 2000; Lockhart et al., 2002; Ciriza et al., 2004; Djebaili et al., 2004; He et al., 2004; Rhodes and Frye, 2004; Rhodes, McCormick, and Frye, 2004; Djebaili et al., 2005; Rhodes and Frye, 2005a;) are partly mediated by its metabolites subsequent to their rapid conversion by 5α-reductase and 3α-hydroxysteroid dehydrogenase to DHP and 3α,5α-THP (Mellon et al., 2001), respectively. Indeed, P4 can have beneficial effects along a continuum of function (i.e. from improving memory to having neuroprotective effects against damage), and these effects are likely due to actions of its metabolites. For example, P4 administration improves spatial memory of young, ovariectomized rats and mice as well as aged female mice (Frye et al., 2007; Frye and Walf, 2008a,b). However, blocking P4 metabolism in the brain with co-administration of s.c. medroxyprogesterone acetate, a clinically-used synthetic progestin, attenuate the mnemonic and neuroprotective effects of P4 in female rodents (Littleton-Kearney et al., 2005; Ciriza et al., 2006; Frye, Koonce, and Walf, 2010) and in cell culture models (Nilsen and Brinton, 2002a,b). Similarly, the 5α-reductase inhibitor, finasteride, and the 3α-hydroxysteroid dehydrogenase inhibitor, indomethacin, administered systemically to female rats blocked conversion of P4 to DHP and 3α,5α-THP and its neuroprotective effects (i.e. increased gliosis) following kainic acid administration (Ciriza et al., 2006). These data, together with the present results, suggest that both DHP and 3α,5α-THP may be necessary for the neuroprotective effects of P4.
The hippocampus is a major central nervous system target for the conversion to, and actions of, P4 metabolites. The hippocampus has high expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase (Li et al., 1997). In the present study, the hippocampus as a potential target for the protective effects of progestogens was investigated in three ways. First, progestogen levels were measured in the grossly-dissected out hippocampus following P4 dosing. P4 treatment produced physiological levels of P4, DHP, and 3α,5α-THP in these hippocampus samples. Second, the hippocampus may be sensitive to damage by kainic acid and other excitotoxins, and amenable to protection by P4. Progestogens protect hilar neurons in the hippocampus from degeneration following administration of kainic acid to female rats (Ciriza et al., 2004) and perforant pathway stimulation in male rats (Frye, 1995a). We have shown that P4 reduces reactive gliosis, a marker of degeneration and inflammation, and neuronal loss in hilar neurons in the hippocampus after kainic acid treatment coincident with formation of DHP and 3α,5α-THP in the hippocampus (Ciriza et al., 2006). Third, the functional effects of P4 were assessed by using the Morris water maze, which is traditionally considered a hippocampus-mediated task. Kainic acid produced performance deficits among rats that were not administered P4 prior to kainic acid-induced seizures. Administration of P4 before kainic acid-induced ictal activity prevented these performances deficits in the water maze when rats were assessed 2.5 weeks after kainic acid administration; albeit, more details about hippocampal involvement may have been gained if a different protocol, with more trials, were used to assess this. Nonetheless, the present effects are congruent with a previous report demonstrating that 3α,5α-THP pre-treatment reduced performance deficits in the water maze two weeks following perforant pathway stimulation-induced seizures of male rats (Frye, 1995a). Of interest in future studies would be to examine the histological damage produced by kainic acid, and effects of P4 dosing. Indeed, whether the functional deficits in the water maze produced by kainic acid, and mitigated by progesterone, are associated with histological differences in the hippocampus, would be of great interest in future studies.
