Abstract
Scope
Selenium has complex effects in vivo on multiple homeostatic mechanisms such as redox balance, methylation balance, and epigenesis, via its interaction with the methionine-homocysteine cycle. In this study, we examined the hypothesis that selenium status would modulate both redox and methylation balance and thereby modulate myocardial structure and function.
Methods and Results
We examined the effects of selenium deficient (<0.025 mg/kg), control (0.15 mg/kg), and selenium supplemented (0.5 mg/kg) diets on myocardial histology, biochemistry and function in adult C57/BL6 mice. Selenium deficiency led to reactive myocardial fibrosis and systolic dysfunction accompanied by increased myocardial oxidant stress. Selenium supplementation significantly reduced methylation potential, DNA methyltransferase activity and DNA methylation. In mice fed the supplemented diet, inspite of lower oxidant stress, myocardial matrix gene expression was significantly altered resulting in reactive myocardial fibrosis and diastolic dysfunction in the absence of myocardial hypertrophy.
Conclusions
Our results indicate that both selenium deficiency and modest selenium supplementation leads to a similar phenotype of abnormal myocardial matrix remodeling and dysfunction in the normal heart. The crucial role selenium plays in maintaining the balance between redox and methylation pathways needs to be taken into account while optimizing selenium status for prevention and treatment of heart failure.
Keywords: Selenium, Collagen, Oxidant Stress, Homocysteine, Methylation
Introduction
Dietary selenium deficiency is considered to be a causative factor in various forms of heart failure such as the endemic cardiomyopathy prevalent in China termed Keshan disease [1], in subjects receiving total parenteral nutrition [2], peripartum cardiomyopathy [3], and in patients with acquired immune deficiency syndrome [4]. Lower blood selenium levels are observed in patients with heart failure with reduced left ventricular contractility or systolic heart failure [5–7]. Selenium, as part of a combination of high dose micronutrients, has been shown to improve left ventricular function and quality of life in systolic heart failure in a small clinical study [8]. However, the exact link between selenium deficiency and cardiac dysfunction is not well understood; one hypothesis is that deficiency of selenium potentiates the pathogenicity of other factors such as viral infection [9]. Even though preclinical studies utilizing models such as ischemia-reperfusion injury and adriamycin-induced cardiomyopathy have demonstrated the benefit of supplemental selenium [10, 11], dietary selenium supplements have not been conclusively shown to prevent cardiovascular events in clinical studies [12]. In fact, recent large clinical studies have shown a potential increased risk of diabetes with selenium supplements [13–15]. Hence, the effects of selenium on myocardial structure and function need to be better understood to allow the use of selenium to prevent and treat heart failure. This is especially important since recent surveys have shown that selenium supplements are widely used [16] inspite of inconclusive data.
Selenium has complex and multiple biological effects derived from its metabolism in vivo as well as through the intersection of selenium with other metabolic and homeostatic pathways including redox balance and epigenetic modification of DNA and histones [10, 17–22]. A major metabolic pathway with which selenium interacts is the methionine-homocysteine cycle [23–25], and this interaction is crucial to the actions of selenium in vivo via effects on both redox balance and on DNA methylation. A better understanding of the biological effects of selenium and its in vivo interaction with the methionine-homocysteine cycle would be crucial to the appropriate clinical use of selenium. Our previous work has shown that perturbations in the methionine-homocysteine cycle can affect myocardial interstitial remodeling and lead to myocardial fibrosis and dysfunction [26–28]. In addition to increasing anti-oxidant defenses via selenoproteins such as glutathione peroxidases (GPx) and thioredoxin reductases (TRRs), selenium has also been shown to affect flux through the methionine-homocysteine cycle and, thereby, affect methylation balance and DNA methylation [22–25, 29–32]. Hence we examined the hypothesis that dietary selenium modulates myocardial matrix remodeling via concomitant effects on redox and methylation status.
Materials and Methods
Animal model
All procedures in this study were approved by the Institutional Animal Care and Use Committee of Boston University School of Medicine (Protocol AN-14822) and Harvard Medical School (Protocol 04782) and conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Male C57BL6 mice (8–10 weeks old) were purchased from Charles River Laboratories (Boston, MA, USA) and were maintained in our institutional Division of Laboratory Animal Medicine on a 12:12 light-to-dark cycle with free access to chow and water. The animals were randomized into 3 groups – control amino-acid defined diet (Control); selenium-deficient diet (SD); and selenium supplemented diet (SS) (Harlan Teklad, Indianapolis, Indiana, USA). The control amino-acid defined diet was similar to that utilized in our prior published studies [28]. A selenium-deficient mineral mix was utilized in all the diets and sodium selenite was added to achieve a selenium content of 0.15 mg/kg in the Control diet and 0.5 mg/kg in the SS diet. The SD diet did not have added sodium selenite; its selenium level was <0.025 mg/kg. Dietary treatment was initiated after acclimatization for 7–10 days to the facility, and continued for 3 or 12 weeks, at the end of which euthanasia was performed by inducing deep anesthesia with a mixture of xylazine (10 mg/kg) and ketamine (90–200mg/kg) intraperitoneally followed by vital organ removal within 3–5 minutes. For histological and biochemical measurements, we studied 5–6 animals per group, while for measurements of cardiac function, 6–8 animals/group were utilized.
Histological analysis of myocardial remodeling
Coronal sections of ventricular myocardium were fixed in 10% neutral buffered formalin, and serial sections (5 μm) were stained with hematoxylin and eosin for estimating myocyte size, and with picrosirius red for estimating fibrillar collagen. Perivascular collagen, coronary arteriolar wall thickening, and interstitial collagen volume fraction were measured as described previously [26]. Immunostaining was performed as described previously [26] utilizing mouse monoclonal anti-4-hydroxynonenal (HNE) antibody (Oxis International, Portland, OR). Intensity of immunostaining was graded on a scale of 0–4 by an investigator who was blinded to the groups.
Measurement of ventricular function
Cardiac function was measured ex vivo utilizing isolated perfused Langendorff heart preparations [26]. Briefly systolic and diastolic function was assessed by measuring developed pressure, +dP/dtmax, diastolic pressure-volume relationship and −dP/dtmax at various preload balloon volumes normalized to heart weight.
Determination of plasma thiols
Total plasma thiol (homocysteine, cysteine, and glutathione) concentrations were determined as previously described [33] (See Supplemental Methods for details).
Determination of liver S-adenosylmethionine and S-adenosylhomocysteine levels
S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) were determined in a 50 ul aliquot of liver homogenate using C-18 reversed phase HPLC and UV detection at 254 nm [34] (See Supplemental Methods for details).
Measurement of the plasma selenium level
Plasma selenium levels were measured using a PerkinElmer model A Analyst 600 Atomic Absorption Spectrometer, equipped with longitudinal Zeeman-effect background correction, a THGA graphite furnace, and an AS800 autosampler (PerkinElmer, Waltham, MA) [35]. Briefly, mouse plasma samples (10ul) were diluted with 0.2% HNO3 (190ul). The diluted samples (20ul) was mixed with a matrix modifier solution containing 0.1% Pb(NO3)2 and 0.01% Mg(NO3)2 (6ul) (PerkinElmer). The matrix modifier solution was included to reduce interference as well as to enhance sensitivity and repeatability. A selenium solution (1,000ug/ml) (PerkinElmer) was used as the external calibration standards (5ug/L and 15ug/L). The limit of the selenium measurement was 0.6ug/L.
Measurement of myocardial glutathione peroxidase activity
Myocardial (glutathione peroxidase) GPx activity was measured as described in prior publications from our laboratory [35] (See Supplemental Methods for details).
Measurement of plasma isoprostanes
Free 8-isoprostane level was determined using a commercially available enzyme immunoassay kit as previously described [36] (See Supplemental Methods for details).
Real-time PCR analysis
Total RNA was extracted from frozen mouse heart sections using RNeasy Fibrous Tissue Mini-columns (catalog # 74704, Qiagen Inc. Germantown, MD) and real-time PCR performed as previously described [35]. Following specific primers were utilized: GPx-1 (catalog # Mm00656767_g1), tissue inhibitor of matrix metalloproteinase (TIMP)-3 (catalog # Mm01224941_m1), alpha 2 chain of type I collagen (COL1A2; catalog # Mm01165187_m1), alpha 1 chain of type III collagen (COL3A1; catalog # Mm01254476_m1), matrix metalloproteinase (MMP)-13 (catalog # Mm00439491_m1), and Thioredoxin reductase-1 (cat# Mm00443675_m1).
DNA Methyltransferase activity assay
Mouse whole heart nuclear extracts were harvested using EpiQuik Nuclear Extraction Kit (Epigentek, New York, NY) following the manufacturer’s protocol. Protein concentrations were quantified using the Bradford assay (BioRad, Hercules, CA), and 20μg of nuclear protein was used to measure DNA methyltransferase activity utilizing the EpiQuik DNA Methyltransferase Activity/Inhibition Colorimetric Assay Kit (catalog# P3001, Epigentek, New York, NY) according to manufacturer’s instructions. The 96 well plate provided in the kit has cytosine-rich DNA substrate embedded in the wells. The nuclear extracts were added to the plate and incubated with the substrate and the provided assay buffers for 1 hour. Subsequently, capture and detection antibodies were added to detect the methylated DNA. DNA methyltransferase activity was calculated from the amount of methylated DNA as per manufacturer’s protocol.
Long Interspersed Nuclear Element (LINE)-1 Methylation Assay
Analysis of global methylation status was performed by measuring the methylation status of five CpG sites in the mouse LINE-1 element that are highly methylated in the normal state, using bisulfite modification and Pyrosequencing by EpigenDx, Inc.(Worcester, MA; http://www.epigendx.com/). Briefly, DNA from ventricular extracts was treated with sodium bisulfite to effect conversion of unmethylated cytosines to uracil, and subsequently DNA sequence was analyzed by PCR amplification and Pyrosequencing [37].
Statistical Analysis
Data were evaluated by ANOVA with a Student-Newman-Keuls post-hoc test or by t-test as appropriate using GraphPad Prism (Graph Pad Software, La Jolla, CA). Two-way ANOVA was utilized to estimate the interaction of dietary selenium content with time (3 week vs. 12 week dietary treatment). The criterion for significance was a p value <0.05. Data are reported as means ±SEM.
Results
Effects of dietary selenium content on myocardial remodeling
As shown in Table 1, neither selenium deficiency nor supplemental selenium affected body weight or heart weight after 12 weeks of dietary treatment. Coronary arteriolar wall thickness did not vary between groups; however, perivascular and interstitial collagen levels in the left ventricle were significantly increased in both the selenium deficient and selenium supplemented groups compared to the control diet (Table 1). Interstitial fibrosis was also increased in the right ventricle in the SD (4.7±0.4%) and SS (4.0±0.5%) groups compared to the control group (2.4±0.2%; p < 0.05 on ANOVA). There was no histological evidence of myocyte hypertrophy, and myocyte size did not vary between groups (data not shown). Figure 1 shows representative sections of the left ventricle from the three groups demonstrating a significant increase in interstitial collagen in the groups with deficient and excess dietary selenium.
Table 1.
Cardiac morphometric indices after 12 weeks of dietary treatment.
| Parameter | Dietary groups (n=7–8/group) | ||
|---|---|---|---|
| Selenium Deficient | Control | Selenium Supplemented | |
| Body weight (g) | 37.2±0.6 | 37.0±1.1 | 35.8±0.7 |
| Heart weight (mg) | 136.6±4.4 | 137.7±3.3 | 132.6±3.8 |
| Heart weight/body weight (mg/g) | 3.67±0.1 | 3.74±0.1 | 3.70±0.1 |
| Coronary arteriolar wall thickness (normalized to lumen area) | 1.40±0.1 | 1.61±0.1 | 1.43±0.1 |
| Perivascular Collagen (normalized to lumen area) | 0.77±0.1** | 0.45±0.04 | 0.64±0.1* |
| Interstitial collagen (% area) | 1.21±0.1* | 0.72±0.1 | 1.22±0.2* |
p<0.05
p<0.01
P<0.001 vs. Control group
Figure 1. Representative sections of the myocardium.