The mechanisms involved in the neuroprotective effects of progestogens are of interest. One target for these effects may be intracellular P4 receptors (PRs), which are expressed throughout the brain and in the hippocampus (Guerra-Araiza et al., 2003), and by which P4 and DHP bind and may have their neuroprotective effects at low, physiological dosages (Rupprecht et al., 1993; Melcangi et al., 1999; Ciriza et al., 2004). As well, of interest is the membrane PRs (Zhu et al., 2003), which we are currently investigating the functional role of in rodents. 3α,5α-THP exerts rapid membrane-mediated effects through the gamma-aminobutyric acid type A receptor complex (Follesa et al., 2001; Lambert et al., 2003; Belelli et al., 2005), which may contribute to the neuroprotective effects of P4 (Frye and Scalise, 2000; Melcangi et al., 2001; Rhodes and Frye, 2005a,b). Indeed, it may be that progestogens have complementary mechanisms for neuroprotection. Progestogens can reduce oxidative stress following injury. In support, P4 reduced maleic dialdehyde levels in the hippocampus and striatum (Ozacmak and Sayan, 2009). Following cerebral ischemia, there is some evidence for reduction in brain lipid peroxidation and increases in protein kinases associated with neuronal survival (Sugino et al., 1996; Reyna-Neyra, Camacho-Arroyo, Ferrera and Arias, 2002; Balasubramanian et al., 2008; Cai et al., 2008). In addition to neuroprotective effects, as observed here, and as discussed above, P4 can also promote trophic actions, such as the growth of nerve processes, and mediate synaptic/neural plasticity (Reyna-Neyra et al., 2002; Foy, Akopian and Thompson, 2008). In addition to reducing the expression of pro-apoptotic proteins (e.g. Bax, Bad and caspase-3; Djebaili et al., 2005; Yao et al., 2005), P4 increases the expression of anti-apoptotic proteins (e.g. Bcl-2 and Bcl-XL; Nilsen and Brinton, 2002b; Yao et al., 2005). Furthermore, progestogens activate signal transduction cascades associated with cell survival (Singh, 2001; Nilsen and Brinton, 2002b, 2003; Singh, 2005) and regulates trophic factor expression (e.g. brain-derived neurotrophic factor; Gonzalez et al., 2005). Together, these data substantiate further investigation into the mechanisms of progestogens for their growth-enhancing and neuroprotective effects.
Acknowledgments
This work was supported by grants from National Institutes of Health (NIMH) and National Science Foundation (IBN03-16083). The assistance provided by Dr. Madeline Rhodes and Jason Paris is greatly appreciated.
References
- Asbury ET, Fritts ME, Horton JE, Isaac WL. Progesterone facilitates the acquisition of avoidance learning and protects against subcortical neuronal death following prefrontal cortex ablation in the rat. Behav Brain Res. 1998;97:99–106. doi: 10.1016/s0166-4328(98)00031-x. [DOI] [PubMed] [Google Scholar]
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: I. Protein kinase C activation in the hypothalamus of female rats. Endocrinology. 2008;149:5509–5517. doi: 10.1210/en.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger A, Cusan L, Caron S, Barden N, Dupont A. Ovarian progestins, androgens and estrogen throughout the 4-day estrous cycle in the rat. Biol Reprod. 1981;24:591–596. doi: 10.1095/biolreprod24.3.591. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Cai W, Zhu Y, Furuya K, Li Z, Sokabe M, Chen L. Two different molecular mechanisms underlying progesterone neuroprotection against ischemic brain damage. Neuropharmacology. 2008;55:127–138. doi: 10.1016/j.neuropharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Pike CJ. Progesterone blocks estrogen neuroprotection from kainate in middle-aged female rats. Neurosci Lett. 2008;445:229–32. doi: 10.1016/j.neulet.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes M, Gonzalez-Vidal MD, Ruelas R, Escobar A, Morali G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33:6–14. doi: 10.1016/s0188-4409(01)00347-2. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–28. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Guo Q, Pettus EH, Hoffman SW, Stein DG. The neurosteroids progesterone and allopregnanolone reduce cell death, gliosis, and functional deficits after traumatic brain injury in rats. J Neurotrauma. 2005;22:106–118. doi: 10.1089/neu.2005.22.106. [DOI] [PubMed] [Google Scholar]
- Follesa P, Concas A, Porcu P, Sanna E, Serra M, Mostallino MC, Purdy RH, Biggio G. Role of allopregnanolone in regulation of GABA(A) receptor plasticity during long-term exposure to and withdrawal from progesterone. Brain Res Brain Res Rev. 2001;37:81–90. doi: 10.1016/s0165-0173(01)00125-4. [DOI] [PubMed] [Google Scholar]
- Foy MR, Akopian G, Thompson RF. Progesterone regulation of synaptic transmission and plasticity in rodent hippocampus. Learn Mem. 2008;15:820–822. doi: 10.1101/lm.1124708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C, Sagratella S. Neuroprotective effects of allopregnenolone on hippocampal irreversible neurotoxicity in vitro. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:1117–1126. doi: 10.1016/s0278-5846(00)00124-x. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neurosteroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995a;696:113–120. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- Frye CA. Estrus-associated decrements in a water maze task are limited to acquisition. Physiol Behav. 1995b;57:5–14. doi: 10.1016/0031-9384(94)00197-d. [DOI] [PubMed] [Google Scholar]