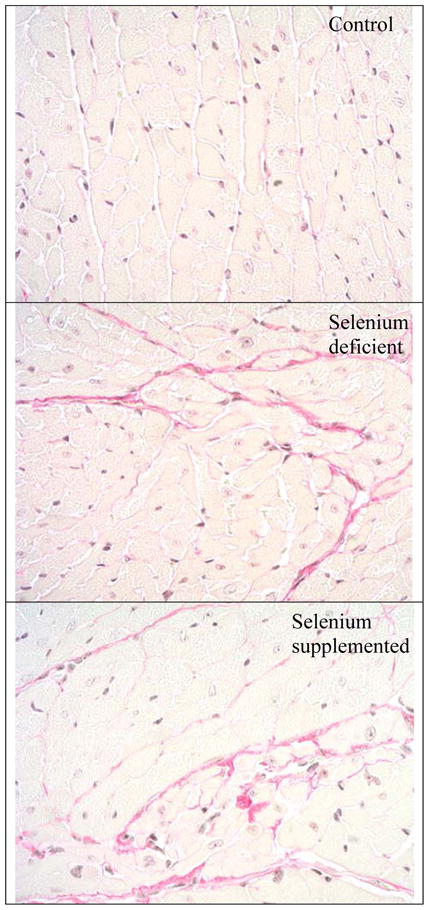
Picrosirius stained sections of the myocardium (X200) demonstrates increased staining for fibrillar collagen in the interstitium (pink staining) in the selenium deficient (middle panel) and selenium supplemented (lower panel) groups compared to the control group (upper panel).
Dietary selenium affects ventricular function
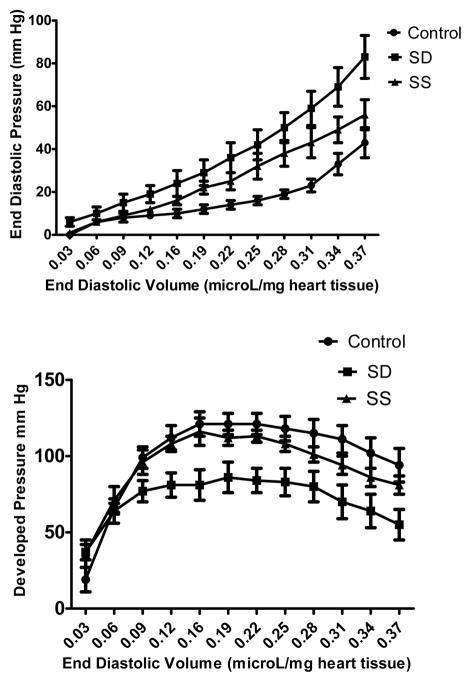
As shown in Figure 2 (upper panel), 12 weeks of treatment with the selenium deficient diet led to a significant upward shift of the diastolic pressure-volume curve compared to the control diet, with a similar but less prominent effect observed in the selenium supplemented group. Maximum observed values for −dP/dt were: control- 4945±117 mm Hg/s; SD-2881±441 mm Hg/s; and SS- 4154±373 mm Hg/s (SD significantly lower compared to the other two groups; p <0.05). Developed pressure was significantly decreased by selenium deficiency compared to control and selenium supplemented groups (Figure 2; lower panel). Maximum +dP/dt values obtained in the various dietary groups were: control- 4463±265 mmHg/s; SD-3582±458 mmHg/s.; and SS- 4505±393 mm Hg/s; (no significant differences between the groups)
Figure 2. Effect of selenium on cardiac function after 12 weeks of dietary treatment.
(n=6–8 animals/group). Upper panel demonstrates the effect of selenium on diastolic function. Compared to control diet, the selenium deficient diet caused a marked upward shift of the relationship of left ventricular end diastolic volume to end diastolic pressure (ANOVA; p<0.05). Selenium supplementation caused a lesser upward shift of the diastolic pressure-volume curve compared to control diet (ANOVA; p<0.05). Lower panel demonstrates the effect of selenium on cardiac systolic function. Developed pressure (peak systolic-diastolic) was significantly decreased by selenium deficiency (ANOVA; p<0.05), while selenium supplementation did not alter systolic function.
Selenium modulates myocardial expression of selenoprotein and matrix genes
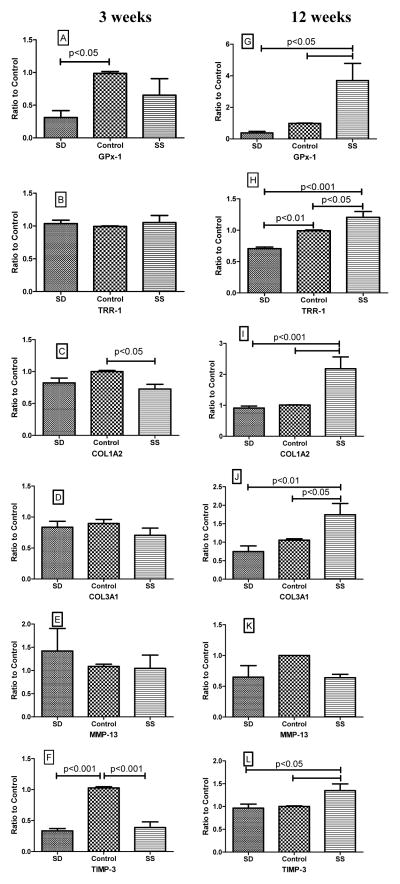
Figure 3 shows the expression of selenoprotein and matrix genes in the myocardium after 3 (short-term) and 12 weeks (long-term) of dietary intervention. We examined the expression of GPx-1, a selenoprotein which is proposed to be highly sensitive to selenium status, and of TRR-1, a “housekeeping” selenoprotein proposed to be less affected by dietary selenium content [38]. At 3 weeks, GPx-1 expression was markedly decreased in the SD group compared to control (Figure 3A), while at 12 weeks GPx-1 expression was significantly increased (more than 3-fold) in the SS group compared to control and SD groups (Figure 3G). There was no effect of the diets on TRR-1 expression at 3 weeks (Figure 3B); however, at 12 weeks, TRR-1 expression was significantly greater in the control group compared to SD, and in the SS group compared to both control and SD groups (Figure 3H).
Figure 3. Effect of selenium on myocardial gene expression.
Figures 3A–F demonstrate the effects after 3 weeks of dietary treatment, while figures 3G–L show the effect of selenium after 12 weeks of dietary intervention. Results are expressed as a ratio to the control group. P values obtained by ANOVA are shown in the figure. GPx-1–glutathione peroxidase-1; TRR-1–thioredoxin reductase-1; COL1A2–collagen I alpha2; COL3A1-collagen III alpha1; MMP-13–matrix metalloproteinase-13; and TIMP-3-tissue inhibitor of matrix metalloproteinase-1.
Dietary selenium also affected myocardial matrix gene expression. As shown in Figures 3C and 3I, selenium supplementation elicited a biphasic response in myocardial expression of COL1A2, with a significant decrease in expression at 3 weeks compared to control group, and a significant increase in expression at 12 weeks compared to the other two groups. COL3A1 expression was not significantly altered at 3 weeks (Figure 3D); at 12 weeks, there was a significant increase in expression in the SS group (Figure 3J). Myocardial expression of the major rodent collagenase MMP-13 was not significantly different in any group at either time point (Figures 3E and 3K). Figures 3F and 3L demonstrate the expression of TIMP-3, a major inhibitor of MMP activity. Both selenium deficiency and selenium supplementation significantly decreased TIMP-3 expression compared to the control group after 3 weeks of dietary intervention, while the expression of TIMP-3 was significantly increased in the SS group at 12 weeks compared to the other 2 groups.
Effects of selenium on redox status
Table 2 shows the effects of the three diets on plasma selenium and redox status at 12 weeks. The plasma selenium level was significantly decreased in the SD group compared to the other two groups. There was no significant difference in the plasma selenium concentration between the SS and control groups. The activity of GPx-1 was significantly decreased in the myocardium of SD mice compared to control (p< 0.05) and SS groups (p< 0.001). The intensity of immunostaining for the lipid peroxidation end-product HNE was significantly increased in the myocardium of the SD group compared to the other two groups. The plasma level of F2-isoprostane, a marker of systemic lipid peroxidation, was also significantly increased in the SD group compared to the other two groups.
Table 2.
Selenium and redox status at 12 weeks.
| Parameter | Dietary groups (n=7–8/group) | ||
|---|---|---|---|
| Selenium Deficient | Control | Selenium Supplemented | |
| Plasma Selenium (μg/L) | 61.8±6.4a | 370.7±38.8 | 403.9±1.2 |
| Myocardial GPx activity(mU/mg protein) | 16.7±4.2 | 38.2±1.4b | 57.3±9.7c |
| Myocardial HNE staining# | 2.37±0.2d | 2.04±0.2 | 1.54±0.2 |
| Plasma isoprostane level(pg/mL) | 320±58a | 83±15 | 102±12 |
p<0.001 vs. other groups;
p<0.05 vs. selenium deficient group;
p<0.001 vs. selenium deficient group;
p<0.05 vs. selenium supplemented group;
arbitrary scale of 0–4
Effect of dietary selenium on plasma thiol levels
The effect of dietary intervention on the plasma levels of homocysteine, cysteine, and glutathione are presented in Figure 4. Plasma homocysteine level did not vary between groups at 3 weeks (Figure 4A). At 12 weeks, the SD group had a significantly lower plasma homocysteine level compared to the control and SS groups (Figure 4D). Similarly, plasma cysteine level was significantly reduced in the SD group compared to the other groups at 12 weeks (Figure 4E), while the levels were similar in the three groups at 3 weeks (Figure 4B). Interestingly, the plasma glutathione level was significantly decreased in the SS group compared to control and SD groups at 3 weeks (Figure 4C), while at 12 weeks there was no significant difference between groups (Figure 4F).
Figure 4. Effect of selenium on plasma thiol levels.
Figures 4A–C demonstrate the effects of dietary intervention at 3 weeks, while panels 4D–F show effects of the three diets at 12 weeks. ANOVA was utilized to analyze for significant differences between groups and p values obtained are shown in the figure.
Selenium affects methylation status
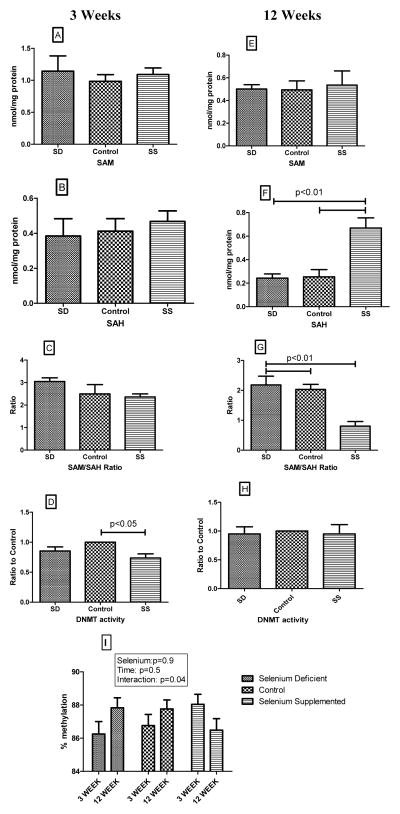
Since selenium affected the status of the methionine-homocysteine cycle in our model, and since selenium is proposed to affect gene expression by altering DNA methylation, we examined the effect of selenium on global methylation status. Figure 5 shows the effects of dietary treatment on liver content of S-adenosyl methionine (SAM), S-adenosyl homocysteine (SAH), the ratio of SAM/SAH, and myocardial DNA methyltransferase (DNMT) activity. None of the dietary treatments altered SAM levels at 3 or 12 weeks (Figures 5A and 5E). SAH level did not vary significantly among groups at 3 weeks (Figure 5B); however, SAH level was significantly increased in the SS groups compared to control and SD groups after 12 weeks of dietary treatment (Figure 5F). Consequently, the ratio of SAM to SAH was decreased significantly in the SS group at 12 weeks (Figure 5G) with no difference noted at 3 weeks (Figure 5C). Interestingly, DNMT activity in the myocardium was decreased significantly in the SS group at 3 weeks compared to the other two groups (Figure 5D); there was no statistically significant difference among the three groups at 12 weeks. Analysis of methylation status of five CpG sites which are normally highly methylated showed a similar differential response to time and selenium status. There was modest increase in methylation between 3 and 12 weeks in the control and selenium deficient groups, while an opposite effect of a decrease in methylation between 3 and 12 weeks was observed in the selenium supplemented group (Figure 5I).
Figure 5. Effect of dietary treatments on methylation potential and DNA methyltransferase activity.
Figures 5A–C demonstrate the effects of diets on liver methylation potential after 3 weeks of treatment, while figures 5E–G show the effects of 12 weeks of dietary intervention. Figures 5D and 5H demonstrate the effects of selenium on myocardial DNA methyltransferase activity. Figure 5I shows the average percentage methylation levels of five CpG sites in the LINE-1 retrotransposon. The p values obtained on ANOVA are shown in the figure; results of 2 way ANOVA to determine the interaction of selenium status and duration of treatment is shown in Figure 5I. SAM - S-adenosyl methionine; SAH – S-adenosyl homocysteine; DNMT- DNA methyltransferase.