- Frye CA. Hormonal influences on seizures: basic neurobiology. Int Rev Neurobiol. 2008;83:27–77. doi: 10.1016/S0074-7742(08)00003-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE. Mating stimuli influence endogenous variations in the neurosteroids 3α,5α-THP and 3α-Diol. J Neuroendocrinol. 1999;11:839–847. doi: 10.1046/j.1365-2826.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Bayon LE, Pursnani NK, Purdy RH. The neurosteroids, progesterone and 3α,5α-THP, enhance sexual motivation, receptivity, and proceptivity in female rats. Brain Res. 1998;808:72–83. doi: 10.1016/s0006-8993(98)00764-1. [DOI] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA. Mnemonic effects of progesterone to mice require formation of 3α,5α-THP. Neuroreport. 2010;21:590–5. doi: 10.1097/WNR.0b013e32833a7e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Scalise TJ. Anti-seizure effects of progesterone and 3α,5α-THP in kainic acid and perforant pathway models of epilepsy. Psychoneuroendocrinology. 2000;25:407–420. doi: 10.1016/s0306-4530(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol Learn Mem. 2008a;90:171–177. doi: 10.1016/j.nlm.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Progesterone enhances performance of aged mice in cortical or hippocampal tasks. Neurosci Lett. 2008b;437:116–120. doi: 10.1016/j.neulet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Effects of progesterone administration and APPswe+PSEN1Deltae9 mutation for cognitive performance of mid-aged mice. Neurobiol Learn Mem. 2008c;89:17–26. doi: 10.1016/j.nlm.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. Spatial working memory and hippocampal size across pregnancy in rats. Horm Behav. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94:143–9. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–242. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C, Villamar-Cruz O, Gonzalez-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15:984–990. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Bennett JC, Frick KM. Effects of estrogen and progesterone on spatial memory consolidation in aged females. Neurobiol Aging. 2007;28:602–610. doi: 10.1016/j.neurobiolaging.2006.02.019. [DOI] [PubMed] [Google Scholar]
- He J, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, enhances behavioral recovery and decreases neuronal loss after traumatic brain injury. Restor Neurol Neurosci. 2004;22:19–31. [PubMed] [Google Scholar]
- Herzog AG. Hormonal therapies: progesterone. Neurotherapeutics. 2009;6:383–91. doi: 10.1016/j.nurt.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–1. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;182:124–134. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- Horikoshi H, Suzuki Y. On circulating sex steroids during the estrous cycle and the early pseudopregnancy in the rat with special reference to its luteal activation. Endocrinol Jpn. 1974;21:69–79. doi: 10.1507/endocrj1954.21.69. [DOI] [PubMed] [Google Scholar]
- Jones NC, Constantin D, Prior MJ, Morris PG, Marsden CA, Murphy S. The neuroprotective effect of progesterone after traumatic brain injury in male mice is independent of both the inflammatory response and growth factor expression. Eur J Neurosci. 2005;21:1547–1554. doi: 10.1111/j.1460-9568.2005.03995.x. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABAA receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Li X, Bertics PJ, Karavolas HJ. Regional distribution of cytosolic and particulate 5α-dihydroprogesterone 3α-hydroxysteroid oxidoreductases in female rat brain. J Steroid Biochem Mol Biol. 1997;60:311–318. doi: 10.1016/s0960-0760(96)00195-1. [DOI] [PubMed] [Google Scholar]
- Littleton-Kearney MT, Klaus JA, Hurn PD. Effects of combined oral conjugated estrogens and medroxyprogesterone acetate on brain infarction size after experimental stroke in rat. J Cereb Blood Flow Metab. 2005;25:421–426. doi: 10.1038/sj.jcbfm.9600052. [DOI] [PubMed] [Google Scholar]
- Lockhart EM, Warner DS, Pearlstein RD, Penning DH, Mehrabani S, Boustany RM. Allopregnanolone attenuates N-methyl-D-aspartate-induced excitotoxicity and apoptosis in the human NT2 cell line in culture. Neurosci Lett. 2002;328:33–36. doi: 10.1016/s0304-3940(02)00448-2. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Magnaghi V, Galbiati M, Martini L. Formation and effects of neuroactive steroids in the central and peripheral nervous system. Int Rev Neurobiol. 2001;46:145–176. doi: 10.1016/s0074-7742(01)46062-4. [DOI] [PubMed] [Google Scholar]
- Melcangi RC, Magnaghi V, Martini L. Steroid metabolism and effects in central and peripheral glial cells. J Neurobiol. 1999;40:471–483. [PubMed] [Google Scholar]
- Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- Morali G, Letechipia-Vallejo G, Lopez-Loeza E, Montes P, Hernandez-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett. 2005;382:286–290. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002a;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. NeuroReport. 2002b;13:825–830. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- O’Connor CA, Cernak I, Johnson F, Vink R. Effects of progesterone on neurologic and morphologic outcome following diffuse traumatic brain injury in rats. Exp Neurol. 2007;205:145–153. doi: 10.1016/j.expneurol.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Ozacmak VH, Sayan H. The effects of 17β estradiol, 17α-estradiol and progesterone on oxidative stress biomarkers in ovariectomized female rat brain subjected to global cerebral ischemia. Physiol Res. 2009;58:909–12. doi: 10.33549/physiolres.931647. [DOI] [PubMed] [Google Scholar]