Discussion
The results of our study provide novel insights into the effects of dietary selenium on myocardial remodeling. Similar to prior reports in cardiac injury models such as ischemia- reperfusion and adriamycin-induced cardiomyopathy, selenium deficiency de novo promoted systolic dysfunction accompanied by diastolic abnormalities. Unexpectedly, selenium supplementation at doses well below toxic levels also led to diastolic dysfunction. Since supplemental selenium altered methylation potential, DNMT activity, and methylation of DNA, the observed effects on matrix gene expression is likely modulated via epigenetic changes. Our study suggests that selenium status influences not only oxidant stress, but also methylation balance and epigenesis, thereby resulting in similar phenotypes with selenium deficiency and modest excess. These results demonstrate the complex biology of selenium in vivo, and have important implications for the use of selenium supplementation in cardioprotection.
In our study selenium deficiency resulted in increased oxidant stress, a reduction in GPx-1 expression and activity, and a reduction in systolic function similar to findings observed in prior reports [10, 39, 40]. While the effect on myocardial contractility could be secondary to previously reported abnormalities in calcium handling and contractile machinery [41], the effects of selenium depletion on myocardial fibrosis have not been well studied. Keshan disease is associated with multifocal myocardial necrosis and replacement fibrosis [42]. Similarly, clinical reports of severe selenium deficiency associated with parenteral nutrition also demonstrated replacement fibrosis similar to that found in Keshan disease [2]. A recent study utilizing a combined selenium and vitamin E deficiency model of Keshan disease demonstrated increased oxidant stress in the myocardium leading to myocardial fibrosis [43]. Since the interstitial fibrosis did not include larger areas, and since histological analysis did not reveal evidence of myocyte necrosis, the most likely reason for the observed fibrosis is reactive and not replacement fibrosis [44].
The novel findings of the study were the direct effects of selenium supplementation on myocardial matrix remodeling and function The dose used in our study (0.5 mg/kg) was similar to doses used in clinical studies, and was modest compared to previous studies that utilized doses as high as 2.5 mg Se/kg diet [11]. Moreover, the SS diet did not elevate the plasma selenium level compared to the normal diet. However, this modest dose of selenium produced favorable effects on myocardial expression of GPx-1, suggesting that the intracellular availability of selenium for selenoprotein synthesis was increased in the myocardium. Myocardial collagen content depends not only on the expression of the major myocardial collagens types I and III, but is also dependent on matrix metalloproteinases (MMPs) which degrade collagen as well as the collagen breakdown products gelatins, and the expression of tissue inhibitors of MMPs or TIMPs which regulate the activity of MMPs. The effects of the SS diet was time-dependent, with transcription of both major myocardial collagens (types I and III) and of TIMP-3 increased by selenium supplementation after 12 weeks of treatment, while at 3 weeks, the major effect was a decrease in TIMP-3 expression. Myocardial compliance decreased, as evidenced by an upward shift in the diastolic pressure-volume relationship without a significant change in the rate of myocardial relaxation measured by −dP/dt. Our observed results on myocardial matrix remodeling are similar to previous studies in non-cardiovascular tissues and cells. For example, a study by Kucharz and colleagues demonstrated that toxic levels of selenium increased total collagen content in the skin [45], while Chen and co-workers have shown that selenium significantly affects matrix turnover in chondrocytes [46]. Recent microarray-based studies on the effects of selenium on gene expression also suggest a significant impact of selenium on matrix gene expression, especially TIMP-3, in rat liver as well as in mouse liver and kidney [47, 48]. Conversely, a study by Carlson and colleagues showed that elimination of selenoprotein sysnthesis in mouse macrophages utilizing genetic mutation of the tRNA for selenocysteine increased collagen and TIMP-3 transcription in mouse macrophages [49]. Our study also suggests that selenium status is an important modulator of matrix metabolism even at modest supplemental doses.
In addition to the well-described effects mediated via changes in oxidant stress, selenium is also implicated in methylation balance and epigenetic modifications. As shown in Figure 6, selenium metabolism is intricately linked with the methionine-homocysteine cycle. In this cycle, methionine is converted to S-adenosyl methionine, which serves as the methyl donor for all methylation reactions with the exception of remethylation of homocysteine to methionine. Donation of a methyl group leads to the conversion S-adenosyl methionine to S-adenosyl homocysteine, which is hydrolyzed to yield the amino-acid homocysteine. Homocysteine can undergo one of two fates, remethylation or transsulfuration. Remethylation is catalyzed by either methionine synthase, which requires vitamins B12 and folate as cofactors, or by betaine homocysteine methyltransferase. Transsulfuration is initiated by cystathionine beta synthase, and irreversibly commits homocysteine to conversion to cysteine, the precursor of glutathione, which is the substrate for glutathione peroxidases. Selenium itself is methylated into mono-, di-, and tri-methyl forms which are less toxic [50], and based on some reports might have greater effect in preventing carcinogenesis than the non-methylated forms [51]. Hence as shown in Figure 6, selenium interacts extensively with the methionine-homocysteine cycle and thereby modulates redox and methylation reactions. Plasma homocysteine level was reduced by selenium deficiency, similar to prior reports in rodent models, possibly via increased flux through the transsulfuration pathway which irreversibly commits homocysteine to the production of cysteine and glutathione [23, 31, 32]. The initial reduction in plasma glutathione levels at 3 weeks is most likely due to the utilization of selenite during normal metabolism of selenite to selenide [52], with compensatory mechanisms over longer period of treatment leading to levels similar to control diet at 12 weeks. Selenium status has also been shown to affect methyl metabolism and DNA methylation [22, 24, 25]. In our study, selenium deficiency did not alter methylation potential represented by the ratio of SAM to SAH, or DNA methyl transferase activity; however, selenium supplementation markedly altered methylation potential at 12 weeks. The level of SAH, a potent inhibitor of all methyltransferases, was increased, while that of SAM did not change in the selenium-supplemented group. DNA methyltransferase activity was decreased in the selenium-supplemented group at 3 weeks. The decrease in DNA methylation between 3 and 12 weeks in the selenium supplemented group suggests that methylation is affected by the interaction between selenium supplementation and duration of treatment. This effect of supplemental selenium on DNA methylation could be the potential mechanism responsible for the marked changes in matrix gene expression seen with modest selenium supplementation as selenium has been reported to have effects on DNA methylation and other epigenetic mechanisms in several in vitro and in vivo studies [19, 24, 25]. Supporting this possibility is the fact that in humans, the TIMP-3 gene, and both genes coding for type I collagen have been shown to be regulated by methylation [53–55]. The effects of methylation imbalance in the adult stage, as in our study may be different from the effects of maternal methyl deprivation on the offspring, which has been shown to lead to a perinatal cardiomyopathy characterized by metabolic abnormalities and cardiac hypertrophy [56].
Figure 6. Interaction of selenium metabolism and the methionine-homocysteine cycle: effects on redox status and methylation.
Selenium in incorporated as the unique amino-acid selenocysteine in the catalytic site of the anti-oxidant enzyme glutathione peroxidase-1, which utilizes glutathione derived from transsulfuration of homocysteine, as a substrate. Homocysteine is derived from S-adenosylhomocysteine, which is formed as a result of methylation reactions including methylation of selenium. ROS-reactive oxygen species; RNS-reactive nitrogen species; B12-vitamin B12; B6-vitamin B6.
Our study examined the de novo effects of selenium supplementation. It is possible that in the presence of significant pathology such as hypertensive heart disease or myocardial infarction and the co-existent oxidant stress and neurohormonal activation, the effects of selenium could be different from the de novo effects observed in our study. For example, in a study by Lymbury and colleagues, normal and high selenium diets markedly reduced mortality in a spontaneously hypertensive rat model of heart failure, while the selenium status did not affect survival in normotensive rats [17].
As mentioned above, endemic selenium deficiency or marked deficiency resulting from parenteral nutrition can result in cardiomyopathy and heart failure [2, 42]. In addition, selenium deficiency is thought to play a role in heart failure associated with other conditions. For example, a reduced cardiac selenium level has been observed in patients suffering from acquired immune deficiency syndrome [4], and selenium supplementation has been shown to reduce Coxsackie virus induced myocardial damage in rodents with this syndrome [57]. However, epidemiologic and clinical studies have yielded conflicting results on the relation of the plasma selenium level to cardiovascular disease [58]. In fact, a U-shaped curve has been demonstrated for all-cause, cancer and cardiovascular mortality in relation to selenium levels; hence a narrow range of “optimal selenium status” could explain the relation of low and high selenium levels to disease states [59, 60]. Hence, the exact mechanisms of action of selenium in normal and pathologic states and the appropriate biologic markers of the myocardial effects of selenium need to be studied in detail to allow the use of selenium as a preventive or cardioprotective agent. It is possible that the effects of selenium supplementation on matrix metabolism and oxidant stress could be beneficial in conditions such as post-infarct remodeling, while these effects on matrix turnover could be deleterious in the context of pathologic hypertrophy with concomitant fibrosis as occurs in hypertensive heart disease.
Limitations
We analyzed global methylation using a surrogate marker, LINE-1. Our results do not address the methylation status of promoter regions or exons of matrix associated genes, which will be the objective of future studies. The changes in LINE-1 methylation are modest in absolute terms and may not correlate with methylation of specific sites, since global hypomethylation can co-exist with site-specific DNA hypermethylation [22]. The analyses reported in this study were conducted utilizing whole heart tissue and, hence, does not allow us to distinguish the effects in different cell types in the heart.
Conclusions
In conclusion, our results suggest that dietary selenium status directly affects myocardial matrix remodeling and function. Our results also suggest that along with redox balance, changes in methylation and epigenetic mechanisms also may play a role in the effects of selenium on cardiovascular biology. These novel findings offer new therapeutic targets in the use of selenium for cardioprotection as well as new biologic markers to monitor potential adverse effects of selenium.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health [grant number HL89734 to J. Joseph], [grant number HL52234 to D.W. Jacobsen], and [grant numbers HL61795, HL70819, HL48743, HL107192, and HL108630 to J. Loscalzo].
Footnotes
Conflicts of Interest:
None of the authors have any conflicts of interest to report.
References
- 1.Ge K, Xue A, Bai J, Wang S. Keshan disease-an endemic cardiomyopathy in China. Virchows Arch A Pathol Anat Histopathol. 1983;401:1–15. doi: 10.1007/BF00644785. [DOI] [PubMed] [Google Scholar]
- 2.Fleming CR, Lie JT, McCall JT, O’Brien JF, et al. Selenium deficiency and fatal cardiomyopathy in a patient on home parenteral nutrition. Gastroenterology. 1982;83:689–693. [PubMed] [Google Scholar]
- 3.Cenac A, Simonoff M, Moretto P, Djibo A. A low plasma selenium is a risk factor for peripartum cardiomyopathy. A comparative study in Sahelian Africa. Int J Cardiol. 1992;36:57–59. doi: 10.1016/0167-5273(92)90108-f. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin BM, Antonecchia PP, Smith F, Weiss L, et al. Reduced cardiac selenium content in the acquired immunodeficiency syndrome. JPEN J Parenter Enteral Nutr. 1989;13:644–647. doi: 10.1177/0148607189013006644. [DOI] [PubMed] [Google Scholar]
- 5.Oster O, Prellwitz W, Kasper W, Meinertz T. Congestive cardiomyopathy and the selenium content of serum. Clin Chim Acta. 1983;128:125–132. doi: 10.1016/0009-8981(83)90062-1. [DOI] [PubMed] [Google Scholar]
- 6.de Lorgeril M, Salen P, Accominotti M, Cadau M, et al. Dietary and blood antioxidants in patients with chronic heart failure. Insights into the potential importance of selenium in heart failure. Eur J Heart Fail. 2001;3:661–669. doi: 10.1016/s1388-9842(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo M, Laguardia SP, Bhattacharya SK, Nelson MD, et al. Micronutrients in African-Americans with decompensated and compensated heart failure. Transl Res. 2006;148:301–308. doi: 10.1016/j.trsl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Witte KK, Nikitin NP, Parker AC, von Haehling S, et al. The effect of micronutrient supplementation on quality-of-life and left ventricular function in elderly patients with chronic heart failure. Eur Heart J. 2005;26:2238–2244. doi: 10.1093/eurheartj/ehi442. [DOI] [PubMed] [Google Scholar]