- Palumbo MA, Salvestroni C, Gallo R, Guo AL, Genazzani AD, Artini PG, Petraglia F, Genazzani AR. Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endocrinol Invest. 1995;18:853–856. doi: 10.1007/BF03349832. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, Rubinow DR, Morrow AL, Paul SM. Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Reyna-Neyra A, Camacho-Arroyo I, Ferrera P, Arias C. Estradiol and progesterone modify microtubule associated protein 2 content in the rat hippocampus. Brain Res Bull. 2002;58:607–612. doi: 10.1016/s0361-9230(02)00829-8. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Progestins in the hippocampus of female rats have antiseizure effects in a pentylenetetrazole seizure model. Epilepsia. 2004;45:1531–1538. doi: 10.1111/j.0013-9580.2004.16504.x. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Attenuating 5α-pregnane-3α-ol-20-one formation in the hippocampus of female rats increases pentylenetetrazole-induced seizures. Epilepsy Behav. 2005a;6:140–6. doi: 10.1016/j.yebeh.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. Actions at GABA(A) receptors in the hippocampus may mediate some antiseizure effects of progestins. Epilepsy Behav. 2005b;6:320–7. doi: 10.1016/j.yebeh.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERβ-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, McCormick CM, Frye CA. 3α,5α-THP mediates progestins’ effects to protect against adrenalectomy-induced cell death in the dentate gyrus of female and male rats. Pharmacol Biochem Behav. 2004;78:505–512. doi: 10.1016/j.pbb.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Robertson CL, Puskar A, Hoffman GE, Murphy AZ, Saraswati M, Fiskum G. Physiologic progesterone reduces mitochondrial dysfunction and hippocampal cell loss after traumatic brain injury in female rats. Exp Neurol. 2006;197:235–243. doi: 10.1016/j.expneurol.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Rodbard D. Statistical quality control and routine data processing for radioimmunoassay and immunoradiometric assays. Clin Chem. 1974;20:1255–1270. [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Heyburn JW, Stein DG. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp Neurol. 1996;138:246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31:1–11. doi: 10.1007/BF02815156. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099:206–10. doi: 10.1016/j.brainres.2006.03.127. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, van SB, Wetzel C, Damm K, Zieglgansberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115:384–393. [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Robert F, Carelli C, Gago N, Ghoumari A, Gonzalez Deniselle MC, Gonzalez SL, Ibanez C, Labombarda F, Coirini H, Baulieu EE, De Nicola AF. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res. 2004;14(Suppl A):S18–S33. doi: 10.1016/j.ghir.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Shaikh AA, Shaikh SA. Adrenal and ovarian steroid secretion in the rat estrous cycle temporally related to gonadotropins and steroid levels found in peripheral plasma. Endocrinology. 1975;96:37–44. doi: 10.1210/endo-96-1-37. [DOI] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Singh M. Ovarian hormones elicit phosphorylation of Akt and extracellular-signal regulated kinase in explants of the cerebral cortex. Endocrine. 2001;14:407–415. doi: 10.1385/ENDO:14:3:407. [DOI] [PubMed] [Google Scholar]
- Singh M. Mechanisms of progesterone-induced neuroprotection. Ann NY Acad Sci. 2005;1052:145–151. doi: 10.1196/annals.1347.010. [DOI] [PubMed] [Google Scholar]
- Stein DG. Progesterone and its metabolites in the treatment of traumatic brain injury and other CNS disorders. International Behavioral Neuroscience Society Conference, 2010, presentation.2010. [Google Scholar]
- Sugino N, Shimamura K, Tamura H, Ono M, Nakamura Y, Ogino K, Kato H. Progesterone inhibits superoxide radical production by mononuclear phagocytes in pseudopregnant rats. Endocrinology. 1996;137:749–754. doi: 10.1210/endo.137.2.8593826. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. Antianxiety and antidepressive behavior produced by physiological estradiol regimen may be modulated by hypothalamic-pituitary-adrenal axis activity. Neuropsychopharmacology. 2005;30:1288–301. doi: 10.1038/sj.npp.1300708. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2006;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KK, Larner SF, Robinson G, Hayes RL. Neuroprotection targets after traumatic brain injury. Curr Opin Neurol. 2006;19:514–519. doi: 10.1097/WCO.0b013e3280102b10. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Yao XL, Liu J, Lee E, Ling GS, McCabe JT. Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J Neurotrauma. 2005;22:656–668. doi: 10.1089/neu.2005.22.656. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]