- 9.Beck MA, Kolbeck PC, Shi Q, Rohr LH, et al. Increased virulence of a human enterovirus (coxsackievirus B3) in selenium-deficient mice. J Infect Dis. 1994;170:351–357. doi: 10.1093/infdis/170.2.351. [DOI] [PubMed] [Google Scholar]
- 10.Toufektsian MC, Boucher F, Pucheu S, Tanguy S, et al. Effects of selenium deficiency on the response of cardiac tissue to ischemia and reperfusion. Toxicology. 2000;148:125–132. doi: 10.1016/s0300-483x(00)00203-1. [DOI] [PubMed] [Google Scholar]
- 11.Boucher F, Coudray C, Tirard V, Barandier C, et al. Oral selenium supplementation in rats reduces cardiac toxicity of adriamycin during ischemia and reperfusion. Nutrition. 1995;11:708–711. [PubMed] [Google Scholar]
- 12.Stranges S, Marshall JR, Trevisan M, Natarajan R, et al. Effects of selenium supplementation on cardiovascular disease incidence and mortality: secondary analyses in a randomized clinical trial. Am J Epidemiol. 2006;163:694–699. doi: 10.1093/aje/kwj097. [DOI] [PubMed] [Google Scholar]
- 13.Stranges S, Marshall JR, Natarajan R, Donahue RP, et al. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 14.Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. Serum selenium concentrations and diabetes in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ Health Perspect. 2009;117:1409–1413. doi: 10.1289/ehp.0900704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stranges S, Sieri S, Vinceti M, Grioni S, et al. A prospective study of dietary selenium intake and risk of type 2 diabetes. BMC Public Health. 2010;10:564. doi: 10.1186/1471-2458-10-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, et al. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–266. doi: 10.3945/jn.110.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lymbury RS, Marino MJ, Perkins AV. Effect of dietary selenium on the progression of heart failure in the ageing spontaneously hypertensive rat. Mol Nutr Food Res. 2010;54:1436–1444. doi: 10.1002/mnfr.201000012. [DOI] [PubMed] [Google Scholar]
- 18.Davis CD, Uthus EO. Dietary selenite and azadeoxycytidine treatments affect dimethylhydrazine-induced aberrant crypt formation in rat colon and DNA methylation in HT-29 cells. J Nutr. 2002;132:292–297. doi: 10.1093/jn/132.2.292. [DOI] [PubMed] [Google Scholar]
- 19.Cox R, Goorha S. A study of the mechanism of selenite-induced hypomethylated DNA and differentiation of Friend erythroleukemic cells. Carcinogenesis. 1986;7:2015–2018. doi: 10.1093/carcin/7.12.2015. [DOI] [PubMed] [Google Scholar]
- 20.Xiang N, Zhao R, Song G, Zhong W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis. 2008;29:2175–2181. doi: 10.1093/carcin/bgn179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis CD, Uthus EO, Finley JW. Dietary selenium and arsenic affect DNA methylation in vitro in Caco-2 cells and in vivo in rat liver and colon. J Nutr. 2000;130:2903–2909. doi: 10.1093/jn/130.12.2903. [DOI] [PubMed] [Google Scholar]
- 22.Zeng H, Yan L, Cheng WH, Uthus EO. Dietary Selenomethionine Increases Exon-Specific DNA Methylation of the p53 Gene in Rat Liver and Colon Mucosa. J Nutr. 2011;141:1464–1468. doi: 10.3945/jn.111.140715. [DOI] [PubMed] [Google Scholar]
- 23.Uthus EO, Yokoi K, Davis CD. Selenium deficiency in Fisher-344 rats decreases plasma and tissue homocysteine concentrations and alters plasma homocysteine and cysteine redox status. J Nutr. 2002;132:1122–1128. doi: 10.1093/jn/132.6.1122. [DOI] [PubMed] [Google Scholar]
- 24.Davis CD, Uthus EO. Dietary folate and selenium affect dimethylhydrazine-induced aberrant crypt formation, global DNA methylation and one-carbon metabolism in rats. J Nutr. 2003;133:2907–2914. doi: 10.1093/jn/133.9.2907. [DOI] [PubMed] [Google Scholar]
- 25.Uthus EO, Ross SA, Davis CD. Differential effects of dietary selenium (se) and folate on methyl metabolism in liver and colon of rats. Biol Trace Elem Res. 2006;109:201–214. doi: 10.1385/BTER:109:3:201. [DOI] [PubMed] [Google Scholar]
- 26.Joseph J, Washington A, Joseph L, Koehler L, et al. Hyperhomocysteinemia leads to adverse cardiac remodeling in hypertensive rats. Am J Physiol Heart Circ Physiol. 2002;283:H2567–2574. doi: 10.1152/ajpheart.00475.2002. [DOI] [PubMed] [Google Scholar]
- 27.Joseph J, Joseph L, Shekhawat NS, Devi S, et al. Hyperhomocysteinemia leads to pathological ventricular hypertrophy in normotensive rats. Am J Physiol Heart Circ Physiol. 2003;285:H679–686. doi: 10.1152/ajpheart.00145.2003. [DOI] [PubMed] [Google Scholar]
- 28.Joseph J, Joseph L, Devi S, Kennedy RH. Effect of anti-oxidant treatment on hyperhomocysteinemia-induced myocardial fibrosis and diastolic dysfunction. J Heart Lung Transplant. 2008;27:1237–1241. doi: 10.1016/j.healun.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 29.Combs GF, Jr, Watts JC, Jackson MI, Johnson LK, et al. Nutr J. 2011;10:75. doi: 10.1186/1475-2891-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis CD, Uthus EO. Does dietary selenium affect plasma homocysteine concentrations in humans? J Nutr. 2003;133:2392. doi: 10.1093/jn/133.7.2392. author reply 2393. [DOI] [PubMed] [Google Scholar]
- 31.Uthus EO, Ross S. Dietary selenium (Se) and copper (Cu) interact to affect homocysteine metabolism in rats. Biol Trace Elem Res. 2009;129:213–220. doi: 10.1007/s12011-008-8295-4. [DOI] [PubMed] [Google Scholar]
- 32.Uthus EO, Ross SA. Dietary selenium affects homocysteine metabolism differently in Fisher-344 rats and CD-1 mice. J Nutr. 2007;137:1132–1136. doi: 10.1093/jn/137.5.1132. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Jhee KH, Hua X, DiBello PM, et al. Modulation of cystathionine beta-synthase level regulates total serum homocysteine in mice. Circ Res. 2004;94:1318–1324. doi: 10.1161/01.RES.0000129182.46440.4a. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Kramer PM, Yang S, Pereira MA, Tao L. Reversed-phase high-performance liquid chromatography procedure for the simultaneous determination of S-adenosyl-L-methionine and S-adenosyl-L-homocysteine in mouse liver and the effect of methionine on their concentrations. J Chromatogr B Biomed Sci Appl. 2001;762:59–65. doi: 10.1016/s0378-4347(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 35.Handy DE, Zhang Y, Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem. 2005;280:15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- 36.Forgione MA, Weiss N, Heydrick S, Cap A, et al. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2002;282:H1255–1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 37.Sheridan SD, Theriault KM, Reis SA, Zhou F, et al. Epigenetic characterization of the FMR1 gene and aberrant neurodevelopment in human induced pluripotent stem cell models of fragile X syndrome. PLoS One. 2011;6:e26203. doi: 10.1371/journal.pone.0026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, et al. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal. 2011;14:2327–2336. doi: 10.1089/ars.2010.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venardos K, Harrison G, Headrick J, Perkins A. Selenium supplementation and ischemia-reperfusion injury in rats. Redox Rep. 2004;9:317–320. doi: 10.1179/135100004225006803. [DOI] [PubMed] [Google Scholar]
- 40.Nakano E, Takeshige K, Toshima Y, Tokunaga K, Minakami S. Oxidative damage in selenium deficient hearts on perfusion with adriamycin: protective role of glutathione peroxidase system. Cardiovasc Res. 1989;23:498–504. doi: 10.1093/cvr/23.6.498. [DOI] [PubMed] [Google Scholar]
- 41.Wang YZ, Jia XA, Zhao JY, Xu GL. Effects of selenium deficiency on Ca transport function of sarcoplasmic reticulum and lipid peroxidation in rat myocardium. Biol Trace Elem Res. 1993;36:159–166. doi: 10.1007/BF02783175. [DOI] [PubMed] [Google Scholar]
- 42.Li GS, Wang F, Kang D, Li C. Keshan disease: an endemic cardiomyopathy in China. Hum Pathol. 1985;16:602–609. doi: 10.1016/s0046-8177(85)80110-6. [DOI] [PubMed] [Google Scholar]
- 43.Jia C, Chen X, Li X, Li M, et al. The effect of DHEA treatment on the oxidative stress and myocardial fibrosis induced by Keshan disease pathogenic factors. J Trace Elem Med Biol. 2011 doi: 10.1016/j.jtemb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 44.Weber KT, Brilla CG. Factors associated with reactive and reparative fibrosis of the myocardium. Basic Res Cardiol. 1992;87(Suppl 1):291–301. doi: 10.1007/978-3-642-72474-9_25. [DOI] [PubMed] [Google Scholar]
- 45.Kucharz EJ, Olczyk K. Influence of chronic intoxication with selenium on collagen and elastin content in tissues of rat. Toxicol Lett. 1993;68:295–299. doi: 10.1016/0378-4274(93)90020-x. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Chu Y, Cao J, Wang W, et al. Effects of T-2 toxin and selenium on chondrocyte expression of matrix metalloproteinases (MMP-1, MMP-13), alpha2-macroglobulin (alpha2M) and TIMPs. Toxicol In Vitro. 2011;25:492–499. doi: 10.1016/j.tiv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 47.Bosse AC, Pallauf J, Hommel B, Sturm M, et al. Impact of selenite and selenate on differentially expressed genes in rat liver examined by microarray analysis. Biosci Rep. 2010;30:293–306. doi: 10.1042/BSR20090089. [DOI] [PubMed] [Google Scholar]
- 48.Raines AM, Sunde RA. Selenium toxicity but not deficient or super-nutritional selenium status vastly alters the transcriptome in rodents. BMC Genomics. 2011;12:26. doi: 10.1186/1471-2164-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlson BA, Yoo MH, Sano Y, Sengupta A, et al. Selenoproteins regulate macrophage invasiveness and extracellular matrix-related gene expression. BMC Immunol. 2009;10:57. doi: 10.1186/1471-2172-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hassoun BS, Palmer IS, Dwivedi C. Selenium detoxification by methylation. Res Commun Mol Pathol Pharmacol. 1995;90:133–142. [PubMed] [Google Scholar]
- 51.Ip C, Ganther HE. Activity of methylated forms of selenium in cancer prevention. Cancer Res. 1990;50:1206–1211. [PubMed] [Google Scholar]
- 52.Suzuki KT, Ogra Y. Metabolic pathway for selenium in the body: speciation by HPLC-ICP MS with enriched Se. Food Addit Contam. 2002;19:974–983. doi: 10.1080/02652030210153578. [DOI] [PubMed] [Google Scholar]
- 53.Bachman KE, Herman JG, Corn PG, Merlo A, et al. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggest a suppressor role in kidney, brain, and other human cancers. Cancer Res. 1999;59:798–802. [PubMed] [Google Scholar]
- 54.Sengupta PK, Smith BD. Methylation in the initiation region of the first exon suppresses collagen pro-alpha2(I) gene transcription. Biochim Biophys Acta. 1998;1443:75–89. doi: 10.1016/s0167-4781(98)00188-2. [DOI] [PubMed] [Google Scholar]
- 55.Sengupta P, Xu Y, Wang L, Widom R, Smith BD. Collagen alpha1(I) gene (COL1A1) is repressed by RFX family. J Biol Chem. 2005;280:21004–21014. doi: 10.1074/jbc.M413191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia MM, Gueant-Rodriguez RM, Pooya S, Brachet P, et al. Methyl donor deficiency induces cardiomyopathy through altered methylation/acetylation of PGC-1alpha by PRMT1 and SIRT1. J Pathol. 2011;225:324–335. doi: 10.1002/path.2881. [DOI] [PubMed] [Google Scholar]
- 57.Sepulveda RT, Zhang J, Watson RR. Selenium supplementation decreases coxsackievirus heart disease during murine AIDS. Cardiovasc Toxicol. 2002;2:53–61. doi: 10.1385/ct:2:1:53. [DOI] [PubMed] [Google Scholar]
- 58.Navas-Acien A, Bleys J, Guallar E. Selenium intake and cardiovascular risk: what is new? Curr Opin Lipidol. 2008;19:43–49. doi: 10.1097/MOL.0b013e3282f2b261. [DOI] [PubMed] [Google Scholar]
- 59.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 60.Bleys J, Navas-Acien A, Laclaustra M, Pastor-Barriuso R, et al. Serum selenium and peripheral arterial disease: results from the national health and nutrition examination survey, 2003–2004. Am J Epidemiol. 2009;169:996–1003. doi: 10.1093/aje/kwn414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.